ISSN : ISSN: 2576-1412
Journal of Applied Microbiology and Biochemistry
Impact of Nationwide Antibiotic Administrative Programmes on Antibiotic Usage and Antibacterial Resistance for Clinical Isolates Over a Two-year Period in 14 Chinese and 5 Swedish Tertiary Hospitals
Yong Yu2, Hua Yu3, XiaoqiangYang4, Qing Yan4, Lars Berglund5, Yonghong Xiao1 and Staffan PE Sylvan6,7*
1State Key laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, China
2Department of Microbiology, The First Hospital Affiliated to General Hospital of PLA, China
3Department of Microbiology, Sichuan Provincial People’s Hospital, China
4The Ministry of Health Center for Antibacterial Surveillance (Mohcas), Pharmacy Administration Commission, Institute of Hospital Administration, National Commission for Healthcare and Family Plan, China
5Uppsala Clinical Research Center, Uppsala, Sweden
6Department of Medical Sciences, Uppsala University, Sweden
7Department of Communicable Disease Control and Prevention, Uppsala County Council, Sweden
- *Corresponding Author:
- Staffan PE Sylvan
Department of Communicable Disease Control and Prevention
Uppsala County Council, Gränby Bilgata 2
751 85 Uppsala, Sweden.
Tel: +46-723167343
Fax: +46-18552901
E-mail: staffan.sylvan@medsci.uu.se; staffan43@spray.se
Received date: July 20, 2017; Accepted date: July 31, 2017; Published date: August 07, 2017
Citation: Yong Yu, Hua Yu, Yang X, Yan Q, Berglund L, et al. (2017) Impact of Nationwide Antibiotic Administrative Programmes on Antibiotic Usage and Antibacterial Resistance for Clinical Isolates Over a Two-year Period in 14 Chinese and 5 Swedish Tertiary Hospitals. J Appl Microbiol Biochem. Vol. 1 No. 4:14
Abstract
The aims of this 2-year multicentre study are to systematically evaluate and establish a reference baseline for the resistance of clinically relevant bacterial isolates in 14 tertiary hospitals in China and 5 Swedish hospitals and to characterise the current use of antibiotics in these hospitals. We also aimed to assess the impact of national antibiotic administrative policies on antibiotic use and resistance patterns of the leading nosocomial pathogens in the hospitals.
The present study was done at a time when Chinese health authorities launched a campaign to discourage over prescription of antibiotics. At present, it seems their efforts are having a meaningful impact. Following the implementation of the intensive control programme in Chinese tertiary hospitals, we found that the consumption levels of parenteral antimicrobials were reduced and accompanied by a significant increase in susceptibility patterns of both Gram-positive and Gram-negative bacteria, including ESBL-producing organisms. During the 2-year study period, no significant change in the level of resistance to commonly used antibiotic groups could be noted in the clinical isolates from the five Swedish tertiary hospitals.
Keywords
Antibiotic administrative programme; Intervention; Bacterial resistance; Antibiotic consumption
Introduction
The emerging problem of antimicrobial resistance (AMR) in bacterial pathogens is a serious threat to global public health. A growing body of evidence has developed showing that the situation is more severe than previously thought, with increasing prevalence of resistant and multi-resistant bacterial strains worldwide [1]. Although poorly quantified, the global burden of resistance is probably concentrated in three major categories: longer duration of illness and higher rates of mortality in patients with resistant infections [2], increasing costs of treatment for resistant infections [3], and an inability to do procedures that rely on effective antibiotics to prevent infection.
The rapid emergence of antibiotic resistance worldwide has led to the mobilisation of the scientific community to promptly address this problem. The strategy developed to confront this task is threefold: first, systematic assessment on the extent to which this problem exists; second, adoption of appropriate measures to control established resistance and limit the emergence of further resistance; and monitoring the overall trends of this established and emerging resistance through surveillance projects on the local, national, and global level.
On World Health Day in April 2011, the World Health Organisation appealed to all member countries to “combat drug resistance: no action today, no cure tomorrow” [4]. In 2006, in a multidisciplinary bilateral cooperation China and Sweden have joined hands to address this challenging issue [5].
The problem is particularly acute in China, where antibiotics are freely available over the counter without prescription for many drugs [6]. It is estimated that 90% of the revenue of public hospitals are currently generated directly from patients for medications and tests and drug sales constitute about half of institutional income and most of the profit, with more than 25% from sales of antimicrobial agents, mostly broad-spectrum antibacterial agents such as cephalosporins and fluoroquinolones [7].
To control AMR the Chinese health administrative authorities have taken a series of actions over the past 10 years, including the development of technical specifications and policies [8]. In 2011, coupled with new health care reforms, the Chinese Ministry of Health (MOH) changed strategy and established a special campaign to promote the rational use of antimicrobials in different health care settings. This change mainly consisted of establishing mandatory management strategies (e.g., target setting, taskforce organisation, and the development of audit and inspection systems). Targets for antibiotic prescription are set at less than 60% and 20% of all prescriptions for hospitalised patients and outpatients, respectively. Antibiotic use in hospitalised patients should be less than 40 daily defined doses (DDD) per 100 patient-days. Adherence to these protocols is to be considered when appointing or dismissing hospital presidents [8,9].
To obtain a more complete picture of bacterial resistance in China in 2004 the Ministry of Health established the National Antimicrobial Resistance Surveillance Net (Mohnarin). Seventeen tertiary hospitals located in 15 cities throughout China participated in the net. Furthermore, to improve guidance about antibiotic use the Chinese MOH Center for Antibacterial Surveillance (Mohcas) was established in 2006. Hospitals were required to set up a drug therapeutics committee to facilitate the rational use of antibiotics.
In Sweden the increasing use of antibiotics and spread of penicillin-resistant pneumococcal clones in the beginning of the 1990s alarmed the medical profession and medical authorities. Strama (The Swedish Strategic Programme against Antibiotic Resistance) started as an informal network between experts and authorities in 1994. In 2000, Strama, in close cooperation with the National Board of Health and Welfare, prepared a proposal for a national plan to contain antibiotic resistance [10]. This proposal was incorporated into a government bill (“Strategy to prevent antibiotic resistance and health care infections”), which was passed in 2006 [11].
Since then, Strama has been institutionalised as an independent governmental body with an annual budget of 10 million Swedish crowns from the Ministry of Health and Social Affairs. Recently, a corresponding Strama VL (Veterinary and Food), coordinated by the National Veterinary Institute, has been inaugurated [12].
The overall aim of Strama’s activities is to preserve the effectiveness of antibiotics in humans and animals. Strama is organised in two levels: locally financed groups in each county and a national executive working group funded by the government. Detailed overviews of the efforts to contain antibiotic resistance in Sweden and of the systems for surveillance of antibiotic consumption and antibiotic resistance have been published [13].
However, little research on the effect of administrative and educational interventions on antibiotic prescribing in tertiary hospitals has been conducted in China and Sweden [14], and particularly, information on the interaction between antibiotic consumption and the development of bacterial resistance to antibiotics is scarce. The aims of this study are therefore to systematically evaluate and establish a reference baseline for the resistance of clinically relevant bacteria in 14 tertiary Chinese hospitals and 5 Swedish and to characterise the current use of antibiotics in these hospitals. In this multicentre study we also aimed to assess the impact of the antibiotic restriction policy on antibiotic use and resistance patterns of the leading nosocomial pathogens in a sample of tertiary hospitals in China and Sweden.
Materials and Methods
Hospital composition
Based on the surveillance hospital definition standards, including geographic distribution, clinical microbiological capability, and hospital willingness, 14 tertiary hospitals located in 14 cities around China were selected as monitoring sites for the study. The distribution of the hospitals was as follows: 1 in the northwest, 1 in the southwest, 4 in the north, 1 in the northeast, 6 in the east and 1 in the southeast of China (Figure 1).
In Sweden, five of seven tertiary hospitals were selected because they had data systems from which data on bacterial resistance could be extracted. The hospitals were mainly located in the middle of Sweden (Figure 2).
Antibacterial agents
Antibiotic consumption data for Chinese hospitals were extracted from the pilot hospitals, all of which are Mohcas member hospitals.
Data on sales of antibiotics to the Swedish hospitals were provided by pharmacists of the local Strama groups of the participating hospitals. Data on the amount of antibiotics used were expressed per DDD/100 patient-days in somatic medical care, according to the WHO Collaborating Centre for Drug Statistics Methodology (www.whocc.no).
The DDDs per 100 patient-days may be applied if drug use by adult inpatients is considered. The same definition should be used when performing comparative studies. As an example, 70 DDDs per 100 patient-days of antibiotics provide an estimate of the therapeutic intensity and suggest that 70% of inpatients might receive a DDD of an antibiotic every day. This unit is quite useful for benchmarking in hospitals.
The following antimicrobial agents were chosen to be included into the surveillance drug panel (Table 1).
Table 1 Antimicrobial agents and their corresponding Anatomical Therapeutic Chemical (ATC) classification codes included into the surveillance drug panel in somatic medical care in participating hospitals.
| Antimicrobial agents | ATC-code |
|---|---|
| Tetracyclines | (J01AA) (JO1CF) (J01CR) |
| Penic-mins with extended spectrum | (JD 1DB-DE) |
| Betalactamase sensitive penicillines | (JO1CE) |
| Betalactamese resistant penicillines | (JO1CF) |
| Combinations of penicillines | (JO1CR) |
| Cephalosporins | (J01DB-DE) |
| Carbapenems | (J01DH) |
| Trimethoprim | (JO1EA) |
| Trimethoprim with sulphonimides | (JO1E E) |
| Macmlides | (J0 1FA) |
| Lincosamides | (J0 1FF) |
| Aminoglycosides | (J0 1G B) |
| Fluoroquinolones | (JO1MA ) |
| Glycopeptides | (JO1XA) |
| lmidazole derivatives | (JO1XD) |
| Methenamine | (JO1XXO5) |
| Linezolid | (JO1XXO8) |
| All agens | (J01) |
Data were collected quarterly and yearly from 1st January 2011 to 31st December 2012.
Bacterial resistance data for Chinese hospitals were provided by the pilot hospitals. In Sweden the data were furnished by the Department of Microbiology at the five university hospitals that participated in the study. Testing of clinical isolates for antibiotic susceptibility in China is routinely performed using the VITEC and K-B methods based on the Clinical and Laboratory Standards Institute (CLSI). Results were described as minimum inhibitory concentration (MIC) or diameter of inhibition zones. The CLSI standard from 2010 was applied in this investigation.
In Sweden the new European disc diffusion method, as described by EUCAST, based on Mueller Hinton agar has been adopted from 2010. For interpretation of MIC determinations and the diameter of inhibition zones, we used EUCAST methodology version 1.3, January 2011. The Scandinavian version used was NordicAST, 2011-04-19. Internal and external quality assurance and quality control of susceptibility testing were performed by all laboratories in accordance with the national guidelines of each country. We collected data on total isolation rate (first isolation) of clinically relevant pathogenic bacteria and the isolation rate of different species of bacteria.
Moreover, we collected data of the bacterial resistance rate and MIC-zone diameter to different antibiotics, focusing on the isolation rate and resistance rate of the bacteria included in the protocol (Table 2) during the same period as was chosen for the antibacterial consumption data. This research was conducted in accordance with the Declaration of Helsinki and national and institutional standards. Written informed consent of the study population was not necessary because this study did not modify the existing diagnosis or the therapeutic strategy. Moreover, all data samples were under code and could not be associated with an identifiable individual by the research team.
Table 2 lnfective pathogens inclucded in the surveillance.
| Pathogens included |
|---|
| Staphylococcus aureus |
| CoaguIase- Negative Staphylocoocci |
| Streptococcus pneumoniae |
| Streptococcus pyogenes |
| Enterococcus faecalis |
| Enterococcus faecium |
| Haemophilus influenzae |
| Escherichia coli |
| Klebsiella pneumoniae |
| Enterobacteriaceae ESBL |
Statistical methods
Statistical description was made of antibiotics usage, the distribution of pathogenic bacteria, the level of resistance to individual antibiotics in each hospital over time, and as congregated data for all hospitals.
Correlation and regression analysis was used to investigate factors related to the increased or decreased levels of bacterial resistance.
All statistical analyses were performed with the individual hospital as the unit of measure. All tests were non-parametric. The use of antibiotics was calculated for each antibiotic agent in the surveillance panel and as total usage.
To measure the impact of the antibiotic restriction policy in Chinese hospitals we calculated the difference between the mean use of antibiotics for quarters 1 and 2 in 2011 and the mean usage in quarters 3 and 4 in 2012 for each hospital and compared usage during these periods with Wilcoxon’s matchedpairs signed-rank test.
Associations between use of antibiotics and bacterial susceptibility were estimated with Spearman’s rank correlation coefficients. Correlations were calculated by each of the years 2011 and 2012. We also calculated correlations between the above-mentioned changes in the use of antibiotics with bacterial susceptibility in 2012.
Results
Results on antibacterial consumption
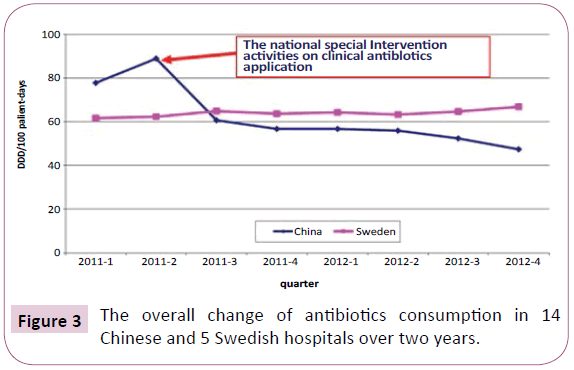
The overall change of antibiotic consumption in 14 Chinese and 5 Swedish tertiary hospitals over 2 years is shown in Figure 3. A substantial reduction of the overall antibiotic use at Chinese tertiary hospitals after the implementation of the national special intervention activities on clinical antibiotics application can be seen. In contrast, a slight increase of antibiotic consumption was observed for the Swedish hospitals during the same period. It can be noted that during the observation period, the use of antimicrobial agents in Chinese pilot hospitals decreased below that of Swedish hospitals. At the end of the 2-year observation period, the mean antibiotic consumption at the 14 Chinese hospitals was 47.33 DDD/100 patient-days, which is almost a 50% reduction of antibiotic use compared with the situation before intervention and approximating the target for antibiotic use in hospitalised patients (e.g. 40 DDD/100 patient-days) that was set by the new guidelines.
Antibiotics were ranked by their frequency (Table 3). In Sweden ß-lactamase resistant penicillin and penicillin´s with extended spectrum were the most used groups of antibiotics, whereas in China fluoroquinolones and cephalosporins were the most frequently prescribed groups. This difference in antibiotic use could partly be explained by the observation that in Swedish hospitals S. aureus and E.coli cultures were significantly more often isolated compared with Chinese hospitals, where significantly more infections caused by pathogens (e.g., P. aeruginosa and E. faecium) were treated (Table 4). A particularly noteworthy finding is that our data demonstrated that 5 Swedish tertiary hospitals together took almost three times the number of bacterial cultures than the 14 pilot hospitals in 2012. Table 5 summarizes the reported resistance rates in common bacterial pathogens isolated in some tertiary hospitals in Sweden and China. A very high resistance rate in several common pathogens was shown for fluoroquinolone, third-generation cephalosporin, methicillin and broad-spectrum penicillin in Chinese isolates.
Table 3 Ranking of antibiotics making up 90% of total antibiotic consumption in 5 Swedish and 14 Chinese tertiary hospitals in 2012.
| Rank | Antibiotic | DDD/100 patient days | Percentage | Cumulative Percentage | |||
|---|---|---|---|---|---|---|---|
| Sweden | China | Sweden | China | Sweden | China | ||
| 1 | J01CF (Betalactamase resistant penicillin) | 10.9 | 0.26 | 18.90 | 0.42 | 18.90 | 0.42 |
| 2 | J01CA (Penicillin with extended spectrum) | 6.0 | 0.26 | 12.20 | 0.42 | 31.10 | 0.84 |
| 3 | J01MA (Fluoroquinolones) | 5.8 | 7.5 | 9.80 | 12.10 | 40.90 | 12.94 |
| 4 | J01DD (Third-generation cephalosporins) | 5.2 | 16.5 | 8.90 | 26.61 | 49.80 | 39.55 |
| 5 | J01CE (Betalactamase sensitive penicillin) | 5.1 | 0.13 | 8.70 | 0.21 | 58.50 | 39.76 |
| 6 | J01AA (Tetracyclines) | 4.5 | 0.2 | 7.90 | 0.32 | 66.40 | 40.08 |
| 7 | J01DH (Carbapenems) | 3.9 | 2.2 | 7.20 | 3.55 | 73.60 | 43.63 |
| 8 | J01CR (Combination of penicillins) | 3.9 | 3.4 | 7.20 | 5.48 | 80.80 | 49.11 |
| 9 | J01EE (Trimethoprim with sulphonamides) | 2.4 | 0.5 | 4.10 | 0.81 | 84.90 | 49.92 |
| 10 | J01FF (Lincosamides) | 1.8 | 0.2 | 3.10 | 0.32 | 88.00 | 50.24 |
| 11 | J01XA (Glycopeptides) | 1.5 | 1 | 2.50 | 1.61 | 90.50 | 51.85 |
| 12 | J01XD (Imidazole derivatives) | 1.2 | 3.2 | 2.10 | 5.16 | 92.60 | 57.01 |
| 13 | J01FA (Macrolides) | 0.9 | 2.2 | 1.50 | 3.55 | 94.10 | 60.56 |
| 14 | J01DB-DE (Cephalosporins) | 1.8 | 19.7 | 3.10 | 31.77 | 97.20 | 92.33 |
| 15 | J01XX (Other antibacterials) | 2.80 | 7.67 | 100.00 | 100.00 | ||
Table 4 The most common bacteria isolated from clinical specimens in 5 Swedish and 14 Chinese tertiary hospitals in 2012.
| Number | Percenatge Sweden | Number | Percenatge china | P-value | |
|---|---|---|---|---|---|
| E. coli | 22218 | 27.1 | 3737 | 13.5 | <0.005 |
| S.aureus | 21003 | 25.6 | 4875 | 17.6 | <0.05 |
| CoNS | 11387 | 13.9 | 4690 | 16.9 | ns |
| P. aureginosa | 6507 | 7.9 | 6197 | 22.3 | <0.005 |
| K. pneumonia | 5040 | 6.2 | 717 | 2.6 | ns |
| E. faecalis | 4910 | 6.0 | 2163 | 7.8 | ns |
| H. influenzae | 3669 | 4.5 | 622 | 2.2 | ns |
| S. pneumonia | 2871 | 3.5 | 728 | 2.6 | ns |
| E. faecium | 1506 | 1.8 | 2124 | 7.7 | <0.005 |
| E. coli (blood) | 1349 | 1.6 | 1224 | 4.4 | ns |
| S. pyogenes | 1113 | 1.4 | 44 | 0.2 | ns |
| K. pneumoniae (blood) | 457 | 0.6 | 626 | 2.3 | ns |
| ns= not significant | 82030 | 27747 |
Table 5 Reported resistance rates in common bacterial pathogens in Swedish and Chinese tertiary hospitals (%).
| Sweden | China | |
|---|---|---|
| Ciprofloxacin resistant E. coli (urine) | 11.0 | 72.6 |
| Metacillin resistant S. aureus (MRSA) | 3.8 | 38.5 |
| Ciprofloxacin resistant S. aureus | 5.3 | 41.8 |
| Cefoxitin resistant CoNS | 46.5 | 76.1 |
| Imipenem resistant P. aureginosa | 14.0 | 28.7 |
| Ciprofloxacin resistant K. pneumoniae (urine) | 7.6 | 47.5 |
| Vancomycin resistant E. faecalis | 0.0 | 1.7 |
| Ampicillin resistant H. Influensae | 19.8 | 47.6 |
| Erythromycin resistant S. pneumoniae | 8.6 | 90.8 |
| Vancomycin resistant E. faecium | 0.0 | 5.1 |
| Third generation cephalosporin resistant E. coli (blood) | 5.0 | 71.2 |
| Third generation cephalosporin resistant K. pneumoniae (blood) | 4.7 | 45.5 |
| Carbapenem resistant K. pneumoniae (blood) | 0.5 | 5.9 |
After the introduction of the special campaign set up by the MOH to promote the rational use of antibiotics in hospital settings, we could observe a significant reduction in total prescription of antimicrobials in the Chinese hospitals, and particularly, in the use of third-generation cephalosporins and fluoroquinolones (Table 6). Although not statistically significant, a trend towards a reduction in the use of first- and second-generation cephalosporin was also observed. In contrast, the participating hospitals in Sweden demonstrated no significant change in the consumption of antibacterial agents when the 2011 and 2012 data were compared (data not shown).
Table 6 Differences in prescription of antibioitics from Q1 and Q2 for 2011 to Q3 and Q4 2012 in Chinese tertiary hospitals.
| Group of antibiotics | Q1 and Q2 for 2011 | Q3 and Q4 2012 | P-value | ||
|---|---|---|---|---|---|
| Mean | Median | Mean | Median | ||
| Total prescription | 83.41 | 64.87 | 55.03 | 54.62 | 0.0085 |
| Fluoroquinolones | 11.59 | 9.6 | 6.79 | 6.77 | 0.0295 |
| Third generation Cephalosporines | 16.43 | 14.52 | 7.84 | 6.93 | 0.0023 |
| First generation Cephalosporines | 6.04 | 4.89 | 3.78 | 3.69 | 0.1352 |
| Second generation Cephalosporines | 8.75 | 7.67 | 7.85 | 7.71 | 0.4631 |
The high prescription rate of antibiotics before the introduction of the special campaign was associated with a significantly increased resistance rate in several bacterial species (Table 7).
Table 7 Total AB prescription correlates with resistance to antibiotic agents in Gram+ and Gram- bacteria in tertiary hospitals in China 2011.
| Antibiotic | Bacteria | P-value | Correlation co efficients |
|---|---|---|---|
| Imipenem | K. pneumoniae (blood) | <0.03 | 0.71204 |
| E. coli (blood) | <0.004 | 0.81935 | |
| Meropenem | E. coli (blood) | <0.05 | 0.08940 |
| Moxifloxacin | S. pneumoniae | <0.04 | 0.90000 |
| Levofloxacin | K. pneumoniae (blood) | <0.01 | 0.85714 |
Decrease in antibiotic resistance
Changes in the consumption of all given antibiotics through the implementation of improved administration of antibiotics resulted in a significantly decreased resistance rate for antibiotics in several clinical pathogens isolated in tertiary hospitals during the 2-year observation period (Table 8). There was a significant increase in the susceptibility of S. aureus to clindamycin and fluoroquinolone, E. coli to TxS and Pip-Taz, K. Pneumoniae to fluoroquinolone and imipenem, P. aureginosa to Pip-Taz and Imipenem and ESBL-producing organisms to ceftazidime, and ciprofloxacin.
Table 8 Correlation between reduction in total AB prescription and increased bacterial sensitivity to some antibacterial agenuin tertiary hospitals in China in 2012.
| Group of antibiotics | Bacteria | Increased sensit ivity to | P-value | Correlation coefficients |
|---|---|---|---|---|
| Total AB prescription | S. aureus | Ciprofloxacin | <0.03 | - 0.70909 |
| Levofloxacin | <0.004 | - 0.79091 | ||
| Clindamycin | <0.03 | - 0.65149 | ||
| E. coli (blood) | Txs | <0.04 | - 0.66061 | |
| Pip-Taz | <0.0006 | - 0.88964 | ||
| K. pneumoniae | Ciprofloxacin | <0.0005 | - 0.96429 | |
| Levofloxacin | <0.0005 | - 0.96429 | ||
| Imipenem | <0.053 | - 0.69048 | ||
| ESBl | Ciprofloxacin | <0.03 | - 0.76190 | |
| Ceftacidime | <0.004 | - 0.88095 | ||
| P. aeuroginosa | lmipenem | <0.04 | - 0.60839 | |
| Pip-Taz | <0.006 | - 0.7355 |
During the 2-year study period, no significant change in the level of resistance to commonly used antibiotic groups could be noted in the clinical isolates from the five Swedish tertiary hospitals (data not shown).
Discussion
The present study was conducted at a time when Chinese health authorities started a campaign to discourage over prescription of antibiotics. The findings here suggest that their efforts appear to be paying off. Antibiotics are among the most frequently prescribed drugs, with patients contributing up to 90% of hospital revenue. In China overprescribing has become a concern in the context of China’s increasing health care expenditure and the growing rates of antimicrobial resistance. Compared with the Swedish hospitals as an example of rational health system in which the use of antimicrobial agents is strictly controlled and prescribed only by qualified physicians without monetary interests, the rates of resistance for the most common pathogens were much higher in Chinese tertiary hospitals participating in this study.
The question of whether prescribing restrictions can bring about reductions in levels of antimicrobial resistance in the community remains undecided [15]. A close association exists between resistance rate and the amount of antimicrobial agents used [16]. Such an association indicates a serious need to control antibiotic consumption. Analyses have indicated that the time scale for emergence of resistance under constant selective pressure is much shorter than the decay time after cessation or decline in the volume of drug use and that significant reductions in resistance require equally significant reductions in drug consumption [17]. During our 2-year investigation period, the total use of antibiotics decreased dramatically from 84 DDDs/100 patient-days to 47.3 DDDs/100 patient-days. The most frequently used antibiotics were cephalosporins and fluoroquinolones. This decrease of antimicrobial usage in 14 tertiary hospitals was accompanied by a markedly enhanced susceptibility in S. aureus for quinolones, E.coli for TxS, and Pip-Taz, K.pneumonia for quinolones and Imipenem, P. aurigenosa for Pip-Taz and ESBL-producing Enterobacteriacae for Ceftazidime, and Ciprofloxacin.
Studies using mathematical models of the epidemiology and population genetics of antibiotic treatment and resistance in hospitals predicted that reduced use of antibiotics coupled to the application of infection control and other measures such as increasing the rate at which patients leave the hospital and by the use of antibiotics for which there is little or no resistance, the response to these measures to control resistance in hospitals is anticipated to be rapid, taking place within months or even weeks, rather than decades or years, as anticipated for open communities [18,19]. These studies support our results showing that restricted prescribing can reduce resistance in hospital setting and may partly explain the much less successful results in decreasing resistance among E. coli by reduced community prescribing in a recent Swedish study [20].
Our data on antibiotic consumption and microorganism resistance changes in 14 Chinese tertiary hospitals show a significant correlation between high prescription of cephalosporins and fluoroquinolones and high resistance rates to these antibiotics. Our analysis shows a positive correlation between high antibiotic consumption and K. pneumoniae resistance to levofloxacin. Similar results have been reported in an analysis of antibiotic consumption and bacterial resistance in Lithuanian tertiary hospitals, which showed a positive correlation between quinolone consumption and K.pneumoniae resistance to ciprofloxacin [21]. Thus, our results and those from other studies support the need for controlled use of these effective antibiotics [22].
Stopping the inappropriate use of third-generation cephalosporin without replacement with a different class of antibiotics in patients without clinical or microbiological evidence of infection is an important restriction method. It can be implemented on a mandatory as in the Chinese example, or by education and recommendation, which has been the main strategy for many years for the Swedish Strama groups [12]. Most importantly, however, is finding a method that is suitable and applicable to the individual hospital for reducing inappropriate use of thirdgeneration cephalosporin.
Since the reduction in antimicrobial consumption was so dramatic during the studied period of 2 years, data on hospital mortality (especially related to infection) before and during the study would besides increasing bacterial susceptibility have been valuable to determine the usefulness of measures to reduce antibiotic consumption. Increasing awareness of antimicrobial resistance and promoting the rational use of antibiotics among prescribers and the public are key components to combat the unnecessary use of these drugs [23]. Some important programmes have been implemented in developed countries, including Strama in Sweden [12], the Get Smart: know when antibiotics work programme of the US Centres for Disease Control and Prevention [24], and recently, the European commission presented a 5-year action plan against the rising threats from antimicrobial resistances in Europe [25]. Lately, despite not being law yet, an innovative model to promote the rational use of medicines and counteract antimicrobial resistance – the Antibiotics Smart Use programme (ASU) - has been initiated in Thailand with some success [26]. In Sweden, Strama’s multidisciplinary coordinated programme has contributed to a reduction of antibiotic use in Sweden without measurable negative consequences. In combination with increasing emphasis on infection control the spread of resistance has been limited [27]. The present study demonstrates that the decline in the consumption of antibiotics has reached a plateau in tertiary hospitals in Sweden.
The long-term sustainability of these programmes will require continued local and national commitment, political support, effective auditing, and integration of the programmes into the routine systems of outpatient and hospital health care management with appropriate financial incentives. Following the implementation of the intensive control programme in Chinese tertiary hospitals, we found that the consumption levels of parenteral antimicrobials were reduced and accompanied by a significant increase in susceptibility patterns of both Grampositive and Gram-negative bacteria, including ESBL-producing organisms. This important finding supports the notion that a strategy of decreasing antimicrobial prescribing to reduce existing antimicrobial resistance can be enforced quickly and efficiently, particularly in the hospital setting [17], when coupled to strict implementation of administrative regulations and the imposition of legal penalties. Optimisation of antibiotic usage not only prevents increased resistance but also reduces health care costs [28]. The financial impact of antimicrobial restriction programmes has been shown in both developed and developing countries [29,30]. The long-term influence on medical budget spending may be weaker than the short-term influence because in hospitals restriction programmes can sometimes have undesirable effects by increasing the prescribing of other more expensive agents and subsequent resistance to them [14]. Our study was not intended to analyse the economic consequences of the special campaign carried out by the MOH to promote the rational use of antibiotics in hospital settings. Further research is required to identify whether all services included in the special campaign are sustainable and provide a reasonable return on investment in Chinese hospitals.
The limitation of this study is that only 14 tertiary hospitals in China participated. By the end of 2011, China was reported to have 1399 tertiary hospitals, which makes it extremely difficult to determine the representativeness of our data for China’s tertiary hospitals as a whole [31]. Previous surveillance results, however, indicate that no difference exists for bacteria resistance between different regions in China [32] and our own antimicrobial data suggest that the level of antibiotic use was comparable in different regions.
Conclusion
In conclusion, these observations suggest that the containment of bacterial resistance requires the implementation of a full-scale national strategy, including research, education, regulations, and international collaboration [33].
Acknowledgments
We wish to express our gratitude to Drs Stephan Stenmark, Christian Giske, Åke Gustafsson, Lennart E. Nilsson, Agneta Johansson, Susanne Sutterlin and Gunilla Skoog from the participating local Strama groups and the pilot hospitals in China for providing data on resistance, typing, and collecting data on antibiotic use during the study period. This paper is previously unpublished and not under simultaneous consideration by another journal.
Funding
This work was supported by the Chinese Ministry of Health and the Swedish International Development Cooperation Agency.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors’ Contributions
These authors contributed equally to this work. All authors participated in the design of the study, collection of data, conducted the data analysis, and prepared and reviewed the manuscript.
References
- Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74: 417-33.
- Lambert ML, Suetens C, Savey A, Palomar M, Hiesmayer M, et al. (2011) Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet infect Dis 11: 30-38.
- Holmberg SD, Solomon SL, Blake PA (1987) Health and economic impacts of antimicrobial resistance. Rev Infect Dis 9: 1065-1078.
- World Health Organization (April 2011) World Health Day-7, WHO, Geneva.
- (2016) Memorandum of Understanding between the Government of the Kingdom of Sweden and the Government of the People´s Republic of China concerning Health Cooperation.
- Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S (2011) Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect Dis 11: 692-701.
- Yip WC-M, Hsiao WC, Chen W, Hu S, Ma J, et al. (2012) Early appraisal of China´s huge and complex health-care reforms. Lancet 376: 833-842.
- Xiao YH, Zhang J, Zheng B, Zhao L, Li S, et al. (2013) Changes in chinese policies to promote the rational use of antibiotics. PlosMed 10: e1001556.
- China MoH (2011) Protocol for special campaign of antibiotic administrative in healthcare institutions. Ministry of Health, May 8, 72011.
- The National Board of Health and Welfare (2000) Swedish plan of action against antibiotic resistance. Stockholm.
- Swedish Ministry of Health and Social Affairs (2006) Strategy to prevent antibiotic resistance and health-care infections. Fact sheet 2008 No.8. Stockholm.
- Molstad S, Cars O, Struwe J (2008) Strama- A Swedish working model for containment of antibiotic resistance. Euro Surveill13: 19041.
- Mölstad S, Erntell M, Hanberger H, Melander E, Norman C, et al. (2008) Sustained reduction of antibiotic use and low bacterial resistance. A 10- year follow-up of the Swedish STRAMA programme. Lancet Infect Dis 8: 125-132.
- Tängden T, Eriksson BM, Melhus A, Svennblad B, Cars O (2011) Radical reduction of cephalosporin use at a tertiary hospital after educational antibiotic intervention during an outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae. J Antimicrob Chemother 66: 1161-1167.
- Enne VI (2010) Reducing antimicrobial resistance in the community by restricting prescribing: Can it be done?J Antimicrob Chemother 65: 179-182.
- Laxminarayan R, Heymann D (2012) Challenges of drug resistance in the developing world. BMJ 344: e1567.
- Austin DJ, Kristinsson KG, Anderson RM (1999) The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci USA 96: 1152-1156.
- Levin BR (2001) Minimizing potential resistance: A population dynamics view. CID33: S161-169.
- Haber M, Levin BR, Kramarz P (2010) Antibiotic control of antibiotic resistance in hospitals: A simulation study. BMC Infectious Diseases 10: 254.
- Sundqvist M, Geli P, Andersson DI, Sjölund-Karlsson M, Runehagen A, et al. (2010) Little evidence for reversibility to trimethoprim resistance after a drastic reduction in trimethoprim use. J Antimicrob Chemother65: 350-360.
- Galinyte D, Maciulaitis R, Budnikas V, Kubilius D, Varanaviciene B, et al. (2008) Analysis of antibiotic consumption and microorganism resistance changes. Medicina (Kaunas) 44: 751-767.
- Chen IL, Lee CH, Su LH, Tang YF, Chang SJ, et al. (2013) Antibiotic consumption and healthcare- associated infections caused by multidrug-resistant gram-negative bacilli at a large medical center in Taiwan from 2002 to 2009: Implicating the importance of antibiotic stewardship. PlosONE 8: e65621.
- Malmvall BE, Mölstad S, Darelid J, Hiselius A, Larsson L, et al. (2007) Reduction of antibiotic sales and sustained low incidence of bacterial resistance: Report on a broad approach during 10 years to implement evidence-based indications for antibiotic prescribing in Jönköping County, Sweden. Manage Health Care 16: 60-67.
- Srinivasan A, Fishman N (2012) Antimicrobial stewardship 2012: Science driving practice. Infect Control Hosp Epidemiol 33: 319-321.
- European Commission (2011) Communication from the Commission to the European Parliament and the Council. Action plan against the rising threats from antimicrobial resistance.
- Sumpradit N, Chongtrakul P, Anuwong K, Pumtong S, Kongsomboon K, et al. (2012) Antibiotics Smart Use: a workable model for promoting the rational use of medicines in thailand. Bull World Health Organ 90: 905-913.
- Struwe J (2011) Fighting antibiotic resistance in Sweden – past, present and future. Wien Klin Wochenschr 120: 268-279.
- Altunsoy A, Aypak C, Azap A, Ergönul Ö, Balik I (2011) The impact of a nationwide antibiotic restriction program on antibiotic usage and resistance against nosocomial pathogens in Turkey. Int J Med Sci 8: 339-344.
- Neidell MJ, Cohen B, Furuya Y, Hill J, Jeon CY, et al. (2012) Cost of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clinical Infectious Diseases 55: 807-815.
- Siddiqui S, Hussein K, Manasia R, Salahuddin N, Zafar A, et al. (2007) Impact on antibiotic restriction on broad spectrum antibiotic usage in ICU of a developing country. J Pak Med Assoc 57: 484-487.
- Ministry of Health of the People´s Republic of China (2012) China health statistical yearbook
- Xiao YH, Wang J, Li Y (2008) Bacterial resistance surveillance in China: A report from Mohnarin 2004-2005. Eur J Clin Microbiol Infect Dis 27: 697-708.
- Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, et al. (2013) Antibiotic resistance- the need for global solutions. Lancet Infect Dis 13: 1057-1098.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences