ISSN : 2348-9502
American Journal of Ethnomedicine
In Vivo Biological Investigation of Methanolic Extract of Thymus linearis Whole Plant
Department of Pharmacy, Abasyn University, Peshawar, Pakistan
- *Corresponding Author:
- Attiqa Naz
Department of Pharmacy
Abasyn University, Peshawar, Pakistan
Tel: 92 912582996
E-mail: tryzine2003@yahoo.com; saeedrph2000@yahoo.com; dr.rghaffar@gmail.com; mehrali05@hotmail.com
Received Date: June 13, 2018; Accepted Date: June 17, 2018; Published Date: July 23, 2018
Citation: Naz A, Saeed M, Ghaffar R, Shirazi M (2018) In Vivo Biological Investigation of Methanolic Extract of Thymus linearis Whole Plant. Am J Ethnomed Vol.5 No.1:2
DOI: 10.21767/2348-9502.10002
Abstract
Background and purpose: For treatment of pain, pyrexia and inflammation, medicinal plants have exhibited enormous capability. Medicinal plants are used as anti-pyretic, analgesic and anti-inflammatory agents having low gastro intestinal tract disturbing adverse effects. In order to tackle and assure the antipyretic, analgesic and anti-inflammatory response of herbs, scientific backdrop is mandatory.
Experimental approach: Abdominal writhing was introduced to experimental mice by intra peritoneal injection (i.p) of 1% acetic acid. Pain is induced thermally by observance the mice on hotplate having temperature around 50°C. For antipyretic activity, pyrexia was induced by 20 ml/kg body weight (b.w) i.p brewer′s yeast injection. Anti-inflammatory activity was examined in mice by Carrageenan. Thymus linearis methanolic extract (TLME) was used at test doses of 100, 200 and 300 mg/kg b. w i.p in mice.
Key results: TLME showed reduced writhing in acetic acid method with dose of 100, 200 and 300 mg/kg b. w by 54.00 ± 1.15, 44.00 ± 1.15 and 32.66 ± 0.66 in comparison of control i.e. 66.66 ± 1.76. The latency times in hotplate method was significantly increased from 13.95% to 21.22% at dose of 100 to 300 mg/kg. Antipyretic effect of TLME exhibited 39.70 ± 0.01 protections at 300 mg/kg after 2 h as similar to standard drug Paracetamol 38.57 ± 0.06. Anti-inflammatory effect of TLME was significantly observed by 36.66% and 35.29% respectively at dose of 300 mg/kg.
Conclusion and implications: Results of TLME showed that it can be used to treat pyrexia, pain and inflammation. This investigation actively reinforced the ethno pharmacological uses of T. linearis as analgesic, antipyretic and anti-inflammatory agent.
Keywords
Thymus linearis; Crude methanolic extract; Inflammation; Brewer’s yeast; Antinociceptive; Pyrexia
Abbreviations
b. w: Body Weight; i. p: Intra Peritoneal, TLME: Thymus linearis Methanolic Extract
Introduction
As medicine, plants have been used from ancient times [1]. Treatment through medicinal plants has been considered valuable on basis of empiric data of thousands of years [2]. Medicinal agents and their derivatives using nowadays clinically worldwide attributed about 50% of the entire drug while 25% of the total obtained from higher plants [3]. Today as a single chemical entities a large number of medicinally active compound are used from traditional herbal remedies [4]. The utilization of plants as a hotspot for new medication has been remarkably improved in human healthcare. Scientists utilized enthobotanical data as the “intimation to which plants are the prime contender for additionally screening and synthetic investigations”. Plants and herbs always are used in various dosage forms such as they can be taken as syrups, ointments, tablets, capsules, tea salves and rubs [5].
Thymus linearis also known as Himalayan Thyme is a small shrublet belongs to the family Lamiaceae [6]. In Urdu language it is called Star Farsi and in Pushto it is known as Tambarak. T. linearis possess antimicrobial anticancer [7], antioxidant [8] and hepatoprotective activity. From different species of Thymus various volatile oils has been isolated which have important medicinal activity [9-12]. T. linearis also contain alkaloids, carbohydrates, sterols, triterpenoids, steroids and tannins [13-15]. The crude methanolic extract of whole plant of T. linearis has not been used in in vivo biological investigations. The aim of the present study was to investigate acute toxicity, antipyretic, antinociceptive and anti-inflammatory activity of crude methanolic extract of whole plant of T. linearis to make it rationalized with its folk uses.
Material and Methods
Collection of plant material
The whole plant of T. linearis was collected from in August 2011 from Lake Saif-ul-malook situated in Naran valley, Hazara division, Khyber Pakhtunkhwa, Pakistan. Plant sample was identified by Plant Taxonomist Professor Dr. M. Ibrar, Department of Botany, University of Peshawar.
Extraction and fractionation
The whole plant material was macerated for 10 days in 25 liter methanol at temperature of 25-30°C. The soaked plant was vigorously stirred twice daily. A coarse cloth was used for filtration of methanol soluble substances and then it was filtered by filter paper (Whatmann filter paper No.1). Rotary evaporator was used to make concentrate the filtrate. After that fractionation of this crude methanolic extract of whole plant was done with n-hexane, Chloroform, ethylacetate, n-butanol and aqueous respectively [16].
In vivo biological screening
Acute toxicity activity: In acute toxicity assay the mice were treated with 500, 1000, 2000 mg/kg body weight of crude methanolic extract of T. linearis. Normal saline was used as negative control given through oral route. The LD 50 was measured for 2 days and animal weight was observed on daily basis for seven days.
Antinociceptive activity
Writhings induced by acetic acid: Albino mice of both sexes having weight 19-23 gms were selected for antinociceptive activity. The whole plant crude extract in particular doses of 100, 200 and 300 mg/kg body weight were given through oral route to selected mice and after 30 minutes of that 3% acetic acid about 0.34 ml was injected intraperitoneal to induce writhings in animals. These writhings or stretching of hind limb along with abdominal constrictions were seemed after 5 min of acetic acid administration intraperitoneal. The anti-nociceptive effect of crude methanolic extract was compared with diclofenac sodium which was used as positive control.
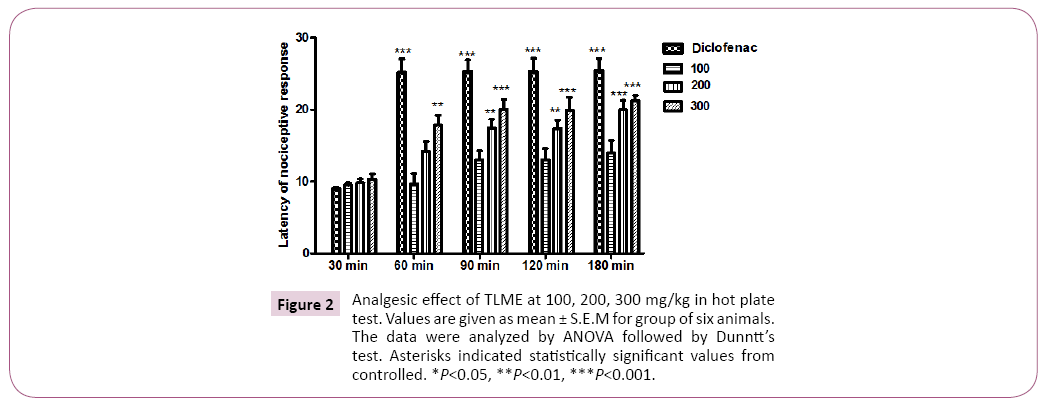
Hot plate method: The crude methanolic extract of T. linearis was given in a dose of 100, 200 and 300 mg/kg and after 30 min of dose administration mice were placed on hot plate. The temperature of hot plate was set at 50-55°C. The latency time was observed at 0, 30, 60, 90, 120 and 180 min by licking the hind paw or by jumping of mice. Percent analgesic effect was measured using the following formula:
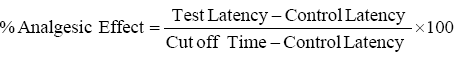
Anti- inflammatory activity
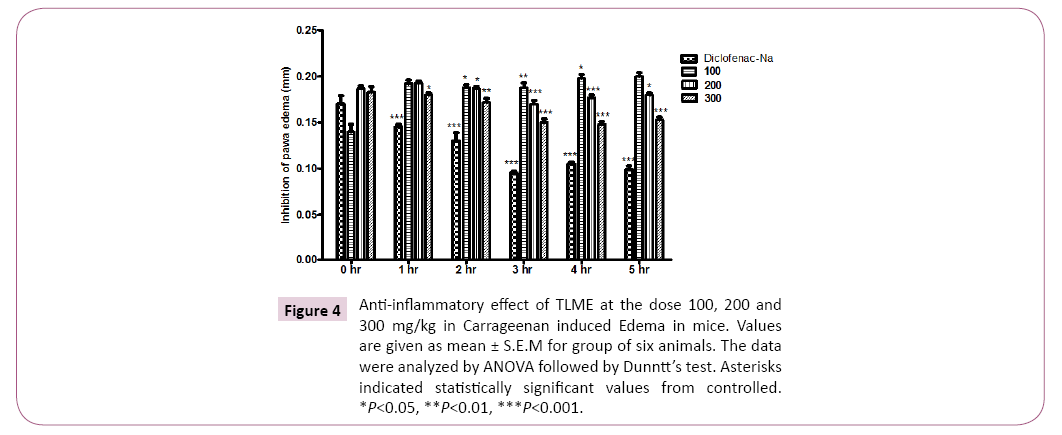
The crude methanolic extract of T. linearis whole plant was evaluated for its anti-inflammatory assay by carrageenan induced paw edema. After 30 min of test doses 100, 200 300 mg/kg body weight of methanolic extract the 0.1 ml of freshly prepared 1% carrageenan suspension was injected sub-plantar of the hind paw of mice. The edema induced by carrageenan and the antiinflammatory effect of extract was measured after 0, 1, 2, 3, 4 and 5 hours. The normal saline was used as negative control and diclofenac sodium in a dose of 10 mg/kg body weight was used as positive control.
Antipyretic activity
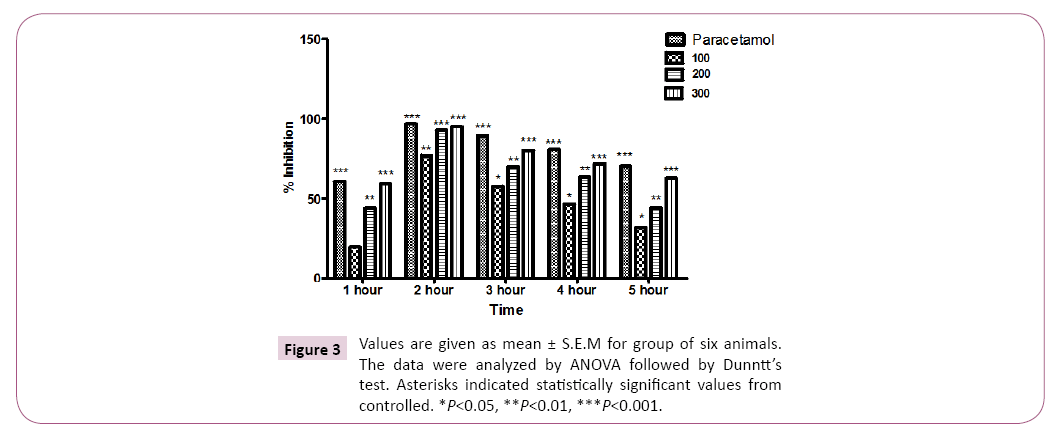
The antipyretic effect of crude methanolic extract of T. linearis whole plant was tested in selected mice by inducing pyrexia against Brewer’s yeast. About 2 ml/kg body weight of 20% w/v aqueous suspension of Brewer’s yeast was given subcutaneously to mice. The rectal temperatures were measured before and after 24 hours of Brewer’s yeast administration. The rectal temperatures of extract treated mice was examined after 1, 2, 3, 4,5 h by administration of 100, 200 and 300 mg /kg body weight of extract of T. linearis. The Salisylic acid was used as standard about 150 mg/kg body weight.
Results and Discussion
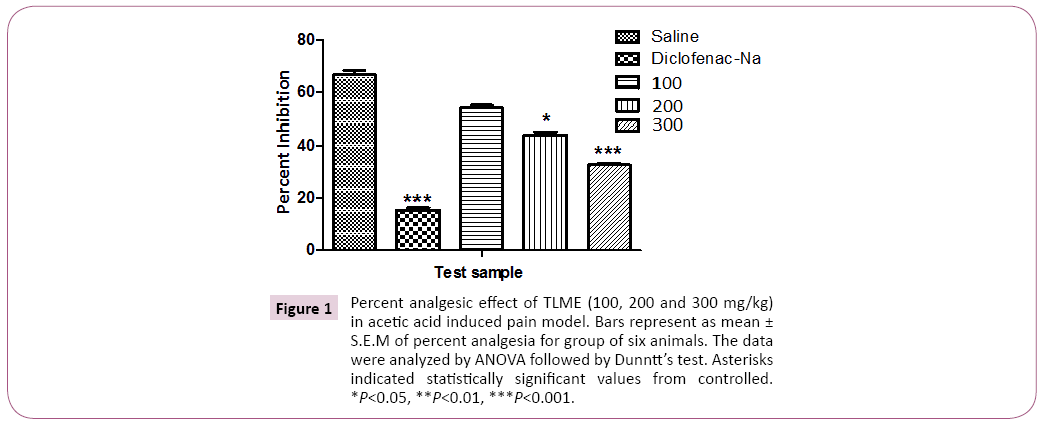
The crude methanolic extract of T. linearis showed dose dependent significant antinociceptive response at the dose of 300 mg/kg. The acetic acid induced writhings were reduced with the dose of 100, 200 and 300 mg/kg body weight by 54.00 ± 1.15, 44.00 ± 1.15 and 32.66 ± 0.66 in comparison of control i.e. 66.66 ± 1.76 as shown in Tables 1 and 2, Figures 1 and 2. In case of hotplate method the latency time was significantly increased from 13.95% to 21.22% at the dose of 100 to 300 mg/kg. The analgesic effect was observed significant after 90 min of extract administration given in Table 3 and Figure 3. In antipyretic activity the maximum antipyretic effect of TLME was observed at 2nd hour which remained significant up to 4th hour of extract administration as shown in Table 4 and Figure 4. The anti-inflammatory effect of TLME was found to be significant (p˂0.01) at dose of 200 and 300 mg/kg as given in Table 5 and Figure 4.
Figure 1: Percent analgesic effect of TLME (100, 200 and 300 mg/kg) in acetic acid induced pain model. Bars represent as mean ± S.E.M of percent analgesia for group of six animals. The data were analyzed by ANOVA followed by Dunntt’s test. Asterisks indicated statistically significant values from controlled. *P<0.05, **P<0.01, ***P<0.001.
Figure 4: Anti-inflammatory effect of TLME at the dose 100, 200 and 300 mg/kg in Carrageenan induced Edema in mice. Values are given as mean ± S.E.M for group of six animals. The data were analyzed by ANOVA followed by Dunntt’s test. Asterisks indicated statistically significant values from controlled. *P<0.05, **P<0.01, ***P<0.001.
Table 1: Acute toxicity test of TLME.
| Samples | Dosage | Response after 4 hours | Death after 24 hours |
|---|---|---|---|
| TLME | 500 | Alive and Normal | Alive and Normal |
| 1000 | Alive and Normal | Alive and Normal | |
| 2000 | Alive and Normal | Alive and Normal |
Table 2: Analgesic effect of TLME at 100, 200, 300 mg/kg in acetic acid induced test.
| Samples | Dose (mg/kgi.p) | No. of writhing (20 min) |
|---|---|---|
| Saline | 10 ml/kg | 66.66 ± 1.76 |
| Diclofenac | 5 | 15.33 ± 0.88*** |
| TLME | 100 | 54.00 ± 1.15 |
*P<0.05, **P<0.01, ***P<0.001
Table 3: Analgesic effect of TLME at 100, 200, 300 mg/kg in hot plate test
| Samples | Treatment/KG | 30 min | 60 min | 90 min | 120 min | 180 min |
|---|---|---|---|---|---|---|
| Saline | 10 ml | 9.30 ± 0.02 | 9.12 ± 0.08 | 9.14 ± 0.09 | 9.20 ± 0.03 | 9.12 ± 0.11 |
| Diclofenac | 30 mg | 9.00 ± 0.25 | 25.15*** ± 1.90 | 25.33*** ± 1.58 | 25.30*** ± 1.86 | 25.38*** ± 1.70 |
| TLME | 100 | 9.50 ± 0.34 | 9.70 ± 1.43 | 13.03 ± 1.26 | 13.02 ± 1.60 | 13.95 ± 1.76 |
| 200 | 9.83 ± 0.54 | 14.20 ± 1.39 | 17.45** ± 1.23 | 17.37** ± 1.17 | 20.00*** ± 1.28 | |
| 300 | 10.33 ± 7.10 | 17.87** ± 1.35 | 20.05*** ± 1.38 | 19.93*** ± 1.78 | 21.22*** ± 0.79 |
*P<0.05, **P<0.01, ***P<0.001
Table 4: Effect of TLME at 100, 200 and 300 mg/kg.i.p in yeast induced pyrexia.
| Sample | Dose | Rectal Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|---|
| After administration of extract | ||||||||
| mg/kg | Normal (A) | after 24 hours (B) | 1 h (C1) | 2 h (C2) | 3 h (C3) | 4 h (C4) | 5 h (C5) | |
| Saline | 10 ml | 36.57 ± 0.05 | 38.93 ± 0.04 | 38.83 ± 0.04 | 38.69 ± 0.01 | 38.69 ± 0.01 | 38.68 ± 0.01 | 38.73 ± 0.02 |
| Paracetamol | 150 mg | 36.82 ± 0.13 | 38.83 ± 0.12 | 38.04*** ± 0.12 | 38.57*** ± 0.06 | 38.63*** ± 0.03 | 38.40*** ± 0.02 | 37.35*** ± 0.19 |
| TLME | 100 | 36.91 ± 0.12 | 39.15 ± 0.14 | 28.86 ± 0.30 | 38.93** ± 0.03 | 38.02* ± 0.01 | 38.13* ± 0.02 | 38.20* ± 0.03 |
| 200 | 36.77 ± 0.14 | 39.20 ± 0.12 | 38.60** ± 0.12 | 38.37** ± 0.04 | 37.67** ± 0.04 | 37.73** ± 0.06 | 37.89** ± 0.03 | |
| 300 | 37.01 ± 0.01 | 39.07 ± 0.12 | 39.70*** ± 0.03 | 38.70*** ± 0.01 | 37.63*** ± 0.01 | 37.76*** ± 0.01 | 37.81*** ± 0.03 | |
*P<0.05, **P<0.01, ***P<0.001
Table 5: Effect of Intraperitonial administration of TLME at 100, 200 and 300 mg/kg in carrageenan induced paw edema test.
| Samples | Dose (mg/kg) | NPS | 0 h | 1 h | 2 h | 3 h | 4 h | 5 h |
|---|---|---|---|---|---|---|---|---|
| Saline | 10 ml | 0.0960 ± 0.22 | 0.177 ± 0.014 | 0.233 ± 0.022 | 0.243 ± 0.019 | 0.246 ± 0.017 | 0.245 ± 0.018 | 0.245 ± 0.22 |
| Diclofenic | 10 mg | 0.0901 ± 0.21 | 0.17 ± 0.009 | 0.145*** ± 0.003 | 0.13*** ± 0.009 | 0.095*** ± 0.002 | 0.105*** ± 0.002 | 0.099*** ± 0.004 |
| TLME | 100 | 0.910 ± 0.23 | 0.180 ± 0.008 | 0.192* ± 0.004 | 0.188* ± 0.003 | 0.188** ± 0.005 | 0.198* ± 0.004 | 0.200 ± 0.004 |
| 200 | 0.970 ± 0.23 | 0.185 ± 0.008 | 0.193 ± 0.004 | 0.187* ± 0.003 | 0.170*** ± 0.005 | 0.177*** ± 0.004 | 0.180* ± 0.004 | |
| 300 | 0.980 ± 0.23 | 0.195 ± 0.008 | 0.180* ± 0.004 | 0.172** ± 0.003 | 0.150*** ± 0.005 | 0.148*** ± 0.004 | 0.153*** ± 0.004 |
*P<0.05, **P<0.01, ***P<0.001
Conclusion
Thymus linearis contains essential oils thymol and p-cymene as main elements [17]. Phytochemical analysis of crude extract of plant showed presence of flavonoids, sterols, steroids and triterpenoids. These essential oils have already been reported to have analgesic, anti-inflammatory and antipyretic activities by inhibition of COX enzyme by a mechanism same like non-steroidal anti-inflammatory drugs [18,19]. Therefore, the analgesic, antiinflammatory and antipyretic activities of Thymus linearis may be due to these compounds.
References
- Alamgeer NM, Ahmad T, Mushtaq MN, Batoo A (2014) Hepatoprotective activity of Thymus linearis against paraceta -mol and carbon tetrachloride-induced hepatotoxicity in albino mice. Bangladesh J Pharmacol 9: 230-234.
- Heinrich M, Barnes J, Gibbons S, Williamson EM (2004) Fundamentals of Pharmacognosy and Phytotherapy. Churchill Livingstone, Elsevier Science Ltd., UK.
- Cragg GM and Newman DJ (2005) Biodiversity: A continuing source of novel drug leads. Pure Appl Chem 77:7-24.
- Pei S (2001) Ethnobotanical Approaches of Traditional Medicine Studies: Some Experiences from Asia. Pharmaceut Biol 39:74-79.
- Kim D, Ahn M, Jung J, Kwon S, Park EJ, et al. (2015) Perspective on the market globalization of Korean herbal manufacturers: A company based survey. Evide Based comple and alter medi 2015:1-12
- Rota MC, Herrera A, Martínez RM, Sotomayor JA, Jordan MJ (2008) Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 19: 681-687.
- Hussain AI, Anwar F, Nigam PS, Sarker SD, Moore JE, et al. (2011) Antibacterial activity of some Lamiaceae essential oils using resazurin as an indicator of cell growth. LWT-Food Sci Technol 44: 1199-1206.
- Janbaz KH, Nizsar U, Ashraf M, Qadir MI (2012) Spasmolytic, bronchodilator and anti-oxidant activities of Erythrinasuperosa Roxb. Acta Pol Pharm 69:1111-1117.
- Karaman S, Digrak M, Ravid U, Ilcim A (2001) Antibacterial and antifungal activity of the essential oils of Thymus revolutus Celak from Turkey. J Ethnopharmacol 76: 183-186.
- Pieroni A (2000) Medicinal plants and food medicines in the folk traditions of Lucca Province, Italy. J. Ethnopharmacol 70: 235-273.
- Rasooli I, Mirmostafa SA (2002) Antibacterial properties of Thymus pubescens and Thymus serpyllum essential oils. Fitoterapia.73: 244-250.
- Veras HN, Araruna MK, Costa JG, Coutinho HD, Kerntopf MR, et al. (2012) Topical anti-inflammatory activity of essential oil of Lippiasidoides Cham: Possible mechanism of action. Phytotherapy Res 27: 179-185.
- Ayoola GA, Coker H, Adesegun SA, Adepoju-Bello AA, Obaweya K, et al. (2008) Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res 7: 1019-1024.
- Kayani AS, Masood A, Achakzai AKK, Anbreen S (2007) Distribution of secondary metabolites in plants of Quetta Balochistan. Pakistan J Bot 39: 1173-1179.
- Gracelin DHS, Britto ADJ, Kumar PBRJ (2012) Antibacterial screening of a few medicinal ferns against antibiotic resistant phytopathogen. Int J Pharm Sci Res 3: 868-873.
- Garba SH, Sambo N, Bala U (2009) The effect of the aqueous extract of Kohautiagraifloraon paracetamol induced liver damage in albino rats. Nig J Physiol Sci 24: 17-23.
- Sharafzadeh S, Bahmani A (2014) Main components in aroma profile of genus thymus: A short review. J Curr Res Sci 2: 158-161.
- Naz A, Saeed M, Hussain MM, Ishaq SM (2015) In vitro phytochemical and antimicrobial screening of Thymus linearis. Bangladesh J Pharmacol 10: 21-26.
- Verma R, Padalia R, Chanotiya C, Chauhan A (2010) Chemical investigation of the essential oil of Thymus linearis (Benth. Ex Benth) from western Himalaya, India. Natural Prod Res 24: 1890-1896.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences