IL-10-Producing Th17 Cells:A Potentially Regulatory Cell Population in Dry Eye Disease
1Schepens Eye Research Institute, Massachusetts Eye and Ear Infirmary, Department of Ophthalmology, Harvard Medical School, Boston, MA, USA
2Peking University Eye Center, Peking University Third Hospital, Beijing, China
- Corresponding Author:
- Reza Dana, M.D., M.P.H., M.Sc
Schepens Eye Research Institute
Massachusetts Eye & Ear Infirmary
Harvard Medical School, 20 Staniford Street
Boston, MA 02114, USA
Tel: +1-617-912-7401
Fax: +617-912-0117
E-mail: reza_dana@meei.harvard.edu
Received Date: November 29, 2017; Accepted Date: December 26, 2017; Published Date: January 02, 2018
Citation: Qi H, Chen Y, Inomata T, Amouzegar A, Dana R (2017) IL-10-Producing Th17 Cells: A Potentially Regulatory Cell Population in Dry Eye Disease. J Immuno Immunother. Vol. 2 No.1:5
Copyright: © 2017 Qi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: To assess the effect of dry eye disease (DED) on regulatory IL-10- producing Th17 cell population.
Methods: DED was induced in female C57BL/6 mice. mRNA and protein expression levels of IL-17 and IL-23 were analyzed in the conjunctivae and draining lymph nodes (DLNs) of mice 14 and 28 days after DED induction using real-time PCR and ELISA, respectively. The frequencies of IL-17-producing Th17 (IL-17+CD4+) cells and IL-10-producing Th17 (IL-10+IL-17+CD4+) cells were determined in the conjunctivae and DLNs of DED mice on days 14 and 28 using flow cytometry analysis.
Results: DED induction significantly upregulated the expression of IL-17 and IL-23 at the ocular surface and draining lymph nodes at day 14. Frequencies of IL-17 producing Th17 cells significantly increased in mice with acute DED, and returned to near normal levels with disease resolution. These changes were accompanied by a significant decrease in frequencies of IL-10-producing Th17 cells in acute DED, and their restoration to baseline levels with resolution of DED.
Conclusions: Decreased frequencies of IL-10-producing Th17 cells concomitant with increased effector Th17 cell frequencies and IL-17 and IL-23 cytokine levels contribute to DED pathogenesis.
Keywords
Dry eye disease; T helper-17 cells; Regulatory; Cornea
Introduction
Dry Eye Disease (DED) is a chronic disease with multifactorial etiology that results in symptoms of ocular discomfort, visual disturbance and tear film instability with potential damage to the ocular surface [1]. The pathogenesis of DED is complex and poorly understood. However, increasing evidence associates DED with loss of immunehomeostasis at the ocular surface and varying degrees of inflammation [2,3].
Emerging data demonstrate that T helper 17 (Th17) cells play a critical role in the pathogenesis of autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, psoriasis, inflammatory bowel disease and DED [4,5]. Th17 cells are considered the principal effector cells responsible for the ongoing ocular surface inflammation and relapsing course of DED [6]. Role of Th17 cells in the immunopathogenesis of DED is supported by a myriad of studies demonstrating increased ocular surface expression of IL-17 and infiltration of Th17 cells to the conjunctivae and draining lymph nodes of DED mice [7,8], and resolution of DED by administration of IL-17 blocking antibodies [9,10].
Naïve CD4+ T cells differentiate into IL-17-producing Th17 cells in the presence of transforming growth factor-β (TGF-β) and IL-6, and subsequent exposure to IL-23 is required for the functional maturation and pathogenic function of Th17 cells [11,12]. Recent studies have revealed that Th17 cells are not a terminally differentiated T helper cell lineage; instead, they are highly plastic and have the ability to acquire the signature phenotypes of other T helper cell lineages in certain microenvironments; specifically, these cells are capable of secreting IFN-γ in the presence of IL-12 and IL-23 [13-15]. Expanding evidence demonstrates the important role of Th17 plasticity in the pathogenesis of a variety of autoimmune inflammatory disorders, such as colitis [13], collagen-induced arthritis (CIA) [16], Experimental Autoimmune Encephalomyelitis (EAE) [17], and DED [18]. On the other hand, the potential anti-inflammatory role of Th17 plasticity in immunoinflammation has not been well studied. It has been reported that in the absence of IL-23, TGF-β plus IL-6 abrogate the pathogenic function of Th17 cells and promote the production of IL-10 by these cells [19]. In addition, in the presence of IL-2 and TGF- β, Th17 cells begin expressing Foxp3 [20]. Foxp3 is a phenotypic and functional marker for a major group of immunosuppressive cells, called regulatory T cells (Tregs) [21]. Tregs are one of the major sources of IL-10, which enables these cells to suppress Th17-induced inflammation [10,22,23]. These findings suggest that Th17 cells can adopt an anti-inflammatory fate under certain conditions, and may contribute to resolution of inflammation.
Recent studies have shown that IL-6 stimulation in the absence of TGF- β reprograms Tregs to become IL-17+Foxp3+ cells and function similar to pathogenic Th17 cells [24,25]. The abrogated suppressive function of Tregs has also been reported in DED and the altered Th17/Treg balance has been linked to DED immunopathogenesis [10]. However, little is known about the potentially regulatory population of IL-10-producing Th17 cells in DED. In the present study, we aimed to explore DED-induced alterations in the IL-10-producing Th17 cell population using a well-established mouse model of DED.
Materials and Methods
Animals
Six- to eight-week-old female C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were used for this study. All animal experiments were approved by the Schepens Eye Research Institute Animal Care and Use Committee, and adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of DED
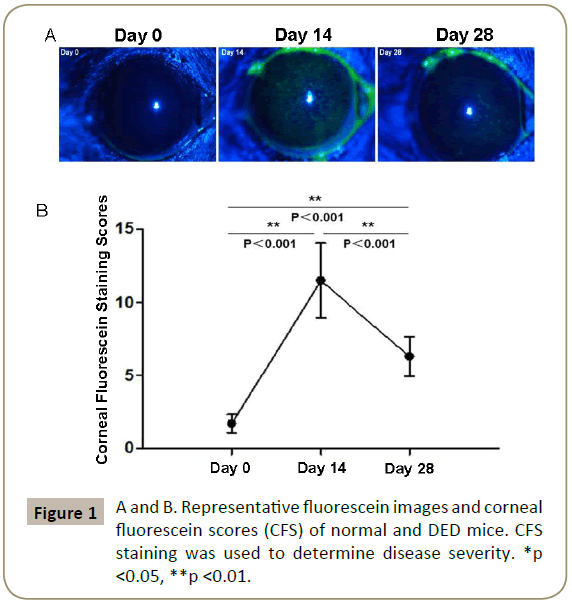
Acute DED was induced in mice by placing them in the Controlled- Environment Chamber (CEC) with a relative humidity of <20%, airflow of 15 L/min and a constant temperature (21-23C) for 14 days, as previously described [9]. To study the resolution phase of DED, mice were subsequently transferred to the standard vivarium and were kept in the normal environment for additional 14 days. Corneal Fluorescein Staining (CFS) was used to evaluate corneal epithelial damage caused by DED. 1 μL of 2.5% fluorescein (Sigma-Aldrich Corp. Darmstadt, Germany) was applied into the lateral conjunctival sac of the mice, and 3 minutes later corneas were examined using a slit-lamp biomicroscope (Topcon SL-D7; Topcon Corp. Tokyo, Japan) under cobalt blue light. Punctate staining was recorded in a masked fashion using the National Eye Institute grading system, scoring 0 to 3 for each of five areas of the cornea: central, superior, inferior, nasal, and temporal [12].
Real-time PCR
Conjunctivaee and draining lymph nodes from mice were harvested, frozen in TRIzol Reagent (Invitrogen, Carlsbad, CA) and stored at −80°C until use. Total RNA was isolated with an RNeasy Micro kit (Qiagen, Valencia, CA) according to the manufacturer’s recommendations and reverse transcribed using a SuperScript III kit (Invitrogen). Real-time PCR was performed using TaqMan Universal PCR Master Mix (Roche, Branchburg, NJ) and predesigned primers for IL-23 (Mm00518984_m1), IL-17 (Mm00439618_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Mm99999915_m1; Applied Biosystems, Foster City, CA) in a LightCycler 480 II System (Roche Applied Science, Indianapolis, IN). The GAPDH gene was used as an endogenous control for each reaction. The results of quantitative PCR were analyzed by the comparative CT method in which the target change was equal to 2−ΔΔCT. The results were normalized by the CT value of GAPDH, and the mean CT of relative mRNA levels in the normal group was used as the calibrator.
Enzyme-linked immunosorbent assay (ELISA)
For IL-23 protein level analysis, single cells were stimulated with 1 μg/ ml lipopolysaccharide (LPS) (Sigma-Aldrich Corp. Darmstadt, Germany) for 24 hours. For IL-17 protein level analysis, single cells were stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin for 24 hours. The supernatant was assayed for the levels of IL-17 and IL-23 using commercial ELISA kits (Biolegend).
Cell isolation and flow cytometry analysis
Single cell suspensions were prepared from conjunctivae and the draining cervical lymph nodes (DLNs) of normal or DED mice by collagenase digestion. Briefly, tissues were removed and cut into small fragments, followed by digestion with 2 mg/mL collagenase type IV (Sigma-Aldrich Corp, Darmstadt, Germany) and 0.05 mg/mL DNase I (Roche, Basel, Switzerland) for 1 hour at 37°C with agitation. The suspension was then filtered through a 70 μm cell strainer (BD Biosciences, Bedford, MA). Single cells were first stimulated with 50ng/ml phorbol 12-myristate 13-acetate (PMA) and 500 ng/ml ionomycin (Sigma-Aldrich Corp, Darmstadt, Germany) for 18h at 37°C and 5% CO2 in the presence of GolgiStop (4 μl per 6 ml cell culture; BD Biosciences, San Jose, CA) to inhibit cytokine secretion, and then stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4. After permeabilization using Permeabilization Buffer (eBioscience, San Diego, CA), cells were stained with the following antibodies: PECy7– conjugated anti–IL-17A and PerCP/Cy5.5-conjugated anti– IL-10 (Biolegend). Control samples were stained with appropriate isotype-matched control antibodies. Stained cells were examined using the LSR II flow cytometer (BD Biosciences), and the results were analyzed using FlowJo vX.0.7 software (TreeStarInc, Ashland, OR).
Statistical analysis
Student’s t test was used to analyze differences between the groups, and p<0.05 was considered significant.
Results
Expression of IL-17 and IL-23 is upregulated in acute ded
We first evaluated disease severity by performing CFS staining on days 14 and 28 after DED induction. Representative images show the corneal fluorescein uptake by normal and DED mice (day 14 and 28) (Figure 1A). The CFS scores increased significantly by day 14 (p<0.001), and decreased to substantially lower levels by day 28 (p<0.001) (Figure 1B), demonstrating the progression of DED from acute to resolution phase by day 28.
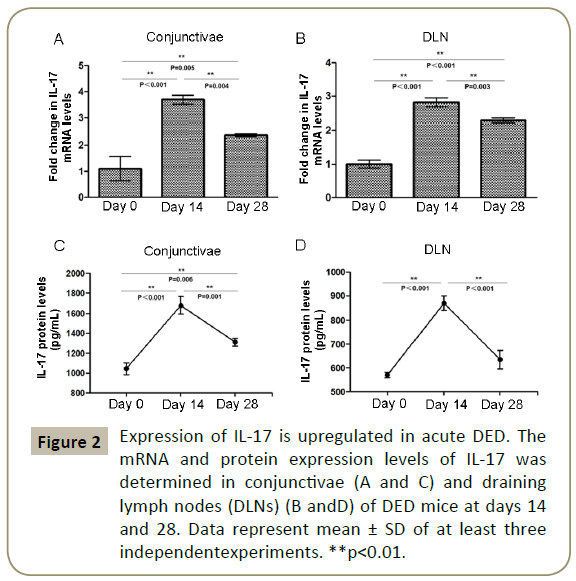
We next determined the expression levels of IL-17 and IL-23 cytokines in the acute and resolution phases of DED using ELISA and real-time PCR. Our data demonstrated that mRNA expression of IL-17 in the conjunctivae and Draining Lymph Nodes (DLN) of mice with DED respectively increased by 3.7 folds (p<0.001) and 2.8 folds (p<0.001) at day 14 compared to non-DED controls. IL-17 mRNA levels decreased by 38% in the conjunctivae and by 20% in the DLN of DED mice at day 28 compared to day 14 (p=0.004 and p=0.003, respectively), but still persisted at higher levels than non-DED mice (p=0.005 and p<0.001, respectively) (Figure 2A and 2B). Similarly, we observed significantly higher IL-17 protein levels in both conjunctivae and DLN of mice with DED at day 14 (871.38 ± 29.24 pg/ml and 1679.11 ± 86.25 pg/ml) compared to normal mice (570.05 ± 11.74 pg/ml and 1044.39 ± 59.13 pg/ ml, respectively; p<0.001). At day 28, IL-17 protein levels in the conjunctivae were higher than baseline (p<0.006), but returned to near normal values in the DLN (Figure 2C and 2D).
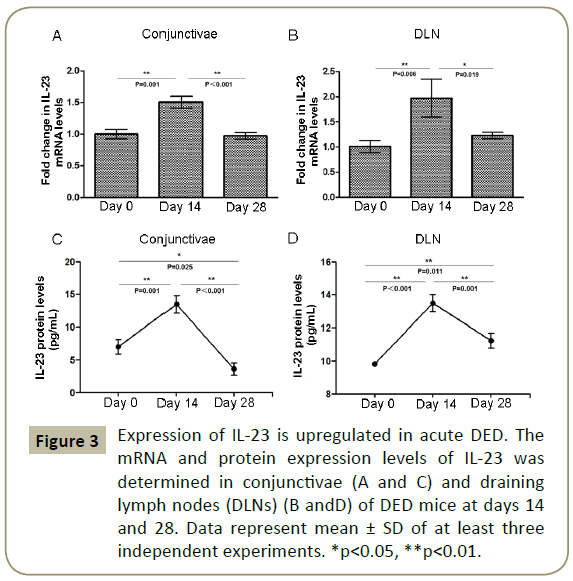
A similar trend was observed for IL-23 expression. The mRNA levels of IL-23 increased to significantly higher levels in both conjunctivae (2-fold increase, p=0.001) and DLN (1.5-fold increase, p=0.006) of DED mice at day 14, and returned to near normal values in both tissues by day 28 (Figure 3A and 3B). The protein levels of IL-23 in conjunctivae (13.45 ± 1.65 pg/ml) and DLN (13.49 ± 0.52 pg/ml) were significantly higher at day 14 compared to non-DED controls (7.01 ± 1.33 pg/ml and 9.81 ± 0.04 pg/ml, respectively; p<0.001), but returned to near baseline levels by day 28 (Figure 3C and 3D).
Figure 3: Expression of IL-23 is upregulated in acute DED. The mRNA and protein expression levels of IL-23 was determined in conjunctivae (A and C) and draining lymph nodes (DLNs) (B andD) of DED mice at days 14 and 28. Data represent mean ± SD of at least three independent experiments. *p<0.05, **p<0.01.
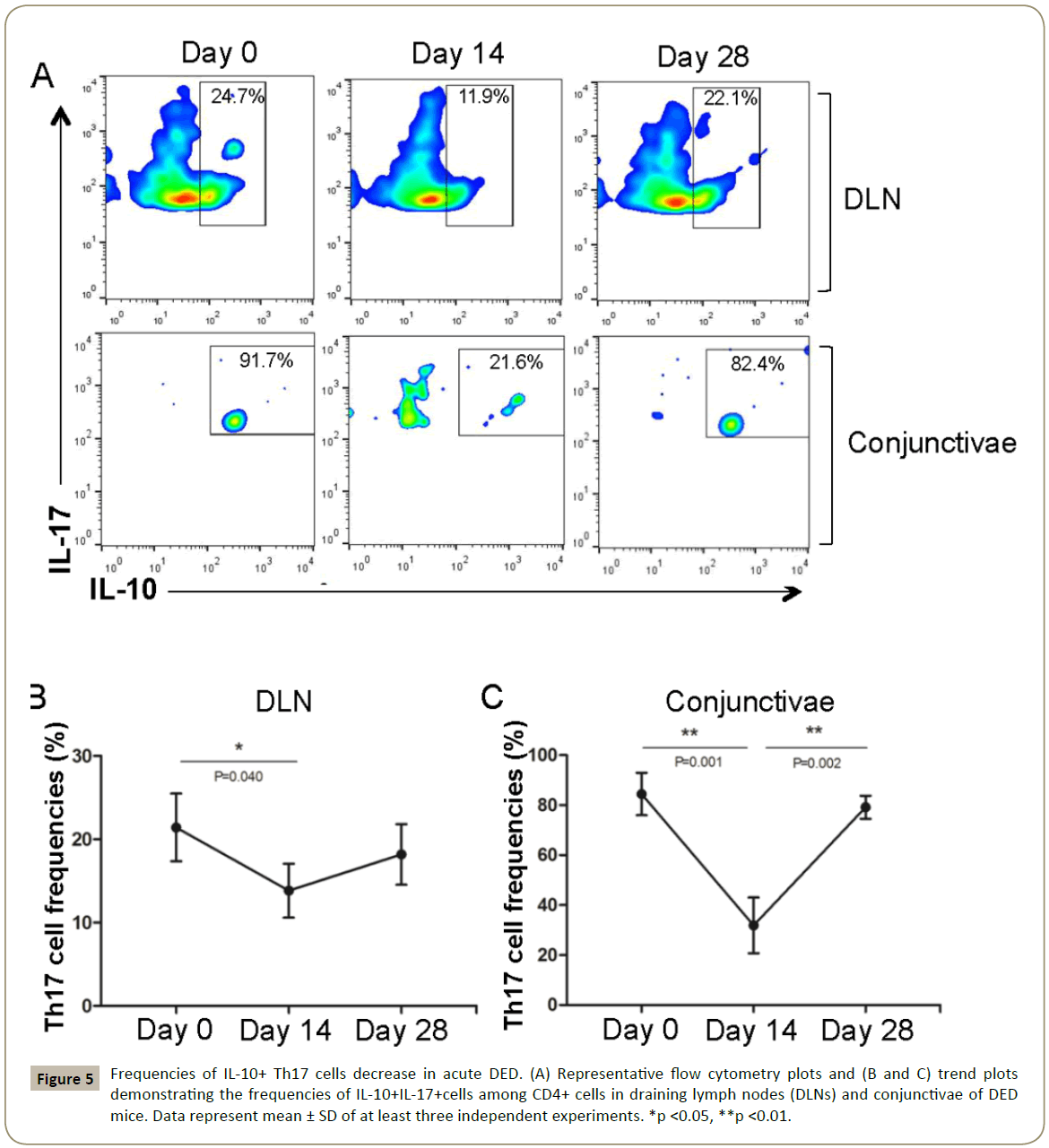
Frequencies of IL-10-producing Th17 cells decrease in acute DED
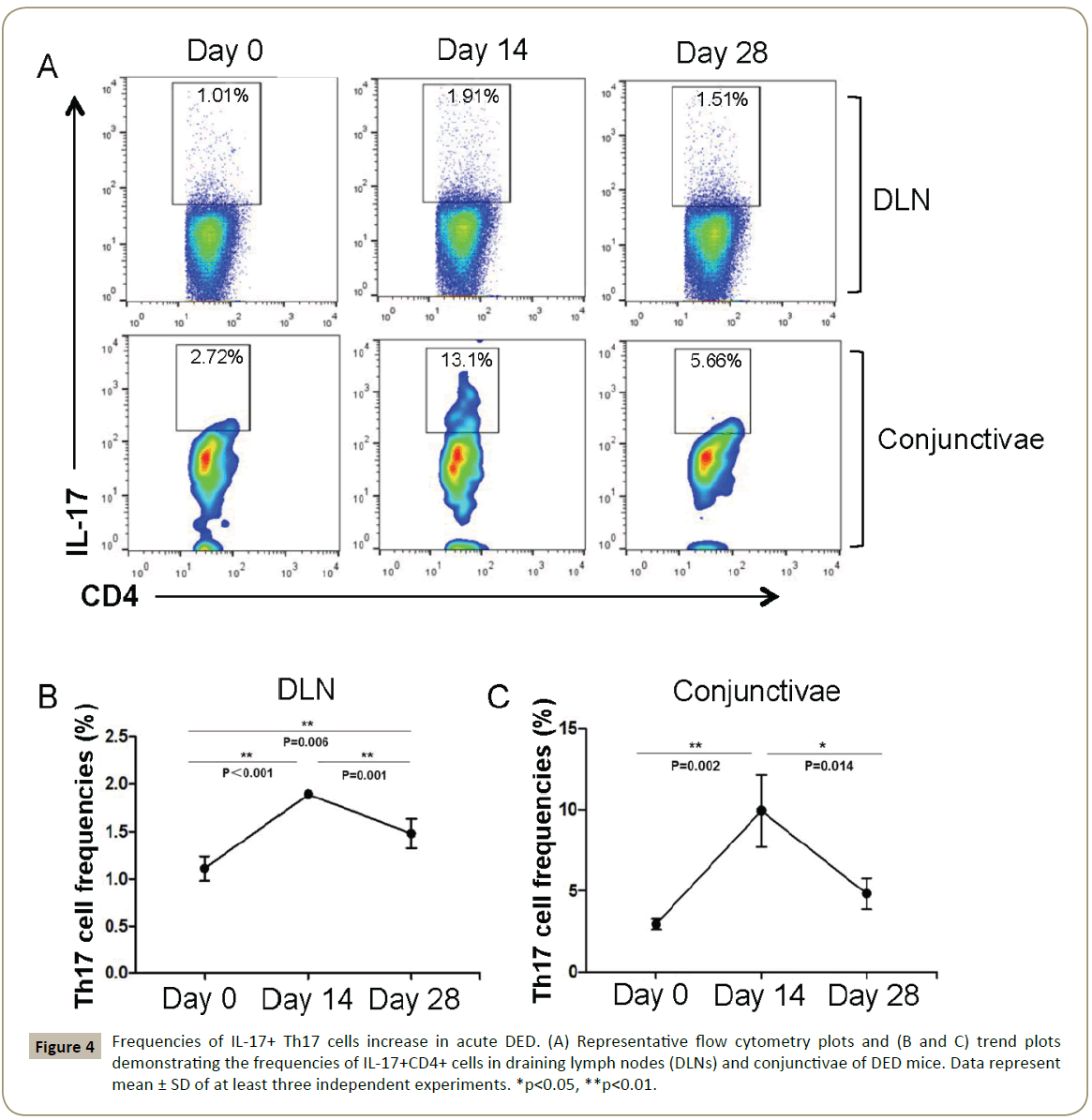
We next determined the frequencies of Th17 cells in the conjunctivae and DLN of DED mice at days 14 and 28 after DED induction. Both conjunctivae and DLN of mice with DED demonstrated significantly higher frequencies of IL-17+ CD4+ T cells compared with normal controls at day 14 (p=0.002 and p<0.001, respectively). By day 28, Th17 frequencies decreased to significantly lower levels in both conjunctivae and DLNs compared to day 14 (p=0.014 and p=0.001, respectively) (Figure 4A-4C).
Figure 4: Frequencies of IL-17+ Th17 cells increase in acute DED. (A) Representative flow cytometry plots and (B and C) trend plots demonstrating the frequencies of IL-17+CD4+ cells in draining lymph nodes (DLNs) and conjunctivae of DED mice. Data represent mean ± SD of at least three independent experiments. *p<0.05, **p<0.01.
IL-10-producing Th17 cells (IL-10+ IL-17+ CD4+) were detected in both conjunctivae and DLNs of normal and DED mice (Figure 5A). The frequencies of IL-10-producing Th17 cells in DLNs decreased from 21.43 ± 4.07% in normal mice to 13.83 ± 3.24% at day 14 after DED induction (p<0.04), and returned to near normal values by day 28 (18.18 ± 3.64%). Similarly, IL-10+ Th17 frequencies in conjunctivae decreased from 84.45 ± 9.81% to 31.9 ± 13.66% at day 14 after DED induction (p=0.001), and returned to baseline levels by day 28 (79.18 ± 5.35%) (Figure 5B and 5C). These data collectively demonstrate that increased frequencies of effector IL-17+ Th17 cells in the acute phase of DED are accompanied by a reduction in the regulatory IL-10+ Th17 cell population.
Figure 5: Frequencies of IL-10+ Th17 cells decrease in acute DED. (A) Representative flow cytometry plots and (B and C) trend plots demonstrating the frequencies of IL-10+IL-17+cells among CD4+ cells in draining lymph nodes (DLNs) and conjunctivae of DED mice. Data represent mean ± SD of at least three independent experiments. *p <0.05, **p <0.01.
Discussion
Th17 cells play a key role in ocular surface autoimmunity [6]. IL- 17 and IL-23 are the principal Th17-associated cytokines involved in the immunopathogenesis of DED. Exposure to IL-23 induces Th17 cells to further differentiate into fully pathogenic IL-17- producing effector cells [26]. IL-17, a potent pro-inflammatory cytokine, is involved in the pathogenesis of a wide array of autoimmune diseases, including DED [27,28]. In the present study, we identified a previously undefined subset of T cells, the IL-10-producing Th17 cells in the immune quiescent status, and demonstrated their decrease in correlation with upregulated IL 17 and IL-23 levels in acute DED, suggesting a role for these cells in maintaining immunohomeostasis.
We first investigated the kinetics of expression of IL-17 and IL- 23 in the course of DED. Our results show that the expression of both IL-17 and IL-23 in conjunctivae and DLNs increase to significantly higher levels in acute DED and decrease to relatively lower levels with disease resolution. These results are consistent with previously reported results from human and animal studies, demonstrating increased levels of IL-17 and IL-23 at the ocular surface and in tears of individuals with DED [29]. In addition, we observe a significant reduction in frequencies of IL-10-producing Th17 cells in the acute phase of DED. This finding was associated with upregulated IL-17 and IL-23 levels and increased frequencies of IL-17 producing effector T cells.
Previous studies have demonstrated that while IL-6 and TGF-β are involved in initial lineage commitment of Th17 cells, they direct Th17 cells to acquire a regulatory phenotype and produce IL-10 [19]. IL-23, on the other hand, has been shown to promote the expression of IL-17 by Th17 cells [19].
Similarly, our results demonstrate that increased levels of IL-23 in acute DED are associated with a decreased ratio of regulatory IL-10+ Th17 cells to pathogenic IL-17+ Th17 cells. Additionally, we demonstrate that concomitant with the decline in IL-17 and IL-23 levels and resolution of DED, IL-10+ Th17 cell population is restored to baseline levels, indicating that the inflammatory cytokine environment in DED can extinguish the expression of anti-inflammatory cytokine IL-10 by the pre-existing IL- 10+Th17 cells. Recent reports have demonstrated the plasticity of Th17 cells during inflammation [30]. It has been shown that in the absence of TGF-β, exposure to IL-12 and IL-23 results in acquisition of a Th1 phenotype and expression of IFNγ by Th17 cells [13]. Our group has also recently demonstrated that the IL- 12 and IL-23 microenvironment is required for the development of double positive Th17/1 cells that contribute to ocular surface inflammation in DED [18]. Taken together, Th17 cells in DED are shaped to be more immunopathogenic with the loss of the anti-inflammatory phenotype. Our findings present the background for novel approaches to treat DED by retaining IL-10+Th17 cells through modifying the cytokine microenvironment, or via transferring the autologous IL-10+Th17 cells to the ocular surface. In fact, acquisition of a regulatory phenotype by Th17 cells has also been demonstrated during resolution of infectious inflammation [30], further supporting a promising perspective to harness the regulatory plasticity of Th17 cells in the development of novel therapeutic strategies for DED.
Conclusion
In conclusion, our findings demonstrate decreased frequencies of IL-10-producing Th17 cells concomitant with increased effector Th17 cell frequencies and IL-17 and IL-23 cytokine expression in acute DED. This is the first study to our knowledge to investigate the alterations in IL-10+Th17 cells in the course of DED. Our results indicate a potential regulatory role for IL-10+ Th17 cells in DED, and could provide the framework for development of potential therapeutic strategies based on expanding IL-10- producing Th17 cells. However, further studies are still required to establish the precise role and function of this Th17 subset in the pathogenesis of DED.
Acknowledgements
The authors would like to thank Rongjun Liu and Caifeng Gao for their help in preparing the manuscript. This study was supported by the National Institutes of Health/National Eye Institute Grant R01 EY 20889 to RD. The first author (HQ) would like to thank the National Natural Science Foundation of China (No. 30872813; No. 81570813), The Grant of China Scholarship Council, and The Scientific Research Foundation for the Returned Overseas Chinese Scholars for their support.
Disclosure
The authors declare no competing financial interests.
References
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5: 75-92.
- Barabino S, Chen Y, Chauhan S, Dana R (2012) Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. ProgRetin Eye Res 31: 271-285.
- Pflugfelder SC (2003) Anti-inflammatory therapy of dry eye. Ocul Surf 1: 31-36.
- Miossec P, Korn T, Kuchroo VK (2009) Interleukin-17 and type 17 helper T cells. N Engl J Med 361: 888-898.
- Bettelli E, Oukka M, KuchrooVK (2007) T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol 8: 345-350.
- Stevenson W, Chauhan SK, Dana R (2012) Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol, 130: 90-100.
- De Paiva CS , Chotikavanich S, Pangelinan SB, Pitcher JD 3rd, Fang B, et al. (2009) IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol 2: 243-253.
- Dohlman TH, Chauhan SK, Kodati S, Hua J, Chen Y,et al. (2013) The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Invest Ophthalmol Vis Sci 54: 4081-4091.
- Chauhan SK, Jin Y, Goyal S, Lee HS, Fuchsluger TA, et al. (2011) A novel pro-lymphangiogenic function for Th17/IL-17. Blood 118: 4630-4634.
- Chauhan SK,Annan J, Ecoiffier T, Goyal S, Zhang Q, et al. (2009) Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol 182: 1247-1252.
- Youjin Lee, AmitAwasthi, NirYosef, Francisco J. Quintana, Sheng Xiao,et al. (2012) Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 13: p. 991-999.
- Zúñiga LA, Jain R, Haines C, Cua DJ (2013) Th17 cell development: from the cradle to the grave. Immunol Rev 252: 78-88.
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D,et al. (2009) Late developmental plasticity in the T helper 17 lineage. Immunity30: 92-107.
- Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF,et al. (2008) Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol 181: 7205-7213.
- Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, et al.(2010) Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity32: 616-627.
- Nistala K, Adams S, Cambrook H, Ursu S, Olivito B,et al. (2010) Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. ProcNatlAcadSci USA 107: 14751-14756.
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, et al. (2011) Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12: 255-263.
- Chen Y, Chauhan SK, Shao C, Omoto M, Inomata T, et al. (2017) IFN-gamma-Expressing Th17 Cells Are Required for Development of Severe Ocular Surface Autoimmunity. J Immunol 199: 1163-1169.
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, et al.(2007) TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 8: 1390-1397.
- Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L,et al. (2011) Human TH17 cells are long-lived effector memory cells. SciTransl Med 3: 104ra100.
- Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057-1061.
- Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T,et al. (2001) Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 182: 18-32.
- Huber S, Gagliani N, Esplugues E, O'Connor W Jr, Huber FJ, et al. (2011) Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34: 554-565.
- Murphy KM, Stockinger B(2010) Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol 11: 674-680.
- Du R, Zhao H, Yan F, Li H (2014) IL-17+Foxp3+ T cells: an intermediate differentiation stage between Th17 cells and regulatory T cells. J LeukocBiol 96: 39-48.
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ,et al. (2010) Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33: 279-88.
- Hashimoto T, Akiyama K, Kobayashi N, Mori A(2005) Comparison of IL-17 production by helper T cells among atopic and nonatopic asthmatics and control subjects. Int Arch Allergy Immunol137: 51-54.
- Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN(2008) IL-17 receptor signaling influences virus-induced corneal inflammation. J LeukocBiol83: 401-408.
- Tan X, Sun S, Liu Y, Zhu T, Wang K, et al. (2014) Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye (Lond) 28: 608-613.
- Gagliani N, AmezcuaVesely MC, Iseppon A, Brockmann L, Xu H,et al. (2015) Th17 cells trans differentiate into regulatory T cells during resolution of inflammation. Nature 523: 221-225.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences