ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
In Vitro Assessment of the Antioxidant and Anti-Inflammatory Effects of Globularia Alypum L . Leaves
Nouir Sahar*, Houda Haddad, Amani Khalifa and Amira Zairi
Department of Biochemistry, University of Sousse, Tunisia
- *Corresponding Author:
- Nouir Sahar
Department of Biochemistry, University of Sousse, Tunisia
E-mail: sanouir@yahoo.fr
Received Date:April 05, 2022, Manuscript No. AJPSKY-22-12173; Editor assigned date: April 07, 2022, PreQC No. AJPSKY-22-12173 (PQ); Reviewed date: April 18, 2022, QC No. AJPSKY-22-12173; Revised date: April 27, 2022, Manuscript No. AJPSKY-22-12173 (R); Published date: May 04, 2022, DOI: 10.36648/2249-7412/22.12.5.220
Citation: Sahar N (2022) In Vitro Assessment of the Antioxidant and Anti-Inflammatory Effects of Globularia Alypum L.Leaves. Asian J Plant Sci Res Vol:12 No:5
Abstract
Objective
This work allowed the valorization of the leaves of Globularia alypum L. by extracting their global biochemical composition and evaluating their biological potential.
Methods
The extraction was based on the use of four different methods (soxhlet, sonication, maceration and infusion) in order to compare their effect on the quality of polyphenols and thus their potential efficacy to exploit in different domains.
Results
The highest composition of polyphenols values was evaluated by infusion and soxhlet extract respectively (89.35–74.25 mg Eq GAE/g ext. While, soxhlet method only assured an important extraction of flavonoids and flavonols (65.45 mg CAT/g ext and 44.16 mg RE/g ext.). The samples were an area under discussion to a screening for their antioxidant activities using the DPPH. FRAP and Nitric oxide assays. Soxhlet and maceration of leaves of G. alypum showed the important antioxidant activity (DPPH and Nitric oxide) with an IC50 (0.28; 0.34 mg/ml and 0.57; 0.67 mg/ml respectively). Concerning, the infusion and the maceration extract, it showed an important Radical-Scavenging Activity and Ferric Reducing (FRAP) with IC50 (0.34 and 0.38 mg/ml respectively). For the first time, the in vitro anti-inflammatory activity of the leaves G. alypum tested by four extraction methods was realized. The soxhlet and maceration methods revealed an important anti-inflammatory effect by stabilization membrane (1.45 and 2.01 mg/ml respectively) and inhibition of protein denaturation (albumin) (1.80 and 1.73 mg/ml respectively).
Conclusion: As a term of exploitation and valorisation, the leaves of G. alypum have antioxidant and anti-inflammatory effects thanks to their richness in phenols, flavonoids, flavonols, anthacynins, alkaloids, saponins and vitamin E.
Keywords
Globularia alypum L; polyphenols; Antioxidant activity; Anti-inflammatory activity
Introduction
The plant kingdom is a treasure house of potential drugs and latterly there has been a swell consciousness about the importance of medicinal plants [1]. Conductive to search for new chemical compounds having a potential antioxidant and anti-inflammatory activities, we have investigated a medicinal plant, namely Globularia alipum L. sometimes called Turbith, belongs to the family Globulariaceae which includes in Tunisia only one genus (Globularia) and two sub-species. One is Arabica L. and the other, less-known, being eu-Alypum L. It’s characteristic of the Mediterranean regions. It is a very branching, upright shrub, 30-60 cm [2]. Reactive Oxygen Species (ROS) such as hydroxyl, superoxide and peroxyl radicals are formed in human cells by endogenous factors and exogenously result in extensive oxidative damage that in turn leads to geriatric degenerative conditions, cancer and a wide range of other human diseases [3]. In recent years, there has been a growing interest in finding phytoconstituents such as phenolics, carotenoids, anthocyanins, and tocopherol. They stabilize cell membranes by reducing lipid peroxidation and scavenging free radicals [4]. For the more they have been found to exert chemopreventive [5], a strong antioxidant activity and may help to protect the cells against the oxidative damage caused by free radicals [6]. Such as carotenoids, the natural pigments from plant origin react rapidly with these free radicals and delay or alleviate the extent of oxidative deterioration [7]. Natural antioxidants, including also volatile chemicals can inhibit oxidative damage and may consequently prevent inflammatory conditions [8]. The chemical composition exhibited the presence of phenolic acid [9]. The iridoid glucosides [10]. In G. alipum. In addition, have demonstrated the extant of secondary metabolites whatever polyphenols, flavonoids and anthocyanins [11]. Many researchers have reported diver’s types of antioxidants in different kinds of higher plants [12]. More recent reports revealed that the extract of G. alipum is used as a source of potential antioxidants [13].
Inflammation is the immune system’s response to harmful stimuli, such as pathogens, damaged cells, toxic compounds, or irradiation [14]. Acts by removing injurious stimuli and initiating the healing process [15]. The rich wealth of the plant kingdom represents novel compounds with significant anti-inflammatory activities [11]. According to the study of [16]. G. alipum L. is widely used in folk medicine for its properties: anti-inflammatory drug, anti-ulcer, antioxidant, and various cancer lesions of the stomach, colon, rectum and oesophagus. The stability of polyphenols in plant extracts relies on numerous factors including the drying and extraction methods. Another relevant aspect of phenolic compound extraction is the selection of an appropriate pH that can influence the yield and stability of phenolic compounds. Acidic conditions are associated with higher extraction yields on different vegetable sources of phenolic compounds [17]. Which may explain that the quality control of herbal medicine and botanical supplements are influenced by the development of different extraction methods in order to analyses these compounds?
Therefore, the aim of this present study is to extract the phenolic compounds present in leaves extract of G. alipum; obtained by different methods in order to evaluate their effect on the biological potential (antioxidant and anti-inflammatory activities) of these compounds.
Material and methods
Plant material
The leaves of G. alypum L. were collected in January 2019 from the Ouardanin region in Tunisia. The botany identification was performed at the Department of Botany, Higher Institute of Biotechnology of Monastir (ISBM, Monastir, Tunisia).
Extraction
In the first step, the fresh leaves were dried at room temperature, and were powdered after dryness. This was carried out in pressurized extractor at the ratio of 20 g of leaves powder with 100 ml ethanol. Each extract was prepared for soxhlet, sonication and maceration methods. However, the infusion extract was prepared by adding 100 ml of boiling distilled water to the sample (10 g) and were left to stand at room temperature for 5 min. Then after filtration, the sample was lyophilized. Afterwards, dried crude concentrated extracts were weighed to calculate the extractive yield and stored in a refrigerator (4°C) in air tight bottles until used for analysis [18].
Phytochemical analysis
The ethanol and aqueous extracts were submitted to phytochemical analysis for secondary metabolites identification using the phytochemical methods, which were previously described by [19]. In general, the presence or absence of saponin, coumarin, Terpenoids, carbohydrate and Quinone molecules were subsequently detected by the addition of an appropriate chemical agent to the preparation in a test tube.
Saponins
The extract (1 ml) was shaken vigorously with distilled water. A stable persistent froth for 20 min. was a positive indicator.
Coumarins
NaOH (2 ml, 10%) was added to 1 mL of extract and formation of yellow color indicates the presence of coumarins.
Terpenoids
The extract (2 ml) was added to acetic anhydride (2 ml) and concentrated H2SO4 drops. Formation of blue, green rings indicated the presence of terpenoids.
Carbohydrates (Molisch’s test)
Few drops of Molisch’s reagent were added to the extract (1 ml), followed by 1 ml of conc. H2SO4 drops. The mixture was allowed to stand for two-three minutes. Formation of a red or dull violet colour at the interphase of the two layers was a positive test.
Quinones
An extract (1 ml) was treated with concentrate HCl drops and observed for the formation of yellow precipitate or coloration.
Determination of Polyphenols Content
Total Phenolic Content (TPC)
The TPC of G. alipum leaf extracts was determined according to the method of [20]. It was based on the reaction with Folin-Ciocalteu’s procedure, using gallic acid as the standard. 0.5 ml of the sample was shared with 2.5 ml of Folin–Ciocalteu reagent and 2 ml of Na2CO3 (75 mg/ml). After incubation at 40°C for 40 min, the absorbance was deliberated at 765 nm. All determinations were performed in triplicate and quantification was done on the basis of the standard curve of gallic acid. The TPC was expressed as mg Gallic Acid Equivalents (GAE) per g of extract.
Total flavonoids
The content of flavonoids was determined by [21]. Method using catechin as a reference compound. 0.5 ml of extract, 2.5 ml of distilled water and 0.15 ml (5%) sodium iodide Na2NO2 were shaken. Then 0.3 ml of AlCl3 (10%) was added and allowed to stand for 6 min before adding 1 ml of NaOH (1 M) and 0.5 ml of distilled water. After incubation for 15 min, the absorbance was then recorded at 510 nm. The total flavonoids content was expressed in milligrams of Catechin equivalent (CAT) per gram of samples. Analysis of each sample was performed in triplicate.
Flavonols content
The content of flavonols was determined by AlCl3 method as described by [22]. Briefly, 0.5 ml of the plant extract was blended with 1 ml aluminium trichloride (2%) and 0.3 ml of acetate of sodium (5%). The mixture was shaken and allowed to rest at room temperature in obscurity for 2 h 30 min. The absorption at 440 nm was then noted. The absorption of rutin solutions was measured under the same conditions. All determinations were carried out in triplicate. The amount of flavonols in plant extracts was calculated in milligrams of Rutin Equivalents (RE) per gram of samples.
Total anthocyanin contents
The Total Anthocyanin Content (TAC) was estimated using the pH-differential method as described by [23]. 1 ml of TAE solution (1 mg/ml) was blended separately with 9 ml buffer at pH 1.0 (0.1 M HCl/4.9 mM KCl) and another at pH 4.5 (24.8 mM sodium acetate). Then, the mixture was incubated for 1 h in the dark. Absorbance was measured at 510 nm and 700 nm in buffers of pH 1.0 and pH 4.5, respectively. The total anthocyanin content was expressed as milligrams cyanidin-3-glucoside equivalents per gram of dry weight purification (mg C-3-G/g DW). For the calculation, we have used this formula:
Anthocyanin content (mg/g)=(A × MW × DF × V × 1000)/(ε × L × Wt)
Where
A:(A515 nm-A750 nm) pH1.0–(A515 nm-A700 nm) pH 4.5/> MW=Cyanidin-3-glucoside molecular weight (449.2)
L=Cell path length (usually 1 cm)
DF=Dilution factor
ε=Cyanidin-3-glucoside molar absorptivity (26,900)
V=the final volume (ml), and Wt=Extract weight (mg).
Estimation of alkaloid content
The total alkaloid content of G. alypum leaves was determined. After pulverization, 5 g of the tested leaves was engrossed in 200 ml of acetic acid in ethanol (10%). After incubation for 4 h at room temperature, the extract was concentrated using a water bath. A drop of concentrated ammonium hydroxide was added. Then, the solution was filtered again after addition of dilute ammonium hydroxide. The residue obtained was first dried and then weighed. To calculate the alkaloid content, this formula was used:
%Alkaloid=Weight of precipitate/Weight of original sample= × 100
Estimation of saponin
The saponin content was evaluated based on the method described by [25]. 5 g of the plant powder was supplemented to 20 ml of ethanol (20%). After extraction for 30 min, the sample was heated over a water bath at 55°C for 4 h. Then, the residue was re-extracted again with 20 ml of ethanol (20%). In a water bath at 90°C. The filtrate was reduced to 40 ml and extracted again twice with 20 ml diethyl ether. After that, n-butanol (60 ml) was added, and the extract was washed twice with 10 ml of 5% aqueous sodium chloride. Finally, the sample was evaporated to dryness to a constant at 40°C. According to this equation, the saponin content was calculated:
%Saponins contents Weight of residue/Weight of sample = × 100
Estimation of phytate
The total phytate content was estimated using the study described by [24]. 2 g of the crushed plant was added to 50 ml of hydrochloric acid (2%) for 3 h. Then, 25 ml of the filtrate was supplanted to 5 ml of ammonium thiocyanate solution (0.3%). To attain the desired acidity, 53.5 ml of distilled water was added. Then 0.05 M of iron III chloride was titrated in order to obtain a reddish-brown colour which persists for 5 min. The formula to calculate the phytate content was:
Phytate (%)=titre value × 0.00195 × 1.19 × 100.
Vitamin A estimation
This method was described by [26], we added 20 ml of petroleum ether to 1 g of pulverized plant on a shaker for about 30 min. After decantation and evaporation of the petroleum ether, 0.2 ml of chloroform-acetic anhydride (1:1 v/v) and 2 ml of trichloroacetic acid-chloroform (1:1 v/v) were added to the residue. The absorbance was calculated at 620 nm. The vitamin A standard was also prepared in the same way at varying concentrations and a standard curve plotted.
Vitamin E estimation
Vitamin E estimation was determined according to the method described by [26]. 20 ml of ethanol was mixed with 0.5 g of the pulverized sample on a shaker for 20 min. After filtration, 1 ml of the filtrate was withdrawed and was added to 1 ml of ferric acid in ethanol (0.2%) and 1 ml of α-α-dipyridine (0.5%). Then, the solution was added up to 5 ml with distilled water. The absorbance was measured at 520 nm. The vitamin E standard was also prepared in the same way at varying concentrations and a standard curve plotted.
Evaluation of Biological Activities
DPPH radical scavenging assay
For the evaluation of antioxidant activity, the DPPH free radical scavenging assay was carried out. This method is based on electron-transfer that produces a purple color that decays in the presence of an antioxidant, which can donate an electron to DPPH. Then the absorbance change is measured at λ=517 nm. The antiradical activity of the plant extract was examined based on the scavenging effect of the stable DPPH free radical activity [27].
According to the method of [28], 180 μl of various concentrations of extracts was added to 1620 μl of DPPH, prepared daily, kept in the darkness at room temperature for 30 min and the absorbance was measured at 517 nm against a blank. The following equation was used to determine the percentage of the radical scavenging activity of each extract. The antiradical activity was expressed as IC50, the extract dose required to induce a 50% inhibition and the value was obtained by interpolation from linear regression analysis. The ability to scavenge the DDPH radical was determined by dint of the following formula:
[(ADPPH-AS)/ADPPH]*100
Where: AS is the absorbance of the solution containing the sample and ADPPH is the absorbance of the DPPH solution.
Determination of Ferric-Reducing Antioxidant Power (FRAP)
The FRAP method was used to determine the total antioxidant activity by measuring the reduction of ferric ion to the ferrous form in the presence of antioxidant compounds [29]. It was revealed to [30] method Briefly, Samples was blended with 2,5 ml sodium phosphate buffer (pH 6.6) and 2.5 ml of potassium ferricyanide (1%). After incubation at 50°C for 20 min, 2.5 ml of trichloroacetic acid (10%), 2.5 ml of distilled water and 0.1 ml of ferric chloride (0.1%) were added, and the mixture was centrifuged. The absorbance was measured at 700 nm. Results were expressed on (IC50).
Nitric radical scavenging activity
At physiological pH, nitric oxide generated from aqueous sodium nitroprusside solution interacts with oxygen to produce nitrite ions, which may be quantified by the Griess Ilosvoy reaction [31]. Nitric oxide scavenging activity was determined according to the method described by [32]. In brief, 2 ml of Sodium nitroprusside (10 mM) was prepared in 0.5 ml phosphate buffer saline (pH 7.4) and varied with 0.5 ml of either plant extracts or ascorbic acid as a standard, at various concentrations (0.009-10 mg/ml). After incubation at 25°C for 2 h 30 min, 0.5 ml of Griess reagent (1.0 ml of 0.33% sulfanilic acid reagent prepared in 20% glacial acetic acid at room temperature for 5 min with 1 ml of naphthylethylenediamine dichloride) was added to 0.5 ml of the incubated solution. After incubation at room temperature for 30 min the absorbance was measured at 540 nm. The amount of nitric oxide radical inhibited by the extracts was determined based on the following equation:
Nitric oxide (NO) radical scavenging activity=(Abs control-Abs sample)/(Abs control) × 100
Where, Abs control was the absorbance of NO radical+methanol; Abs sample was the absorbance of NO radical+sample extract or standards (Vitamin C).
Anti‑inflammatory Activity
Preparation of blood samples for membrane stabilization assays
The Human Red Blood Cell (HRBC) membrane stabilization method has been used as a method to study the in vitro anti-inflammatory activity [33]. The blood was taken from a healthy human volunteer who did not undergo any Non-Steroidal Anti-Inflammatory Medicines (NSAIDS) for 2 weeks. Then it was varied with identical volume of Alsever solution (2% dextrose, 0.8% sodium citrate, 0.5% citric acid and 0.42% NaCl). The samples were stored at 4 °C for 24 h and the supernatant was removed after a centrifugation at 2500 rpm for 5 min. After washing with sterile saline solution (0.9%w/v NaCl), the cell suspension was centrifuged at 2500 rpm for 5 min for three times till the supernatant was clear and colourless. The cellular component was reconstituted to a 40% suspension (v/v) with phosphate buffered saline (10 mM, pH 7.4) and was used in the assays.
Hypotonicity solution induced haemolysis
- alypum leaves were prepared (0.039 to 10 mg/ml), respectively using distilled water. 1 ml of phosphate buffer, 2 ml hyposaline and 0.5 ml of HRBC suspension were added to each concentration. After incubation at 37°C for 30 min and centrifugation at 3000 rpm for 20 min, the haemoglobin content of the supernatant solution was mesured spectrophotometrically at 560 nm. As a standard reference, we used Aspirin. The percentage inhibition of haemolysis or membrane stabilization was calculated according to the method posted by [34].
%Inhibition of haemolysis=100 × {OD1−OD2/OD1}
Where:
OD1=Optical density of hypotonic-buffered saline solution alone.
OD2 =Optical density of test sample in hypotonic solution.
Inhibition of protein denaturation assay
Protein denaturation beget loose of biological properties of protein molecules. Protein denaturation has been correlated with the formation of inflammatory disorders similar to rheumatoid arthritis, diabetes as well as cancer. Therefore, the ability of a substance to prevent the protein denaturation may also help to prevent the inflammatory disorders [35]. In this assay egg albumin is used as protein [36]. Denaturation of protein is induced by keeping the reaction mixture at 70°C in a water bath for 10 minutes [37]. A reaction mixture consists of various concentrations of plant extract 10 mg/ml (cascade dilution), 200 μl of egg albumin was added to 1400 μl of phosphate buffered saline. As a negative control, distilled water instead of extracts with above mixture is used. After incubation at 37°C for 15 min, the mixed solution was heated at 70°C for 5 min. Following cooling under running tap water, their absorbances were measured at 660 nm. Diclofenac is taken as a positive control [36]. The experiment is carried out in triplicates and percent inhibition for protein denaturation is calculated using following equations [36].
% Inhibition of denaturation=(1-D/C) × 100
With: D is the absorbance of extract and C is the absorbance of negative control.
Statistical analysis
Results are presented as mean values ± standard deviation. Statistical analyses, using excel, of experimental results are based on SPSS.
Results and Discussion
Yield’s G. alypum determination
The extraction yield is presented in (Table 1). Results revealed that different extraction methods of G. alypum give different amounts of extractable soluble compounds, which was an expected result. The highest extraction yield was exhibited by the Soxhlet method (71.9%) and it was approximately 1.5 times higher than that of the maceration method (48.8%); 2.4 times higher than that of the infusion method (30%) and 1.1 times higher than that of the sonication method (64.8%) [38]. Evaluated the influence of different plants residues on extraction yield and observed that the type of residue was more influential than the solvent system on extraction yield. In addition, [39] demonstrated that sonication’s has better performance than maceration. This conclusion was confirmed by the results of scanning electron microscope, in which more cell surface damage and deeper gaps were found [39]. That can increase phenolic compounds extraction from plants. The acidity can influence the quality of phenolic compounds. (Table2) showed that soxhlet and maceration’s extraction presented the highest acidity middle (5.42 and 5.5 respectively). The more the middle is acidic, the more phenolic compounds are stable and the more their oxidation is weaker. It is worth noting that although the high pH used to inactivate antinutrients in cowpeas reduced their polyphenol content by up to 67%, no concurrent formation of lysinoalanine was observed [40]. Compared to the study of [41], our study showed the highest values of yield obtained by soxhlet and maceration of the leaves of G. alypum (91.90% and 48.80%; 43.50% and 30.40%). Hence, the difference in yield registered between the two extracts could be owing to the impact of the heat, which has a straight action on various thermo-sensitive phenolics.
| Aqueous extract(g) Organic extract (g) | ||||
|---|---|---|---|---|
| Methods | Infusion | Maceration | Sonication | Soxhlet |
| pH | 6.38 | 5.5 | 5.90 | 5.42 |
Table 1: Comparison between the different pH of G. alypum leaves.
| Aqueous extract(g) Organic extract (g) | ||||
|---|---|---|---|---|
| Methods | Infusion | Maceration | Sonication | Soxhlet |
| 30% | 48,8% | 64,8% | 71,9% | |
Table 2: Comparison between the different yield’s method of G. alypum leaves.
Phytochemical screening
The presence of bioactive constituents was assessed by the qualitative phytochemical screening of the ethanol or water extracts and results of screening are presented in Table 3. The phytochemical screening of all extracts obtained by different methods exhibited the presence of phenolic compounds, saponins, terpenoids, coumarins, carbohydrates and Quinons in the leaves of G. alypum. However, saponins are detected only in the infusion extracts, see (Table 3). On the other hand, Sonication’s method presented richness on carbohydrates and terpenoids. But soxhlet’s method demonstrated richness on coumarins. This plant is rich in biomolecules which can be valorised and exploited in various biological activities. This finding corroborates with the previous study on the G. alypum leaves extracts obtained with methanol using maceration extraction [42].
| Phenolic compounds | Soxhlet | Sonication | Maceration | Infusion |
|---|---|---|---|---|
| Saponins | - | - | - | + ++ |
| Coumarins | ++ | + | + | + + |
| Terpenoids | + | ++ | + | _ |
| Carbohydrates | ++ | ++ + | ++ | + |
| Quinons | - | - | - | _ |
Table 3: Phytochemical screening tests of Globularia alypum leaves.
The results of phytochemical screening test. Key: (+) Presence and (-) Absence.
Determination of phenolic compounds
Phenolic compounds are one of the most effective antioxidative constituents that contribute to the antioxidant activity [43]. Play a role in free radical scavenging capacities [44]. Total Phenolic Content (TPC) of G. alypum leaves was determined by the Folin-Ciocalteu assay. The result showed that the leaves of G. alypum are rich on phenolic compounds. According to (Table 4). The highest TPC and flavonols were obtained from the leaves by infusion and soxhlet of G. alypum (89.35; 74.25 mg GAE/g ext and 44.16 and 32.31 mg Eq RE/g ext, respectively). While soxhlet and maceration methods showed good extraction of flavonoids (65.45 and 58.50 mg CAT/g ext). However, infusion, sonication and soxhlet allowed a large quantity of anthocyanin’s extraction (2.84; 2.066 and 1.95 mg Eq Cyanidin-3-glucoside/g ext). In another work, [45] obtained the highest rate of polyphenols 139 g GAE/g of dry plant extract and from macerated ethanol extract of G. alypum. On the contrary, our study showed the richness of polyphenols obtained by infusion (89.35 mg GAE/g ext.) with the highest rate compared with the study of [46] (55.10 mg GAE/g ext.). This result suggests that the amount of polyphenols was 1.62 times lower than the one we established. This variability can be elucidated by the difference among the extracting solvents (water or ethanol), the quality of the soil where the plant is planted, and the climate. These factors can influence the richness of G. alypum. In fact, many studies have notified that polar fractions have more phenolic contents. Water availability, temperature, altitude, UV, soil and humidity constitute important factors that affect metabolism and secondary metabolite accumulation [47]. Thus, as a survival strategy, environmental variations in different provenances lead to the variation of the phenolic compounds of plants [48]. Therefore, it is important to note that many factors influence the quality of polyphenols, as a consequence, their biological activities. Processing techniques involving extraction solvent, pH, light and heat can markedly influence the levels and efficacy of bioactive compounds of dietary supplements such as polyphenolic compounds [49].
| Phenolic compounds | soxhlet | Sonication | Maceration | Infusion |
|---|---|---|---|---|
| Phenols (mg GAE/g ext) | 74.25 ± 0.08b | 52.68 ± 0.08d | 6 61 ± 0.10c | 89.35 ± 0.02a |
| Flavonoids (mg CAT/g ext) | 65.45 ± 1.82ac | 42.53 ± 1.36d | 58.50 ± 0.00b | 51.22 ± 0.80c |
| Flavonols (mg RE/g ext) | 44.16 ± 0.30a | 28.87 ± 0.79c | 23.87 ± 0.14d | 32.31 ± 0.10b |
| Anthocyanins ( mg cyanidin-3-glucoside/ L) | 1.95 ± 0.02ca | 2.066 ± 0.02b | 0.24 ± 0.08d | 2.84 ± 0.02a |
Table 4: Phytochemical constituents identified in the various extracts of G. Alypum leaves.
Results of the ANOVA test are significantly different at p<0.05 and each data point is represented by the average of three repetitions ± SD. Values with different superscripts within the same column are significantly different (P<0.05), as determined by the Student-Newman-Keuls test.
The ANOVA test confirmed that the interaction between the different methods of extraction and the quantity of phenolic compounds had a significant effect.
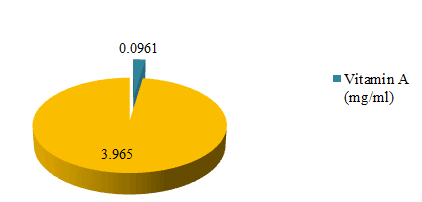
Vitamins analysis of Globularia alypum
This study observed vitamins A and E in the dehydrated leaves of Globularia alypum (Figure 1). The uppermost vitamin content was vitamin E at 3.965 mg/g of dried extract. Whereas the vitamin content was 0.0961 mg/g of dried extract. Vitamins are renowned nutrient that abetted to health and well-being. Although needed in small amounts they play a vital role in normal body physiology and their deficiencies have been linked to some diseases [50]. Vitamin E is well-known antioxidant that plays a major role in cells that display oxidative stress including cancer cells [51]. Vitamin A derivatives such as all trans-retinoic acid are used in the management of acute promyelocytic leukaemia [52]. Some of these vitamins and their derivatives have been mooted as chemopreventive agents [52]. The discovery of more sources of these vitamins and their derivatives like Globularia alypum can be an approach to supervise chronic diseases.
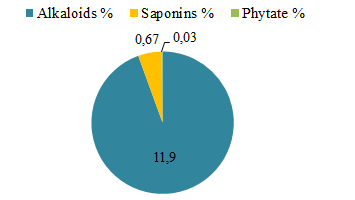
Alkaloids, saponins and phytate analysis of Globularia alypum
Our results showed that the leaves of Globularia alypum contain alkaloids, saponins and phytate (Figure 2). These leaves are rich of alkaloids (11.90%), but have a trace of phytate (0.03%) and an average amount of saponins (0.67%). Alkaloids are a group of important secondary metabolites among those [53]. Alkaloids give dynamic biological activities in human or animal Body [54]. It also give anti-inflammatory, demulcent, ganglionic blocking, anti-plasmodic activity, insecticidal and a hepatoprotective activity. Among the natural ailments used for providing, a major source of pharmaceuticals or promoted for marketed form [54].
DPPH scavenging activity
The different methods of extraction of Globularia alypum evaluated scavenged DPPH efficiently. Their scavenging activity was dose dependent. The maceration and soxhlet extraction exhibited the highest scavenging activity with the lowest IC50 (0.28 and 0.33 mg/ml). All the extracts had lower activity than the standards ascorbic acid (0.01 mg/ml) sees Table 4. In addition, the lowest antioxidant activity is displayed by sonication method (0.40 mg/ml). It is known that sonication, is a method of applying sound energy in order to agitate some particles in a sample. According to the study of [55]. Ultrasound could inhibit the browning of the fresh apple juice to a certain extent, but at the same time it accelerated the degradation of polyphenols and decrease of antioxidant activity of fresh apple (Malus pumila Mill) juice [55]. Results are in agreement with previous data indicating high antioxidant effect of G. alypum [56]. This potentiality is mostly correlated with the type of phenolic compounds according to plant part. Flavonol aglycones such as quercetin, myricetin and kaempferol, containing multiple hydroxyl groups, had higher antioxidant activity than their glycosides such as rutin, myricitrin, astragalin [57]. Generally, phenolic compounds such as flavonoids, phenolic acid and tannins are considered to be a major contributor to the antioxidant activity in medicinal plants [58]. The study of revealed that the aqueous extract of the leaves of G. alypum has an important antioxidant activity with an IC50 (0.164 mg/ml). The richness of G. alypum leaves on phenolic coumpounds can enlighten their significant and powerfully antioxidant potential. On the other hand, it has been reported that antioxidants possess diverse biological activities, such as anti-inflammatory, anti-carcinogenic and anti-atherosclerotic anti-genetoxic activities. These activities may be related to their antioxidant activity [59].
Determination of Ferric-Reducing Antioxidant Power (FRAP)
Our results revealed considerable antioxidant capacity by the FRAP method, (Table 5). Interestingly, the higher FRAP value was established on the leaves from samples obtained by infusion and maceration (IC50 are 0.34 and 0.38 mg/ml. These samples with highest values of polyphenols and anthocyanins (infusion) and flavonoids (maceration), explain the important antioxidant activity than the other samples. Demonstrated that aqueous extract of the leaves of G. alypum has an antioxidant activity with an IC50 (8.9 mM trolox equivalent).
Determination of nitric radical scavenging activity
Nitric oxide is an extremely unstable specy beneath the aerobic condition. It reacts with O2 to create the stable products nitrates and nitrite through the intermediates NO2, N2O4 and N3O4. It is expected by using the Griess reagent. In the presence of test compound, which is a scavenger, the quantity of nitrous acid decreases? The amount of decrease reflects the extent of scavenging. Our result revealed that only the ethanolic extracts obtained by maceration and soxhlet exhibited a high capacity to scavenge nitrous oxide radicals in a dose-dependent decreasing manner i.e., the concentration was the inverse of the scavenging activity. These extracts showed the highest activity with the lowest IC50 respectively (0.57 mg/ml and 0.67 mg/ml), comparing with a standard ascorbic acid (0.09 mg/ml). Nitric oxide is a well-known free radical with pleiotropic effects across some physiological processes in the body [60]. It is known to play a role in vasodilatation, smooth muscle relaxation, inhibition of platelet aggregation, immunity [61]. It is constitutively produced by the body in nanomolar concentration to maintain normal cellular function [62] (Table 5).
| IC50 mg/ml | ||||||
|---|---|---|---|---|---|---|
| Control | Methods | |||||
| Vit C | BHT | Soxhlet | Sonication | Maceration | Infusion | |
| DPPH | 0.01 ± 0.006a | - | 0.34 ± 0.003c | 0.40 ± 0.006e | 0.28 ± 0.003b | 0.38 ± 0.003d |
| FRAP | 0.01 ± 0.00a | - | 0.90 ± 0.003e | 0.55 ± 0.006d | 0.38 ± 0.006c | 0.34 ± 0.006b |
| Nitric oxide | 0.09 ± 0.00a | - | 0.67 ± 0.006c | 0.90 ± 0.010d | 0.57 ± 0.006b | 1.20 ± 0.058e |
Table 5: Antioxidant activity of the leaves of G. alypum.
Results of the ANOVA test are significantly different at p<0.05 and each data point is represented by the average of three repetitions ± SD. Values with different superscripts within the same column are significantly different (P<0.05), as determined by the Student-Newman-Keuls test.
The ANOVA test confirmed that the interaction between the different methods of extraction and the antioxidant activity had a significant effect.
| Polyphenols | DPPH testIC50 (mg/ml) | FRAP testIC50 (mg/ml) | Nitric oxide testIC50 (mg/ml) |
|---|---|---|---|
| Phenols (mg GAE/gext) | -0.404 | -0.663* | -0.413 |
| Flavonoids(mg CAT/g ext) | -0.610* | -0.865** | -0.491 |
| Flavonols (mg RE/g ext) | -0.989** | -0.785** | -0.994** |
| Anthocyanins mg cyanidin-3-glucoside/ L | -0.385 | -0.131 | -0.507 |
Table 6: Correlation between antioxidant compounds and activities.
**The correlation is significant at 0.01(bilateral).
* The correlation is significant at 0.05 (bilateral).
Results of the Person test confirmed that the interaction between the different antioxidants and activities had a significant effect.
Correlation between antioxidant compounds and activities
The correlation was calculated using Person correlation coefficient; (Table 6). The DPPH radical activity showed an important correlation with the flavonoids and flavonols (r=- 0.610, r =-0.989). Additionally, the FRAP radical activity showed a higher correlation with the flavonoids and flavonols (r= -0.865, r =-0.785). For themore, the nitric oxide test correlated strongly with the flavonols (r =-0.994). In contrast, DPPH, FRAP and nitric oxide tests showed a weak correlation with the anthocynins (r=-0.385; r=-0.131; r=-0.507). Person's coefficient is negative, which means that there is a strong correlation between phenolic compounds and antioxidant activities. This also allows us to conclude that the lower the IC50, the greater the antioxidant activity and the richer the plant is in phenolic compounds.
Evaluation of Anti-inflammatory Activity
Hypotonicity solution induced haemolysis
- alypum leaves showed a concentration dependent anti-inflammatory activity, and the protection percent increased with increase in the concentration of the samples of each method. The investigation recommended good ability of the ethanolic leaves extract obtained by soxhlet to resist the cell lysis in small IC50 (2.35 mg/ml) as compared to the standard drug Ascorbic acid (0.77 mg/ml), though not greater than Ascorbic acid (see Table 5). These extracts exposed membrane stabilization effect by inhibiting hypotonicity provoked lyses of erythrocyte membrane. The erythrocyte membrane is analogous to the lysosomal membrane [63]. Its stabilization explains that the extract can be well stabilizing lysosomal membranes. Stabilization of lysosomal membrane is important in limiting the inflammatory response by preventing the release of lysosomal constituents of activated neutrophil such as bactericidal enzymes and proteases, which cause further tissue inflammation and damage upon extra cellular release [64]. Various NSAIDs are recognized to have membrane stabilization effects owing to osmotic loss of intracellular electrolyte and fluid components. The extract may inhibit the processes, which may stimulate or enhance the efflux of these intracellular components [65]. From the study of this experiment, it may be concluded that the ethanolic extract of the leaves of G. alypum obtained by soxhlet has good membrane stability, hence good anti-inflammatory activities. According to the study of [66]. Our study can explain the importance of phytochemical constituents present in G. alypum leaves. The richness on flavonoids and flavonols (65,459 mg CAT/g ext. and 44 mg Eq RE/g ext.) can explain the highest anti-inflammatory charachteristics of this extract. Flavonoids, also known as nature’s tender drugs, possess various biological/pharmacological activities including anticancer, antimicrobial, antiviral, anti-inflammatory, immunomodulatory, and antithrombotic activities [66]. Of these biological activities, the anti-inflammatory capacity of flavonoids has long been utilized in Chinese medicine and the cosmetic industry as a form of crude plant extracts [67].
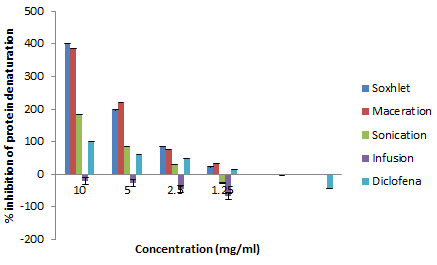
Inhibition of protein denaturation
The anti-inflammatory activity of medicinal plants is used to prevent several adverse effects associated with synthetic anti-inflammatory drugs (Table 7). Many investigations indicate that the anti-inflammatory activities of plants could be attributed to their content in phytochemicals. The anti-inflammatory activity in vitro aqueous and ethanolic extracts of leaves of G. alypum is estimated by calculating the percentages inhibition of protein denaturation. The spatial structures of proteins are sensitive to the environment (heat, pH, ionic strength, solvents etc.) and then change irreversible in form: denaturation. Egg albumin is a reserve of protein, globular, soluble in water. It is sensitive to the rise in temperature (coagulation thermal). The formation of inter or intramolecular disulphide bridges during heating by irreversible exchanges and the resulting disorderly polymerization leads to a decrease in its solubility. In inflammatory syndromes, a hypoalbuminemia is seen, which suggests its denaturation during inflammation [68]. Our results revealed that ethanolic extracts obtained by maceration and soxhlet have the highest inhibition of albumin (protein) denaturation effect with an IC50 (1.73 and 1.80 mg/ml). These extracts presented an anti-inflammatory effect 0.59 and 0.61 times bigger than diclofenac (control). The infusion extract doesn’t have an anti-inflammatory activity at 10 mg/ml (the percentage of inhibition of protein denaturation is -20.37%). As a consequence, these extracts have an important anti-inflammatory effect. This could be due to the richness of these extracts of G.alypum in bioactive compounds. Mainly the flavonoids which exert a strong inhibition on Cyclo Oxygenase (COX) and lipoxygenase [69]. According to our knowledge, most of the works consulted on this biological activity are carried out in vivo by the plantar edema method. Similarly, to our result, the study of [70] showed also that the leaves of G. alypum have an anti-inflammatory activity in vivo. GAME (methanolic extract of the leaves of G. alypum) demonstrated richness and especially various chemical compounds which are the source of these different biological activities (antioxidant, anti-inflammatory, anti-microbial and wound healing effect [70].
| IC50 (mg/ml) | Inhibition of HY haemolysis | Inhibition of prot. denaturation |
|---|---|---|
| Diclo | - | 2.91 ± 0.003c |
| Asc A | 0.76 ± 0.003a | - |
| GA | 0.93 ± 0.003b | - |
| SO | 1.45 ± 0.003c | 1.80 ± 0.006a |
| SON | 2.22 ± 0.006e | 3.04 ± 0.001d |
| MA | 2.01 ± 0.003d | 1.73 ± 0.003b |
| IN | 2.94 ± 0.006f | NF |
Table 7: In vitro anti-inflammatory activity of G. alypum leaves.
Control: Diclofenac/Ascorbic acid/Gallic acid/ Methods: SO: Soxhlet/SON: Sonication/MA: maceration/IN: Infusion/NF: Not found Inhibition of HY haemolysis: Inhibition of hypotonicity solution induced haemolysis/Inhibition of prot. denat: Inhibition of protein denaturation assay. NF: Not found. Results of the ANOVA test are significantly different at p<0.05 and each data point is represented by the average of three repetitions ± SD. Values with different superscripts within the same column are significantly different (P<0.05), as determined by the Student-Newman-Keuls test.
The ANOVA test confirmed that the interaction between the different methods of extraction and the anti-inflammatory activity had a significant effect.
| Polyphenols | Inhibition of HY haemolysisIC50 (mg/ml) | Inhibition of prot.denaturationIC50 (mg/ml) |
|---|---|---|
| Phenols (mg GAE/gext) | 0.048 | -0.209 |
| Flavonoids(mg CAT/g ext) | -0.318 | 0.806** |
| Flavonols (mg RE/g ext) | -0.863** | 0.087 |
| Anthocyanins mg cyanidin-3-glucoside/ L | -0.324 | -0.808** |
Table 8: Correlation between antioxidant compounds and activities.
**The correlation is significant at 0.01(bilateral).
* The correlation is significant at 0.05 (bilateral).
Results of the Person test confirmed that the interaction between the different antioxidants and activities had a significant effect.
Correlation between antioxidant compounds and activities
The correlation was calculated using Person correlation coefficient; (Table 8). The inhibition of hypotonicity solution induced haemolysis activity showed an important correlation with the flavonols (r=-0.863). Additionally, the inhibition of protein denaturation assay showed a higher correlation with the flavonoids and anthocyanins (r=-0.806, r=-0.808). Person's coefficient is negative, which means that there is a strong correlation between phenolic compounds and anti-inflammatory activity. This also allows us to conclude that the lower the IC50, the greater the anti-inflammatory activity (inhibition of hypotonicity solution induced haemolysis activity and inhibition of protein denaturation assay) and the richer the plant is in phenolic compounds (Figure 3).
Conclusion
The leaves of G. alypum demonstrated richness and particularly a variety of chemical components which are the source of these different biological activities (antioxidant and anti-inflammatory). These results give good reason for its traditional use to treat infections and injuries. Furthermore, laboratory study and chemical isolation of this plant leaves strength corroborate an effectual drug molecule in pharmacologic aspects effectively, in both types of pharmaceutical stadiums. In addition, our results can explain the importance of different methods extraction of phenolic compounds. The acidity can alter the quality of polyphenols as a consequence the biological potentials of medicinal plants, especially the aerial parts (leaves and flowers) which contain a vulnerable biomolecule to environmental conditions (temperature, etc.).
References
- Yadav RNS, Munin A (2011) Phytochemical analysis of some medicinal plants. J Phytol 3:10-14
- Pottier Alapetite G, Flore de la Tunisie (1981) Angiospermes-dicotylédones, Gamopétales, Programme flore et végétation tunisienne 655-1190
- Aruoma OI (1999) Free Radicals, Antioxidants and International Nutrition. Asia Pac J Clin Nutr 8:53-63
[Crossref] [Google Scholor] [Pubmed]
- Chaudhuri A, Bowling K, Funderburk CH, Lawal, Inamdar A, et al. (2007) Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci 27:2457-2467
[Crossref] [Google Scholor] [Pubmed]
- Dragsted LO, Strube M, Larsen CJ, (1993) Cancer-protective factors in fruits and vegetables,Biochemical and biological background. Pharmacol Toxicol 72:116 -135
[Crossref] [Google Scholor] [Pubmed]
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha J, ihlaja K et al. (1999) Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J Agric Food Chem 47:3954-3962
[Crossref] [Google Scholor] [Pubmed]
- Akoh CC, Min BD (1997) Food lipid chemistry In Nutrition and biotechnology New York. Marcel Dekker Inc
- Rajesh K, Qi Li, Joerg B, Elise F, Stanley (2007) The presynaptic CaV2.2 channel–transmitter release site core complex. EJN 547-559
[Crossref] [Google Scholor] [Pubmed]
- Djeridane, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, et al. (2006) Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chem 97:654-660
- Es-Safi NE, Khlifi S, Kollmann A, Kerhoas L, El Abbouyi A, et al. (2006) Iridoid glucosides from the aerial parts of Globularia alypum L. Chem Pharm Bull (Tokyo). 54:85–8
[Crossref] [Google Scholor] [Pubmed]
- Touaibia M, Chaouch FZ (2016) Global chemical composition and antioxidant effect of the ethanol extracts prepared from Globularia alypum leaves. Nat Technol 14:2-6
- Mi-Yae Shon, Tae-Hun Kim, Nak Ju Sung (2003) Antioxidant and free radical scavenging activity of Phellinus baumii (Phellinus Hymenochaetacea) extracts. Food Chem 82:593-597
- Riadh BM, Imtinen BJ, Mohammed B, Bochra G, Nesrine E, et al. (2012) Investigation of antioxidant activity of alcoholic extract of Globularia alypum L. J Med Plant Res 4193-4199
- Medzhitov R (2010) Inflammation new adventures of an old flame. Cell 140:771–776
[Crossref] [Google Scholor] [Pubmed]
- Ferrero-Miliani L, Nielsen O, Andersen P, Girardin S (2007) Chronic inflammation importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol 147:227–235
[Crossref] [Google Scholor] [Pubmed]
- Chandra S,Dey P, Bhattacharya S (2012) Preliminary in vitro assessment of anti-inflammatory property of Mikaniascandens flower extract. J Adv Pharm Edu Res 2:25–31
[Crossref]
- Wang C, Shi L, Fan L, Ding Y, Zhao S, et al. (2013) Optimization of extraction and enrichment of phenolics from pomegranate (Punica granatum L) leaves. Ind Crop Prod 42:587–594
- Mital K, Sumitra C (2012) Evaluation of antioxidant and antimicrobial properties of Manilkara zapota L. (chiku) leaves by sequential soxhlet extraction method. Asian Pac J Trop Biom S1526- S1533
- Harsha N, Sridevi V, Lakshmi MVVC, Rani K, Divya N, et al. (2013) Phytochemical analysis of some selected spices. Inter J Innov Res Sci Eng Technol 2:6618-6621
- Awah FM, Uzoegwu PN, Ifeonu P, Oyugi JO, Rutherford J, et al. (2012) Free radical scavenging activity, phenolic contents and cytotoxicity of selected Nigerian medicinal plants. Food Chemi 131:1279-1286
[Crossref] [Google Scholor] [Indexed in]
- Barros CRM, Ferreira MML, Nunes FM, Bezerra RMF, Dias AA, et al. (2011) The potential of white-rot fungi to degrade phorbol esters of Jatropha curcas L. seed cake Eng Life Sci 11:107–110
- Miliauskas G, Yenkutonis PR, Van beek TA (2004) Screening of radical scavenging activity of some medicinal and aromatic plants extracts. Food Chem 85:231-237
- Li HY, Deng ZY, Zhu HH, CL Hu, Liu RH (2012) Highly pigmented vegetables Anthocyanin compositions and their role in antioxidant activities. Food Res Int 46:250-259
- Unuofin JO, Otunola GA, Afolayan JA (2017) phytochemical screening and in vitro evaluation of antioxidant and antimicrobial activities of Kedrostis africana (L). Cogn Asian Pac J Trop Biomed 7:901-908
- Omoruyi BE, Bradley G, Afolayan AJ (2012) Antioxidant and phytochemical properties of Carpobrotus edulis (L.) bolus leaf used for the management of common infection in HIV/AIDS patients in Eastern Cape Province. BMC Complement Altern 12:215
[Crossref] [Google Scholor] [Pubmed]
- Onyesife CO, Ogugua VN, Anaduaka EG (2014) Investigation of some important phytochemicals vitamins and mineral constituents of ethanol leaves extract of Piper Nigrum. Ann Biol Res 5:20-25
- Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC et al. (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15:127-130
[Crossref] [Google Scholor] [Pubmed]
- Kartal N, Sokmen M, Tepe B, Dafereras D, Polissiou M (2007) Investigation of the antioxidant properties of Ferula orienlis L. using a suitable extraction procedure. Food Chem 100:584-589
- Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power the FRAP assay. Anal Biochem 239:70-76
[Crossref] [Google Scholor] [Pubmed]
- Li HY, Deng ZY, Zhu HH, Hu CL, Liu RH (2012) Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res Int 46:250-259
- Griess LC, Wanger DA, Glogowski J, Skipper PL, Wishnok JS, et al. (1982) Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem 126:131-138
[Crossref] [Google Scholor] [Pubmed]
- Boora F, Chirisa E, Mukanganyama S (2014) Evaluation of Nitrite Radical Scavenging Properties of Selected Zimbabwean Plant Extracts and Their Phytoconstituents. J Food Process 7
[Google Scholor] [PubMed]
- Gandhisan R, Thamaraichelvan A, Baburaj (1991) Anti-inflammatory action of Lannea coromandelica HRBC membrane stabilization. Fitotherapia 62:82–83
- Shinde UA, Phadke AS, Nair AM, Mungantiwar AA, Dikshit VJ, et al. (1999) Membrane stabilizing activity—a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 70:251–257
- Sangeetha G, Vidhya R (2016) In vitro anti-inflammatory activity of different parts of Pedalium murex (L.). Inter J of Herb Med 4:31-36
- Osman NI, Sidik NJ, Awal A, Adam NAM, Rezali NI, et al. (2016) In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L and total phenolic content analysis for potential anti-infl ammatory use in gouty arthritis. J Intercult Ethnopharmacol 5:343-349
[Crossref] [Google Scholor] [Pubmed]
- Panda N, Patro VJ, Panda PK, Neelamadhab (2013) Comparative in vitro anti-inflammatory activity of leaf extracts of Limonia acidissima and Callistemon salignus of Similipal Biosphere Researve Odisha India. J Adv Pharm Res 4:96-100
- Babbar N, Neha, Oberoi HS, Uppal DS, Patil RT (2011) Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Int Food Res 44:391-396
- Gusti A, Kadek DP, Yustinus M, Supriyadi, Ria A (2018) Comparison of Sonication with Maceration on Antioxidant Potency of Anthocyanin and Karotenoid of Tamarillo (Solanum betaceaum Cav). Agritech 38:304-312
- Uzogara SG, Morton ID, Daniel JW (1990) Changes in some antinutrients in cowpeas (Vigna unguiculata) processed with “kanwa” alkaline salt. Plant Foods Hum Nutr 40:249-258
[Crossref] [Google Scholor] [Pubmed]
- Touaibia M, Chaouch FZ (2016) Global chemical composition and antioxidant effect of the ethanol extracts prepared from Globularia alypum leaves. Nat Technol 14:2-6
- Bengleil M, Elshibani F, Alshalmani S, Fallah G (2020) Phytochemical, Pharmacological study on east Libyan originated Globularia alypum. Glob Sci Res 8:2320-9186
- Velioglu YW, Mazza G, Gao L, Oomah BD (1998) Antioxidant Activity and Total Phenolics in Selected Fruits Vegetables and Grain Products. J Agric Food Chem 46:4113-4117
- Govindarajan M, Kwon SW, Weon HY (2007) Isolation, molecular characterization and growth-promoting activities of endophytic sugarcane diazotroph Klebsiella sp GR9 World. J Microbiol Biotechnol 23:997–1006
- Khantouche L, Guesmi F, Motri D, Abderabb M (2018) Preparatory Institute for Scientific and Technical Studies La Marsa 2075 Tunisia Nutritional Composition Analysis of Secondary Metabolites and Antioxidative Effects of the Leaves of Globularia alypum L. Indian J Pharm Sci 80:274-281
- Daycem K, Moktar H, Akrem El H, Sylvie C, Jean Pierre S, et al. (2001) Global Chemical Composition and Antioxidant and Anti-Tuberculosis Activities of Various Extracts ofGlobularia alypum L (Globulariaceae) Leaves Molecules. 16:10592-10603
[Crossref] [Google Scholor] [Pubmed]
- Connor AM, Finn CE, Alspach PA (2005) Genotypic and environmental variation in antioxidant activity and total phenolic content among blackberry and hybridberry cultivars. J Am Soc Horti Sci 130:527–533
- Kowalska I (2007) Flavonoids from barrel medic (Medicago truncatula) aerial parts. J Agric Food chem 55:2645–2652
[Crossref] [Google Scholor] [Pubmed]
- Akowuah GA, Mariam A, Chin JH (2009) The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf Pharmacognosy Magazine. 4:81-85 [Crossref]
- Maret T, Jan FS (2011) Vitamins C and E Beneficial effects from a mechanistic perspective. Free Radic Biol Med 51:1000-13
[Crossref] [Google Scholor] [Pubmed]
- Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, et al. (2014) The Role of Vitamin E in Human Health and Some Diseases Sultan Qaboos University. Med J 14:157
- Udensi UK, Tchounwou PB (2014) Dual effect of oxidative stress on leukemia cancer induction and treatment. J Exp Clin Cancer Res. 33:106
[Crossref] [Google Scholor] [Pubmed]
- Acamovic T, Stewart TW (2003) Pennycott Poisonous Plants and Related Toxins CABI Wallingford UK. 608
- Bikash D, Waikhom SS, Manik D, Sanchari G, Mahesh KS (2018) Role of plant alkaloids on human health A review of biological Activities Mater Today Chem. 9:56-72
- Yujing S, Liezhou Z, Lianfei C, Wenwen L, Xingqian YE (2015) Sonication inhibited browning but decreased polyphenols contents and antioxidant activity of fresh apple (malus pumilamill cv Red Fuji) juice. J Food Sci Technol 52:8336–8342
[Crossref] [Google Scholor] [Pubmed]
- Oran SA, Raies A (1999) Biosystematic of the genus Globularia in Jordan and Tunisia. Dirasat 26:203–210
- Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184
[Crossref] [Google Scholor] [Pubmed]
- Hanene JH, Aicha N, Ines S, Eya M, Leila CG, et al. (2010) Antioxidant and antigenotoxic activities of Globularia alypum leaves extracts. J Med Plants Res 4:2048-2053
- Chi-Chun W, Hua-Bin li, Ka-Wing C, Chen F (2006) A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem 97:705–711
- Bescós R, Sureda A, Tur JA, Pons A (2012) The effect of nitric-oxide-related supplements on human performance. Sports Med 42:99-117
[Crossref] [Google Scholor] [Pubmed]
- Jagetia GC, Baliga MS, Malagi KJ, Kamath MS (2002) The evaluation of the radioprotective effect of Triphala (an ayurvedic rejuvenating drug) in the mice exposed to γ-radiation. Phytomedicine 9:99-108
[Crossref] [Google Scholor] [Pubmed]
- Izuegbuna O, Otunola G, Bradley G (2019) Estimation of Phytochemical, Vitamins Composition and Antioxidant Activity of Pelargonium inquinans Leaves. J Nat Prod Pharmacogn 11:237-244
- Chou CT (1997) The anti-inflammatory effect of Tripterygium wilfordii Hook F on adjuvant-induced paw edema in rats and inflammatory mediators release. Phytother Res 11:152-154
- Murugasan N, Vember S, Damodharanm C (1981) Studies on erythrocyte membrane IV In vitro haemolytic activity of Oleandet extract. Toxicol Lett 8:33–38
[Crossref] [Google Scholor] [Pubmed]
- Iwueke AV, Nwodo OF, Okoli CO (2006) Evaluation of the anti-inflammatory and analgesic activities of Vitexdoinana leaves. Afr J Biotech 5:1929–1935
[Crossref]
- Havsteen B (1983) Flavonoids a class of natural products of high pharmacological potency. Biochem Pharmacol 32:1141–1148
[Crossref] [Google Scholor] [Pubmed]
- Pyo Kim H, Ho Son K, Wook Chang H, Sik Kang S (2004) Anti-inflammatory Plant Flavonoids and Cellular Action Mechanisms. J Pharmacol Sci 96:229–245
[Crossref] [Google Scholor] [Pubmed]
- Cuq JL (2006) Biochemistry of proteins. Department of Science and Technology Food Industries University of Montpellier.
- Kim HP, Mani I, Iversen L, Ziboh VA (1998) Effects of naturally-occurringflavonoids and biflavonoids on epidermal cyclooxygenase and Iipoxygenasefrom guinea-pigs Prostaglandins. Leukot Essent Fatty Acids 58:17–24
[Crossref] [Google Scholor] [Pubmed]
- Zohra G, Rim K, Assaad S, Bahira H, Rim A et al. (2016) Globularia alypum methanolic extract improves burn wound healing process and inflammation in rats and possesses antibacterial and antioxidant activities. Biomed Pharmacother 8
[Crossref] [Google Scholor] [Pubmed]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences