ISSN : 0976 - 8688
Der Pharmacia Sinica
Impurity Profiling of Anti-Inflammatory Drugs
Shubhangi Shelke*, Suchitra Patil, Vaishnavi Adake, Vaishnavi Mohite, Pramod Patil and Sachinkumar Patil
Department of Pharmaceutical Quality Assurance, Ashokrao Mane College of Pharmacy, PethVadgaon, Maharashtra, India
- *Corresponding Author:
- Shubhangi Shelke
Department of Pharmaceutical Quality Assurance,
Ashokrao Mane College of Pharmacy,
PethVadgaon, Maharashtra,
India
E-mail: shubhangishelke1999@gmail.com
Received date: January 12, 2023, Manuscript No. IPDPS-22-15631; Editor assigned date: January 16, 2023, PreQC No. IPDPS-22-15631 (PQ); Reviewed date: January 30, 2023, QC No. IPDPS-22-15631; Revised date: April 17, 2023, Manuscript No. IPDPS-22-15631 (R); Published date: April 24, 2023, DOI: 10.36648/0976-8688.14.1.006
Citation: Shelke S, Patil S, Adake V, Mohite V, Patil P, et al. (2023) Impurity Profiling of Anti-Inflammatory Drugs. Der Pharmacia Sinica Vol:14 No:1
Abstract
The goal of the collection of analytical operations known as "impurity profiling" is to detect, characterise the structure of, and quantify organic and inorganic impurities as well as residual solvents in bulk pharmaceuticals and pharmaceutical formulations. Impurity control is presently a crucial problem for healthcare production. Diverse strategies, which include capillary electrophoresis, gas liquid chromatography, high performance liquid chromatography, solid phase extraction methods, ultraviolet spectrometry, and infrared spectroscopy. Thousands of tons of pharmacologically active substances are used annually to treat or to prevent illnesses, or to help people with the stress of modern life. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are the group most often used in human health care, since they are available without prescription for treatment of fever and minor pain. The present review addresses the use of various techniques for the analysis of impurities of NSAIDs. Numerous chemical mediators, including prostaglandins, leukotrienes, and platelet activating factor, cause both acute and chronic inflammations. A variety of activity mechanisms are used by anti-inflammatory drugs to demonstrate their effects. The most often given medications for treating inflammatory illnesses are Non-Steroid Anti- Inflammatory medicines (NSAIDs). The patients receive symptomatic relief from the NSAIDs, but they do not alter the pathogenesis of inflammation. Furthermore, due to serious adverse effects, particularly on the gastrointestinal mucosa, prolonged administration should be avoided.
Keywords
Anti-inflammatory drugs; Impurity profiling; ICH guidelines; Retention time; Gastrointestinal mucosa
Introduction
Impurity profiling
• The ICH (International Conference on Harmonization) describes an impurity profile of a drug material as "a description of the identified and unidentified impurities present in a new drug substance" [1]. The phrase "impurity profiling" refers to analytical techniques that have as their principal aims the detection, identification, structural characterization, and quantitative quantification of organic and inorganic impurities, as well as any residual solvents, in bulk pharmaceuticals and medications [2].
• Any raw material in a new drug substance that is not the chemical entity described as the ingredient in the new drug substance, any ingredient in a drug product that is not the chemical entity described as the ingredient in the drug product, or any excipient in a drug product are all recognised as impurities [3].
Classification of impurities
Impurities can be classified into the following categories:
• Organic impurities (process and drug related).
• Inorganic impurities.
• Residual solvents.
Organic impurities: It can arise during the manufacturing process and/or storage of the new drug substance. These organic impurities can be identified or unidentified, volatile or non-volatile and also include starting materials, by products, intermediates, degradation products, reagents, ligands and catalysts.
Inorganic impurities: Inorganic impurities are usually detected and quantified using pharmacopeial or other appropriate principles. Carryover of catalysts to the drug substance should be evaluated throughout development. These kinds of impurities can result from the manufacturing progression (Table 1). These are normally known and identified and include reagents, ligands and catalysts, heavy metals or other residual metals, inorganic salts. Other materials (e.g., filter aids, charcoal).
Residual solvents: Solvents are inorganic or organic liquids used as vehicles for the preparation of solutions or suspensions in the synthesis of a new drug substance [4].
| Solvent | Risk assessment | Example |
|---|---|---|
| Class I | Solvents to be avoided | Benzene (2 ppm), carbon tetrachloride (4 ppm), methylene chloride (600 ppm), methanol (3000 ppm), Pyridine (200 ppm), toluene (890 ppm) |
| Class II | Solvents to be avoided | N,N-dimethyl formamide (880 ppm), acetonitrile (410 ppm) |
| Class III | solvents with low toxic potential | Acetic acid, ethanol, acetone has permitted daily exposure of ≤ 50 mg/day. |
Table 1: Classification of residual solvents.
Sources of impurities
The impurities can originate from several sources; such as:
• Crystallization related impurities.
• Stereochemistry related impurities.
• Impurities arising during storage.
• Method related impurity.
• Residual solvents.
• Synthetic intermediates and by products.
• Functional group related typical degradation.
• Mutual interaction amongst ingredients [5-7].
Materials and Methods
Analytical methods for identification of impurities
The impurities can be identified by following different methods like:
• Reference standard method: The main purpose of this method is to provides the basic information for evaluating process and product performance of drug substances, drug products, impurities, degradation products, starting materials, intermediates, and excipients.
• Spectroscopic method: The UV, IR, MS, NMR and Raman spectroscopic methods are abundantly used for the identification of impurities.
• Separation method: The separation method includes chromatographic techniques like TLC, HPTLC, HPLC, Gas Chromatography (GC), Supercritical Fluid Chromatography (SFC), electrophoresis techniques like capillary electrophoresis, gel permeation chromatography etc.
• Isolation method: Number of methods can be used for isolation and characterization of impurities i.e. solid-phase extraction methods, liquid-liquid extraction methods, accelerated solvent extraction methods, column chromatography, flash chromatography, TLC, GC, HPLC, HPTLC, Capillary Electrophoresis (CE), Supercritical Fluid Chromatography (SFC).
• Characterization method: For characterization of impurities, different techniques are used;
*HPLC-UV studies, *HPLC-MS studies, *GC-MS studies, *TLC-MS studies, *CE-MS studies, *MEKC-MS and CEC-MS studies *HPLC-NMR studies [8,9].
Anti-inflammatory drugs
Although acetylsalicylic acid the first of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) ha s be en with us since the beginning of the century, it was never so called because anti-inflammatory therapy in rheumatologically practice was not demonstrated clinically until the advent of cortisone in 1949. The first substance to be called a NSAID was phenylbutazone which was introduced in 1952. It was the dramatic reduction of swelling in inflamed joint structures induced by cortisone and other corticosteroids introduced later, and the dramatic relief of swelling and stiffness with decrease, in pain and improved mobility that led to the corticosteroids being termed the 'miracle drugs' of the time. The first 3, aspirin, phenylbutazone/oxyphenbutazone and indomethacin, did in deed demonstrably reduce joint tissue swelling but usually much less dramatically than did corticosteroids at medium or high dosage levels. The idea of reducing pain and swelling by a peripheral action in the affected tissues rather than by reducing pain centrally in the central nervous system [10].
Mechanism of action of anti-inflammatory drugs
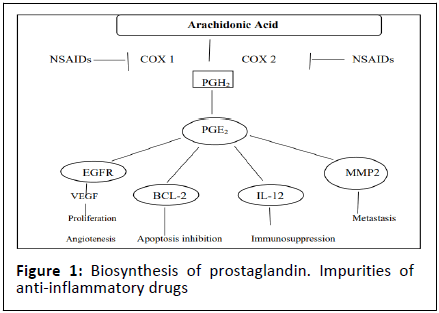
NSAIDs probably act in most cases by inhibiting the synthesis of prostaglandins in inflamed tissues, thereby preventing sensitisation of pain receptors to mediators of inflammation. These mediators histamine, serotonin, kinins and free oxygen radicals-play some part in the inflammatory arthritis process, but playa variable role in different types of inflammation [11]. Arachidonic acid is the precursor substrate for both cyclo oxygenase (prostaglandin synthetase) and lipoxygenase enzymes, the former leading to the production of prostaglandins F, D and E, prostacyclin and the thromboxanes, the latter via the unstable intermediate 5-HPETE to 5-HETE and the leukotrienes (Figure 1) [12]. There is a reasonably good correlation between an NSAID's potency in reducing experimentally induced oedema in laboratory animals and its ability to inhibit prostaglandin production, but it is possible that immediate symptomatic relief of chronic inflammation is produced by blocking cyclo-oxygenase metabolism and a more permanent effect by blocking lipoxygenase metabolism (Table 2).
| Sr. no | Drug name | Impurities |
|---|---|---|
| 1 | Aspirin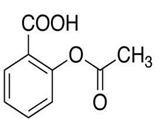 |
Impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, acetylsalicylic acid-d4, acetylsalicylic acid-d3, acetylsalicylic acid-d7, acetylsalicylic anhydride-d8. |
| 2 | Mefenamic acid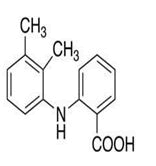 |
Impurity A, impurity B, impurity C, impurity D, 3-Chloro-2-methylaniline, 2-Chloro-5-iodobenzoic acid, 3-Carboxy mefenamic acid, 3-Carboxy mefenamic acid acyl-β-D- glucuronide, 3-Hydroxymethyl mefenamic acid acyl-β-D- glucuronide, Mefenamic acyl-β-D-glucuronide. |
| 3 | Meclofenamic acid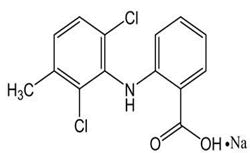 |
2-Fluorobenzoic acid-d4, 3-Hydroxymethyl meclofenamic acid-d4, Meclofenamic acid-d4, Meclofenamate sodium. |
| 4 | Flurbiprofen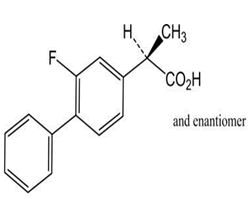 |
Impurity A, impurity B, impurity C, impurity D, impurity E, 3',4'-Dihydroxy Flurbiprofen, 3',4'-Dimethoxy α-Desmethyl flurbiprofen, flurbiprofen axetil, flurbiprofen sulfate, flurbiprofen acyl-β-D-glucuronide-d3, 4'-Hydroxy flurbiprofen-d3. |
| 5 | Diclofenac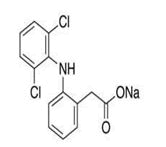 |
Impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, diclofenac methyl ester, diclofenac diethylamine, N-Nitroso-diclofenac. |
| 6 | Naproxen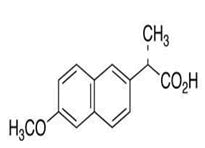 |
Impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity G, impurity H, impurity I, impurity J, impurity K, impurity L, impurity M, impurity N, impurity O, isopropyl 2-hydroxy-2-(6-methoxynaphthalen-2-yl)acetate, rac-5-Bromo naproxen, 1-Deoxy-1-(octylamino)-D-glucitol. |
| 7 | Ibuprofen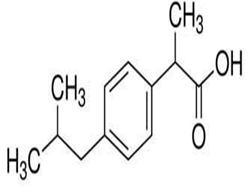 |
Impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity G, impurity H, impurity I, impurity J, impurity K, impurity L, impurity M, impurity N, impurity O, impurity P, impurity Q, impurity R, α-Methyl-4-propylphenylacetic Acid, 2-(4-n-Propylphenyl)propionic Acid, ibuprofen Lysinate, ibuprofen 1,4-Sorbitan ester, race 2-(tert-Butyldimethylsilyloxy) ibuprofen-d6. |
| 8 | Tolmetin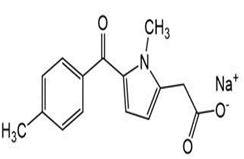 |
Amtolmetin guacil-d3, Tolmetin-d3, Tolmetin-d3 ethyl ester. |
| 9 | Indomethacin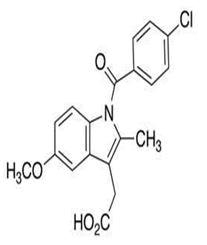 |
Impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity G, impurity H, impurity I, impurity J, indomethacin isopropyl ester, O-Desmethyl indomethacin-d4, Indomethacin-d4 methyl ester, α-Glucametacin-d4. |
| 10 | Sulindac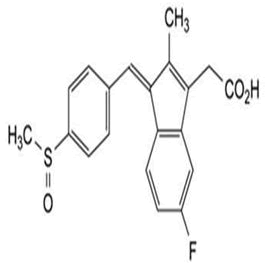 |
Impurity A, impurity B, impurity C, sulindac acyl-β-D- Glucuronide, sulindac sulfone acyl-β-D-Glucuronide, sulindac sulfide-d6, sulindac sulfide methyl ester, sulindac sulfone. |
| 11 | Phenylbutazone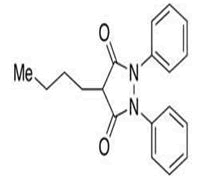 |
Impurity A, impurity B, impurity C, impurity D, N- Acetylaminoisonitrosoacetanilide, 1-Acetyl-2- phenylhydrazine, oxyphenbutazone, nCaproylhydrazobenzene, 2-(1,2-Diphenylhydrazine-1-carbonyl)-2 hydroxyhexanoic acid. |
| 12 | Piroxicam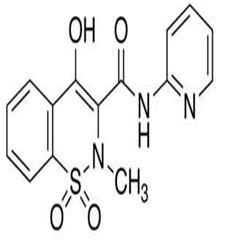 |
Impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity G, impurity H, impurity I, impurity J, impurity K, impurity L, N-(6-Methyl-2-pyridyl)-4-hydroxy-2-methyl-2 H- 1,2-benzothiazine-3-carboxamide 1,1-Dioxide, N-(2- Pyridyl)oxamic Acid, 1,3-Dipalmitoyl-2 chloropropanediol-d5, piroxicam-d3 O-β-D-Glucuronide, 2-Chloro-N-(pyridin-2- yl)acetamide, 2-(1,1-Dioxido-3-oxobenzo (d) isothiazol-2 (3H)-ylN- (pyridin-2-yl)acetamide. |
Table 2: Impurities of NSAID.
Results and Discussion
Validation of impurities
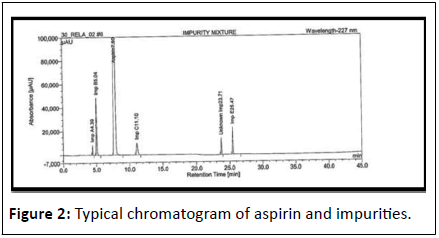
Aspirin: Chemically, Aspirin (ASP) is known as 2-acetyloxybenzoic acid (acetylsalicylic acid). ASP is frequently employed to treat fever, inflammatory diseases, and mild to moderate discomfort [13]. For the measurement of ASP impurities in ASP and DPY capsules, an internal LC gradient technique was created using an inertsil ODS-3 C18, 250 4.6 mm, 5 mm column with a mobile phase made up of 0.01 M Na2HPO4, pH set to 2.5 and orthophosphoric acid as mobile phase-A. Acetonitrile served as mobile phase-B. Salicylic acid (impurity-C), salsalate (impurity-E), 4-hydroxybenzoic acid (impurity-A), 4-hydroxyisophthalic acid (impurity-B), and unknown were isolated using this technique impurities of ASP and Dipyridamole capsule (Figure 2) [14].
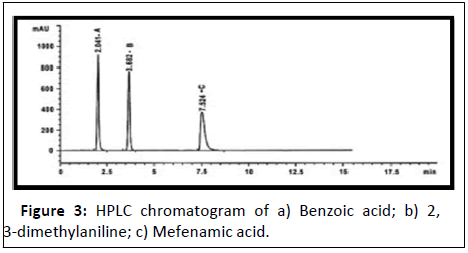
Mefenamic acid: Mefenamic acid (MEF) is 2-(2,3-dimethylphenyl)amino benzoic acid [15]. It is an anthranilic acid derivative and a member of the fenamate group of Non-Steroidal Anti-In lammatory Drugs (NSAIDs). It is used in mild to moderate pain including headache, dental pain, post-operative, postpartum pain and dysmenorrheal. TLC-densitometric method and RP-HPLC-DAD method, were developed and validated for the simultaneous determination of Mefenamic acid (MEF) and its two toxic impurities, Benzoic Acid (BA) and 2,3-Dimethylaniline (DMA) (Figure 3) [16]. In the proposed TLC-densitometric method a developing system consisting of chloroform: Acetone: Acetic acid: Ammonia solution (70:30:2:2, v/v/v/v) was used, TLC aluminum plates 60 F254 was used as a stationary phase and the separated bands were UV scanned at 225 nm.
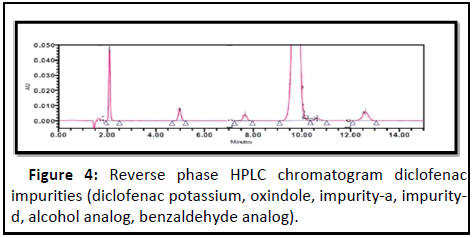
Diclofenac: Diclofenac (DIC) (2-(2,6-dichlorophenyl) aminophenyl acetic acid is an essential Non-Steroidal Anti-Inflammatory Drug (NSAID) which is clinically prescribed for the treatment of inflammatory disorders, such as ankylosing spondylitis, osteoarthritis, and rheumatoid arthritis [17]. Development and validation of RP-HPLC method for simultaneous determination of diclofenac potassium and its process related impurities was carried out by using 150 × 4.6 mm, i.d., 5 μm C-18 column with prepared mobile phase-A consisting 800:200 (v/v) of 0.01 M ammonium acetate adjusted pH 5.3 with acetic acid and acetonitrile and mobile phase-B consisting 200:800 (v/v) of 0.01 M ammonium acetate adjusted pH 5.3 with acetic acid and acetonitrile (Figure 4) [18].
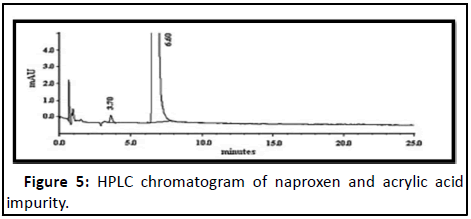
Naproxen: Naproxen (S)-2-(6-methoxynapthalen-2-yl) propanoic acid is a non-steroidal anti-inflammatory drug used in reduction of pain, fever, inflammation and treatment of rheumatoid arthritis, degenerative joint disease, ankylosing spondylitis, acute gout and primary dysmenorrhea (Figure 5). The HPLC analysis was performed on an Agilent 1100 series LC system, Agilent Technologies Inc., Santa Clara, CA, USA [19]. Hypersil ODS column (4.6 mm × 100 mm, 5 m) and mobile phase consisting of a mixture of sodium acetate trihydrate (pH 4.7; 0.04 M)-methanol (60:40, v/v), UV detection at 254 nm, flow rate of 1.5 mL/min was used for resolution of all the impurities i.e. 2-(6-methoxynaphthalen-2-yl)acrylic acid.
Ibuprofen: Ibuprofen has many impurities: A, B, C, D, E, F, G, H, I, J, K, L, M, N, O, P, Q, R. The main impurity is Ibuprofen impurity B: 2-(4-butylphenyl) propionic acid is considered a degradation product, β-isomer, guaifenesin, dioxylonone. HPLC gradient method is developed for the determination of related substances by using Acetonitrile HPLC grade, Potassium dihydrogenate phosphate-AR grade as mobile phase, with column C18, 150 × 4.6 mm, 5 μm (Devolosil ODS or equivalent) and detected at wave length 220 nm.
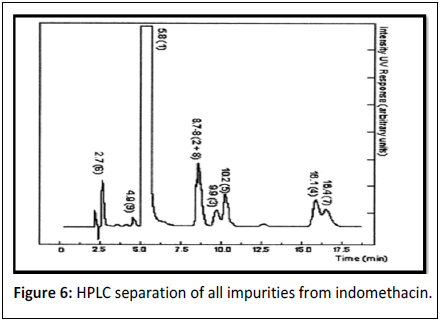
Indomethacin: Indomethacin (1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl acetic acid it has been widely used observed when investigated with compendial methods. In musculoskeletal and joint disorders such as rheumatoid arthritis. It may also be used in inflammation, pain and in the oedema [20]. y, an HPLC–UV method was by Merck developed which allowed for the separation and quantification of the possible process impurities 2-9 from indomethacin. HPLC separation of all impurities from indomethacin. Conditions: Isocratic HPLC, stationary phase: Nucleosil 120-5, C, 250 mm 18 34.6 mm ID; mobile phase: 75 (v/v) methanol and 25 (v/v) 0.2% phosphoric mobile phase was acid, flow rate 1.5 ml/min, UV detection at 320 nm (Figure 6).
Phenylbutazone: Phenylbutazone is an anti-inflammatory, antipyretic, and analgesic activities. It is known to be effective especially in the treatment of ankylosing spondylitis (Table 3). Degraded PBZ results in several types of impurities where benzidine is one of the impurities which are known to be a GTI. HPLC method was validated for impurities detection carried out by using buffer (potassium dihydrogen phosphate): Acetonitrile: methanol (30:50:20 v/v/v) at pH 3.5 in isocratic mode as mobile phase.
| Sr. no. | Drug | Name of impurity | Retention time (min.) |
|---|---|---|---|
| 1 | Aspirin | Imp-A | 4.3 |
| Imp-B | 5.04 | ||
| Imp-C | 11.1 | ||
| Imp-E | 25.47 | ||
| Imp-I | 23.72 | ||
| 2 | Mefenamic acid | Benzoic acid | 2.041 |
| Dimethylaniline | 3.682 | ||
| 3 | Diclofenac sodium | Oxindole | 1.75 |
| Impurity A | 2.25 | ||
| Benzaldehyde | 4.7 | ||
| Alcohol analog | 7.55 | ||
| Impurity D | 10.01 | ||
| 4 | Naproxen | Acrylic acid A | 3.5 |
| Acrylic acid B | 6.6 | ||
| 5 | Ibuprofen | B-Isomer | 0.74 |
| Guaifenesin | 0.85 | ||
| Dioxylonone | 1.84 | ||
| 2-(4-Isobutyryl phenyl)propionic acid | 2.01 | ||
| 6 | Indomethacin | 1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl-2. acetic acid methylate | 8.8 |
| 1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl-acetic acid ethylate | 9.9 | ||
| 1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl-acetic acid tert Butylate | 16.1 | ||
| 4-chlorobenzoic acid 2-1-(4-chlorobenzoyl)-5 methoxy-2-methylindol-3-yl acetylj-N-4-methoxy-6 phenyl)-hydrazide | 10.2 | ||
| 5-methoxy-2-methyl-3-indoleacetic acid | 2.7 | ||
| 1-(4-chlorobenzoyl)-5-methoxy-2,3-dimethylindole | 16.4 | ||
| 1-(3,4-dichlorobenzoyl)-5-methoxy-2-methylindol-3yl-acetic acid | 8.7 | ||
| 4-chlorobenzoic acid N9-(4-chlorobenzoyl)-N-(4 methoxyphenyl)-hydrazide | 4.9 | ||
| 7 | Phenylbuta zone | Benzidine | 2.046 |
Table 3: Validated impurities of anti-inflammatory drugs.
Conclusion
Recently, impurity profiling of pharmaceutical products has attracted significant attention because impurities can be detri mental to human health and can adversely affect the quality of pharmaceuticals. Currently, various methods are available for impurity profiling and other hyphenated techniques. In this review we have discussed the present state of the art of HPLC for determination of impurities of some important anti-inflammatory agent in brief. Impurities present in pharmaceuticals can originate from many sources. They can stem from starting materials, can be introduced or formed during synthesis or can be caused from excipients or due to degradation. As they can be the cause of undesirable pharmacological effects, their content is liable to the control of regulatory authorities. Accordingly, the pharmaceutical impurities must be declared in the range as low as 0.01%-0.10% relative to the API.
References
- Bartos D, Gorog S (2008) Recent advances in the impurity profiling of drugs. Current J Pharm Anal 4:215-230
- Chanda A, Ramalakshmi N, Nalini C, Mahabubi S (2015) Impurity profiling an emerging trend in Pharmaceuticals: A Review. Pharma Tutor 3:29-35
- Prabu SL, Suriyaprakash TN (2010) Impurities and its importance in pharmacy. Int J Pharm Sci Rev Res 3:66-71
- Ahuja S (1998) Impurities evaluation of pharmaceuticals. Informa Health Care 27.
- Parimoo P (1998) A text book of pharmaceutical analysis. 3rd edition. CBS publishers and distributors, New Delhi 20:21
- Ingale SJ, Sahu CM, Paliwal RT, Vaidya S, Singhai AK (2011) Advance approaches for the impurity profiling of pharmaceutical drugs: A review. Int J Pharm Life Sci 2:955-962
- Patil P, Vaidya D (2013) Overview on impurity profiling. Int J For Pharm Res Sch 2:54-65
- Hart FD, Huskisson EC (1984) Non-steroidal anti-inflammatory drugs. Drugs 27:232-255
- Ambanelli U, Fenaccioli GF (1982) Allopurinol like action of diflunisal. Arthritis Rheum 25:474-475
- Superio-Cabuslay E, Ward MM, Lorig KR (1996) Patient education interventions in osteoarthritis and rheumatoid arthritis: A meta-analytic comparison with non-steroidal antiinflammatory drug treatment. Arthritis Care Res 9:292-301
[Crossref] [Google Scholar] [PubMed]
- Baber N, Halliday LD, Van Den Heuvel WJ, Walker RW, Sibeon R, et al. (1979) Indomethacin in rheumatoid arthritis: clinical effects, pharmacokinetics, and platelet studies in responders and non-responders. Ann Rheum Dis 38:128-136
[Crossref] [Google Scholar] [PubMed]
- Berry H, Ford-Hutchinson AW, Camp AV, Heywood D, Molloy MG, et al. (1980) Antirheumatic activity of fenclofenac. Ann Rheum Dis 39:473-475
[Crossref] [Google Scholar] [PubMed]
- Macia A, Borrull F, Calull M, Aguilar C (2007) Capillary electrophoresis for the analysis of non-steroidal anti-inflammatory drugs. TrAC Trends Anal Chem 26:133-153
- Acharya S, Daniel A, Gyadangi B, Ramsamy S (2015) Isolation, characterization of a potential degradation product of aspirin and an HPLC method for quantitative estimation of its impurities. J Chromatogr Sci 53:1491-1497
[Crossref] [Google Scholar] [PubMed]
- Mullani AK, Shinde AS, Mote GD, Sutar GV, Sajne SJ, et al. (2020) Impurity profile study of aspirin in bulk and tablet dosage forms. J Pharm Negat 14:2457-66.
- Zoglio MA, Maulding HV, Haller RM, Briggen S (1968) Pharmaceutical heterogeneous systems III: Inhibition of stearate lubricant induced degradation of aspirin by the use of certain organic acids. J Pharm Sci 57:1877-80
[Crossref] [Google Scholar] [PubMed]
- Hackmann ER, Vals NR, Santoro MI (1997) Determination of acetylsalicylic acids in effervescent tablets by high performance liquid chromatography and UV multicomponent spectrophotometry. Rev Farm Bioquim Univ Sao Paulo 1997:53-58
- Gowri Sankar D, Kondaveni R, Raju TV, Vasi Krishna M (2009) Impurity profiling of aspirin in tablet dosage forms by reverse phase high performance liquid chromatography. Asian J Chem 21:4289-4293
- Morcoss MM, Abdelwahab NS, Ali NW, Elsaady MT (2017) Different chromatographic methods for simultaneous determination of mefenamic acid and two of its toxic impurities. J Chromatogr Sci 55:766-772
[Crossref] [Google Scholar] [PubMed]
- Niopas I, Mamzoridi K (1994) Determination of indomethacin and mefenamic acid in plasma by high performance liquid chromatography. J Chromatogr B Biomed Appl 656:447-450
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences