ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Idiopathic Pulmonary Fibrosis and Progressive Pulmonary Fibrosis: A Call for Collaborative Drug Development
Peter Kiely1*, Botond Nagy2 and Anthony Bohnert3
1Parexel International, Dublin, Ireland
2Parexel International, BucureÈ?ti, Romania
3Parexel International, Massachusetts, USA
- *Corresponding Author:
- Peter Kiely
Parexel International, Dublin,
Ireland,
E-mail: peter.kiely@parexel.com
Received date: June 29, 2024, Manuscript No. IPAPP-24-19229; Editor assigned date: July 01, 2024, PreQC No. IPAPP-24-19229 (PQ); Reviewed date: July 15, 2024, QC No. IPAPP-24-19229; Revised date: July 22, 2024, Manuscript No. IPAPP-24-19229 (R); Published date: July 29, 2024, DOI: 10.36648/2393-8862.11.3.186
Citation: Kiely P, Nagy B, Bohnert A ( 2024) Idiopathic Pulmonary Fibrosis and Progressive Pulmonary Fibrosis: A Call for Collaborative Drug Development. Am J Pharmacol Pharmacother Vol.11 No.3: 186.
Abstract
Fibrosing Interstitial Lung Diseases (ILDs) are a heterogeneous group of conditions primarily affecting people aged ≥ 50 years. Idiopathic Pulmonary Fibrosis (IPF), the most common fibrosing ILD is a chronic progressive and irreversible condition with a high risk of mortality, especially in individuals with comorbidities. The term Progressive Pulmonary Fibrosis (PPF) covers ILDs other than IPF that also show progressive fibrosing phenotype. IPF and PPF are devastating parenchymal lung diseases with poor prognosis, resulting in progressive respiratory failure and death. IPF and PPF share common pathological, radiological and histologic features and have similar clinical course of disease progression. However despite their similarities, drug development appears to be progressing separately. Chronic administration of anti-fibrotic drugs, i.e., nintedanib and pirfenidone, has shown a significant reduction in the rate of forced vital capacity decline and mortality and are currently approved for IPF treatment. Recent studies have also demonstrated the clinical efficacy of these drugs in PPF patients. We conducted a non-systematic literature search for peer reviewed articles and clinical trials to review available data regarding the similarities in pathogenesis and progression pathways between IPF and PPF and discuss current challenges in the management of these conditions. Based on the available scientific evidence, we propose it is timely to treat IPF and PPF as similar conditions, at least in terms of patient management and unified approaches are reasonable to be implemented in future clinical trials for both IPF and PPF.
Keywords
Idiopathic pulmonary fibrosis; Progressive pulmonary fibrosis; Usual interstitial pneumonia; Nintedanib; Pirfenidone
Introduction
Interstitial Lung Disease (ILD) is an umbrella term comprising more than 200 types of diffuse parenchymal lung disorders with varied clinical, radiographic and pathological manifestations. A subset of ILDs show progressive fibrotic phenotype. Progressive fibrotic ILDs are characterized by worsening respiratory symptoms, radiological progression and decline in lung function, poor response to therapies, poor quality of life and early mortality [1]. Idiopathic Pulmonary Fibrosis (IPF) is the most common progressive fibrosing ILD of unknown cause that shows a Usual Interstitial Pneumonia (UIP) pattern on radiology and histopathology examinations. IPF is characterized by progressive worsening of lung function and has a high risk of mortality due to respiratory failure, especially in patients with comorbidities such as infection, lung cancer, ischemic heart disease [2]. Risk factors associated with the development and worsening of IPF were occupational and environmental exposures to metal and wood dusts, pesticide, agricultural work, smoking, as well as the presence of viral or bacterial infections [3,4]. Diagnosing IPF requires excluding alternative causes of fibrosing ILD, broadly categorized into systemic and exposure-related disorders [5,6]. Classified as a rare disease, IPF has an estimated adjusted incidence and prevalence ranging from 0.09 to 1.30 and from 0.33 to 4.51 per 10,000 persons, respectively [7].
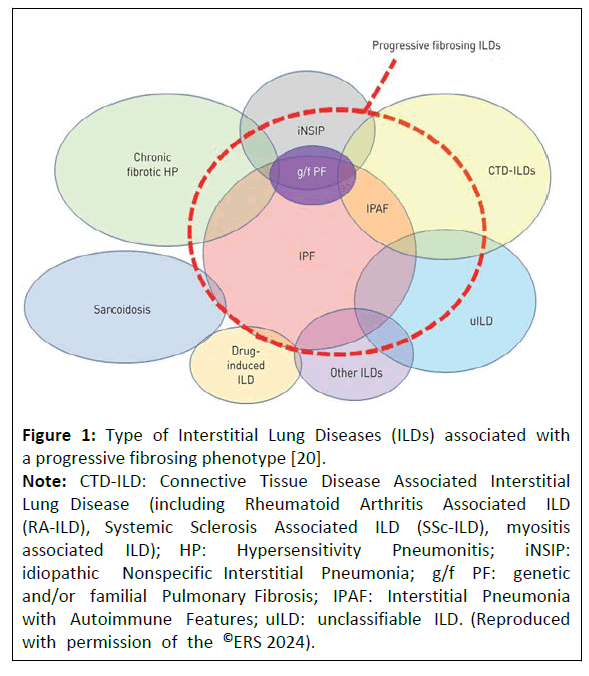
There are a number of ILD conditions which can develop progressive fibrosis (Figure 1). The term Progressive Pulmonary Fibrosis (PPF) covers subtypes of ILDs other than IPF that also show progressive fibrotic features. A significant proportion (18%-32%) of patients with ILDs other than IPF developed PPF despite initial treatment, mirroring the observed patterns in IPF [8-10]. Age, smoking, dyspnoea at disease onset, low lung functions and fibrotic hypersensitivity pneumonitis were reported among the potential risk factors [11,12]. The current international clinical practice guideline published by the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society and Latin American Thoracic Society (ATS/ERS/JRS/ALAT) in 2022 defined PPF separately from IPF; PPF was defined as at least two of the following 3 criteria occurring during the first year after diagnosis: (i) worsened respiratory symptoms (cough and/or dyspnoea); (ii) either a decline of ≥ 5% absolute Forced Vital Capacity (FVC) or a decline of ≥ 10% absolute diffusing capacity for carbon monoxide; (iii) increase in fibrosis on High Resolution Computed Tomography (HRCT) [6]. The exact prevalence of PPF is unknown. The recent real-world cohort study in patients with ILDs has reported progressive phenotype in approximately 25% of non-IPF fibrosing ILDs [13]. In 2021, the estimated global prevalence of PPF ranged from 6.9 to 70.3 per 100,000 individuals, with an estimated incidence of 2.1 to 32.6 per 100,000 person-years [14]. According to a health economic study conducted in 2022, approximately 87,000 individuals were estimated to be affected by PPF other than IPF in the European Economic Area, annual exacerbations were between 22,401 and 31,181 and deaths between 5791 and 6171. The annual aggregate income loss was estimated at €1433 million which suggest a significant economic impact of non-IPF and PFF [15].
Figure 1: Type of Interstitial Lung Diseases (ILDs) associated with a progressive fibrosing phenotype [20].
Note: CTD-ILD: Connective Tissue Disease Associated Interstitial Lung Disease (including Rheumatoid Arthritis Associated ILD (RA-ILD), Systemic Sclerosis Associated ILD (SSc-ILD), myositis associated ILD); HP: Hypersensitivity Pneumonitis; iNSIP: idiopathic Nonspecific Interstitial Pneumonia; g/f PF: genetic and/or familial Pulmonary Fibrosis; IPAF: Interstitial Pneumonia with Autoimmune Features; uILD: unclassifiable ILD. (Reproduced with permission of the ©ERS 2024).
IPF and PPF share common pathological mechanisms, prognosis and radiological and histologic features, however, drug development seems mainly focused on IPF alone. While antifibrotic drugs are currently recommended for the treatment of patients with IPF, recent studies have shown potential clinical effect in slowing disease progression in patients with PPF [16-19]. Considering these similarities and the unmet need for new therapies for both patient populations, we advocate for a common drug development approach.
Literature Review
We conducted a non-systematic PubMed search for peerreviewed articles published over the past 5 years using the following search terms (individually and in combination): Idiopathic pulmonary fibrosis, progressive pulmonary fibrosis, progressive interstitial lung disease, pulmonary fibrosis, pathogenesis, diagnosis, treatment and co-development in IPF and PPF. Following an initial screening of titles and abstracts, relevant papers were retrieved and subjected to full-text screening. Although the literature search was mainly focused on review articles and meta-analyses, further scrutiny of publications was performed during the full-text screening and original papers deemed relevant for the topic were also included. To determine the number of clinical trials assessing interventions for IPF, PPF and both PPF and IPF, an additional search was conducted on the U.S. National Library of Medicine’s ClinicalTrials.gov. This search included all interventional clinical trials completed, withdrawn, or ongoing up to 17 November 2023. The results identified 481 trials for IPF alone, 45 for PPF alone and 11 for both IPF and PPF. Among these 11 trials, five investigated medicinal products, two of which were already licensed for use in patients with IPF and/or PPF.
Similarities between IPF and PPF
The pathogenesis of PPF is yet to be fully elucidated however it is suggested to be similar to IPF in which normal epithelial repair becomes disrupted with proliferation of keratin-17 positive cells in areas of fibrosis. Injured epithelial cells induce activation of transforming growth factor β which results in differentiation of fibroblasts into myofibroblasts and leads to excessive matrix proteins and remodeling [21]. Macrophages are recruited and activated, releasing cytokines which drive the fibrotic process, including Platlet Derived Growth Factor (PDGF). The development of a fibrotic response in different ILDs likely shares a similar core fibrotic pathway. The transcriptional signatures of IPF and other ILDs exhibit similar upregulation of numerous profibrotic genes and pathways, including collagen metabolism and collagen fibril organisation. Similar genetic susceptibility and common genomic risk factors (gene polymorphism, telomere shortening) have been identified between IPF and Rheumatoid Arthritis-Associated ILD (RA-ILD) [22], Hypersensitivity Pneumonitis (HP) [23] and autoimmune ILD [24]. Lung epithelial cell glycoproteins KL-6 and MMP7 could be considered as serum biomarkers indicative of ILD progression in both IPF and different fibrosing ILDs [25,26]. Cellular senescence, mitochondrial dysfunction and immune dysregulation have also been considered among common pathological mechanisms to induce disease progression [27,28].
IPF and PPF share common radiological and histologic features of Usual Interstitial Pneumonia (UIP) [29]. HRCT reveals fibrotic changes, including irregular reticulation, ground glass opacities, traction bronchiectasis, honeycombing and volume loss, meeting radiological criteria for UIP [6,30]. The histological features of UIP are well known in IPF are also well described in PPF. It is characterized by a patchwork appearance of alternating scarred and normal lung tissue, architectural distortion and fibroblastic foci. When patterns of UIP are seen on biopsy or HRCT in non-IPF processes, such as RA-ILD, chronic HP, and interstitial pneumonia with autoimmune features populations, the disease survival is similar to that in IPF [24,31].
Current therapeutical approaches
Despite extensive research, curative drug therapies remain unavailable. Progressive fibrosing ILDs have been treated with corticosteroid and immunosuppressive therapies. However, the lack of clear effectiveness and associated long-term adverse effects have challenged the adequacy of these therapies, indicating the need for an efficacious treatment.
Nintedanib and pirfenidone are two authorised anti-fibrotic drugs for IPF treatment. Nintedanib is a tyrosine kinase inhibitor that targets the adenosine triphosphate-binding pocket of enzymes, thereby blocking intracellular signaling and action of multiple growth factors receptors such as Platelet-Derived Growth Factor Receptor (PDGFR), the Vascular Endothelial Growth Factor Receptor (VEGFR) and the Fibroblast Growth Factor Receptor (FGFR) [32]. Pirfenidone, a pleiotropic drug with anti-inflammatory and anti-fibrotic effects, mitigates fibroblast proliferation [33]. Both drugs have demonstrated a significant reduction in the rate of FVC decline by approximately 50% over 1 year, accompanied by a reduction in mortality [32,33].
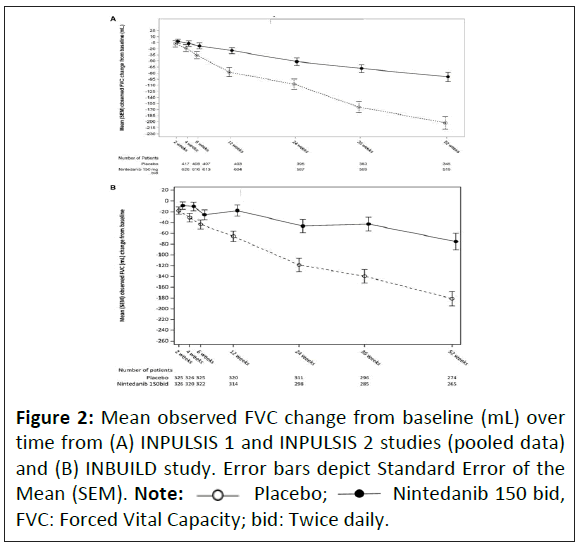
Given the similarities in fibrotic pathways between IPF and PPF, anti-fibrotic therapy has been considered a promising therapy to slow disease progression in non-IPF disorders. Considerable efforts have been made in this regard and results from recent studies have showed that both nintedanib and pirfenidone reduce the FVC decline and slow disease progression. In the INBUILD study, a phase 3, randomized, placebo-controlled study, the annual rate of decline in FVC was -80.8 ml per year in the nintedanib group, significantly lower than in the placebo group (-187.8 mL per year) [34]. The effect of nintedanib compared with placebo on attenuating the FVC decline was consistent across the ILD subgroups [35]. A study comparing placebo recipients from the pivotal phase 3 trials in patients with IPF, INPULSIS 1 and 2, and INBUILD (patients with PPF other than IPF) trial has showed that the annual rate of decline in FVC (Figure 2) and the proportion of patients with 10% or >5% decline in FVC at 12 months were similar [36].
The phase 2b RELIEF trial, although prematurely terminated due to futility caused by slow recruitment, has shown that pirfenidone can reduce disease progression (i.e., FVC decline) in patients with PPF [37]. In the SENSCIS trial, nintedanib treatment of patients with systemic sclerosis associated ILD over 52 weeks resulted in an annual decline in FVC of 41.0 mL per year (p=0.04) as compared with placebo [38]. In a pre-specified subgroup analysis of the SENSCIS trial, the annual rate of decline in FVC in patients who received nintedanib in combination with mycophenolate was lower (26.3 mL per year) than in those who only received nintedanib (55.4 mL per year). Safety and tolerability profiles of nintedanib and pirfenidone in these trials were acceptable. In patients with connective tissue diseaseassociated ILD and RA-ILD, meta-analysis of available results has shown a significant reduction of 61.35 mL per year in annual FVC decline with nintedanib and 80.00 mL per year with pirfenidone [19]. Another recent meta-analysis of pooled data from randomized controlled trials with nintedanib and pirfenidone have shown similar efficacy between anti-fibrotic drugs and a significant reduction in mortality in both IPF and non-IPF populations [16].
Real-world data from a multicenter, prospective, observational registry supported the beneficial effect of anti-fibrotic therapy on survival and transplant-free survival, irrespective of baseline disease severity, compared with no anti-fibrotic therapy [39]. IPF and PPF were independent predictors of transplant-free survival and no difference in transplant-free survival were seen between IPF and PPF [40,41]. On the other hand, anti-fibrotic drugs used as monotherapy or in combination with immunosuppressive therapy have shown to be beneficial in IPF and PPF patients undergoing lung transplantation [42]. A recent European expert group consensus statement on pulmonary fibrosis has highlighted the importance of using anti-fibrotic therapy early in PPF, particularly when initial management fails to prevent progression, a viewpoint increasingly supported by scientific evidence [43]. Additionally, ATS practice guideline has also recommended nintedanib for PPF in patients who have failed standard management for fibrotic ILD [9,44].
Systematic literature reviews and meta-analyses have been conducted to assess the impact of nintedanib [18] and pirfenidone [17] on disease progression, lung function, respiratory symptoms, adverse effects and mortality in PPF populations. Although the quality of evidence was considered low, mainly due to the low number of studies and small sample sizes, a mean reduction in FVC decline of 107 mL and 100 mL were observed with nintedanib and pirfenidone, respectively, as compared with placebo. There was no statistically significant difference in other lung function parameters, respiratory symptoms, or mortality. Based on these and other published evidence, the ATS/ERS/JRS/ALAT committee has made a conditional recommendation for the use of nintedanib in PPF considering the significant reduction in disease progression and the reversibility of common adverse events upon treatment discontinuation. No recommendation has been made for pirfenidone [6].
Discussion
Future perspectives in drug development
Despite extensive research efforts, no curative drug therapies are available for patients with progressive ILDs. In the IPF setting, initiation of anti-fibrotic therapy is well established and recommended even before significant progression occurs and considering the available literature data, this could be a reasonable approach also in the PPF setting. Efficacy and safety of anti-fibrotic therapy have been demonstrated to be comparable between IPF or non-IPF settings with progressive fibrosis, supporting the hypothesis of a common pathogenesis.
PPF exhibits heterogeneity in its aetiology, and currently, no established biomarkers for disease progression exist. Consequently, predicting which patients will experience progression of lung fibrosis poses a significant challenge. In the absence of biomarkers, long-term clinical trials are required to demonstrate the prevention of disease progression, which may present feasibility challenges. Previous trials enrolled patients with documented progression; however, administering treatment earlier could be more beneficial for patients by further delaying lung function decline. Moreover, as licenced therapies become available, investigational products may need to be added to the licenced treatments, potentially resulting in a smaller effect size.
While developers could undertake separate studies in IPF or non-IPF populations, such development strategy could have feasibility issues. The low incidence of IPF and PPF and the competition between sponsors for patients create challenges in recruiting and retaining participants. Moreover, the assessment of disease progression in individual fibrotic ILDs is complicated by the selection of underpowered study groups, needs multicenter collaboration and clear definitions of endpoints. Patient fatigue and breathlessness can pose challenges to travel, visit length and completion of on-site assessments. Additionally, a lack of information and support can amplify the anxiety and negativity experienced by participants [45].
Comparing investigational drugs with a true placebo would be considered unethical. Alternatively, a new drug candidate could still be tested against a placebo in clinical trials limited to a significant minority of patients who discontinue licenced therapy due to tolerability issues [46].
Considering the urgent and unmet need for new and innovative treatments, it would be beneficial and timely to treat both IPF and PPF (UIP pattern) as similar diseases, at least in the context of clinical trials and drug development. Beside the ATS/ ERS/JRS/ALAT clinical practice guideline, other national agencies have also issued recommendations and position statements related to the diagnosis and treatment of IPF and PPF, admitting the importance of multidisciplinary collaborative management of patients [47,48].
Drug development strategies combining progressive fibrotic phenotypes, something similar to basket trial design in oncology, would enhance clinical trial feasibility and address the current high therapeutic need in these conditions. Although a dosefinding study could be conducted in either population, a confirmatory phase 3 study should enrol both populations. The analysis could report an overall treatment effect in both subgroups. Additionally, the use of adaptive study design could further enhance drug development. Future drug development should extend beyond slowing the decline in lung function to include arresting the process of fibrosis, early identification of patients at risk of PPF, and the development of predictive biomarkers [49]. New therapies targeting various pathways of disease progression and promoting lung repair or regeneration will provide additional advancements for patients.
Conclusion
There is an increasing understanding of the similarities between IPF and PPF (especially with UIP pattern) in terms of disease progression, prognosis, histological changes and radiographic and clinical features. International consensus guidelines recommend commencing anti-fibrotic therapies early following diagnosis in IPF and PPF patients once initial management has failed. Based on the current understanding of both conditions, this article suggested a unified development pathway should be considered from both clinical and regulatory perspective. While we admit the potential limitations of the present work, e.g., non-systematic nature of the literature search and the paucity of authorized therapies, we consider that such collaborative development would aid the authorization of new therapies in a timely manner for the benefit of patients and society in general. Ultimately, a common development would help address a high unmet therapy need in both populations to improve life expectancy and quality of life of the patients.
Competing Interest
The authors declare no competing interest. Peter Kiely is a former alternate member of the Committee for Medicinal Products for Human use (CHMP), European Medicines Agency. Anthony Bohnert is the global respiratory lead and pulmonologist. Botond Nagy is a regulatory affairs consultant.
Funding
No funding was received for the conduct of this research or the preparation of the manuscript.
References
- Kang HK, Song JW (2023) Progressive pulmonary fibrosis: Where are we now? Tuberc Respir Dis 87: 123-133.
[Crossref], [Google Scholar], [Indexed]
- Raghu G, Amatto VC, Behr J, Susanne S (2015) Comorbidities in idiopathic pulmonary fibrosis patients: A systematic literature review. Eur Respir J 46: 1113-1130.
[Crossref], [Google Scholar], [Indexed]
- Mostafaei S, Sayad B, Azar MEF (2021) The role of viral and bacterial infections in the pathogenesis of IPF: A systematic review and meta-analysis. Respir Res 22: 53.
[Crossref], [Google Scholar], [Indexed]
- Park Y, Ahn C, Kim TH (2021) Occupational and environmental risk factors of idiopathic pulmonary fibrosis: A systematic review and meta-analyses. Sci Rep 11: 4318.
[Crossref], [Google Scholar], [Indexed]
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson C, et al. (2018) Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 198: e44-e68.
[Crossref], [Google Scholar], [Indexed]
- Raghu G, Remy-Jardin M, Richeldi L, Thomson C, Inoue Y, et al. (2022) Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An Official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 205: e18-e47.
[Crossref], [Google Scholar], [Indexed]
- Maher TM, Bendstrup E, Dron L, Langley J, Smith G, et al. (2021) Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res 22: 197.
[Crossref], [Google Scholar], [Indexed]
- Guler SA, Winstone TA, Murphy D, Hague C , Soon J, et al. (2018) Does systemic sclerosis-associated interstitial lung disease burn out? Specific phenotypes of disease progression. Ann Am Thorac Soc 15: 1427-1433.
[Crossref], [Google Scholar], [Indexed]
- Reiseter S, Gunnarsson R, Mogens AT, Lund M, Mynarek G, et al. (2018) Progression and mortality of interstitial lung disease in mixed connective tissue disease: A long-term observational nationwide cohort study. Rheumatology (Oxford) 57: 255-262.
[Crossref], [Google Scholar], [Indexed]
- Zamora-Legoff JA, Krause ML, Crowson CS, Ryu J, Matteson E, et al. (2017) Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 69: 542-549.
[Crossref], [Google Scholar], [Indexed]
- Hambly N, Farooqi MM, Dvorkin-Gheva A, Garlick K, Scallan C, et al. (2022) Prevalence and characteristics of progressive fibrosing interstitial lung disease in a prospective registry. Eur Respir J 60: 2102571.
[Crossref], [Google Scholar], [Indexed]
- Nashatyreva MS, Trofimenko IN, Chernyak BA, Avdeev S (2023) Pulmonary fibrosis and progressive pulmonary fibrosis in a prospective registry of interstitial lung diseases in Eastern Siberia. Life (Basel) 13: 212.
[Crossref], [Google Scholar], [Indexed]
- Nasser M, Larrieu S, Si-Mohamed S, Ahmad K, Boussel L, et al. (2021) Progressive fibrosing interstitial lung disease: A clinical cohort (the PROGRESS study). Eur Respir J 57: 2002718.
[Crossref], [Google Scholar], [Indexed]
- Cottin V, Teague R, Nicholson L, Langham S, Baldwin M, et al. (2022) The burden of progressive-fibrosing interstitial lung diseases. Front Med (Lausanne) 9: 799912.
[Crossref], [Google Scholar], [Indexed]
- Løkke A, Castello L, Pinheiro MP, Soulard S, Hilberg O, et al. (2023) Burden of disease and productivity loss in the European Economic Area in patients affected by fibrosing interstitial lung disease. Adv Ther 40: 5502-5518.
[Crossref], [Google Scholar], [Indexed]
- Finnerty JP, Ponnuswamy A, Dutta P, Abdelaziz A, Kamil H, et al. (2021) Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both Idiopathic Pulmonary Fibrosis (IPF) and non-IPF: A systematic review and meta-analysis. BMC Pulm Med 21: 411.
[Crossref], [Google Scholar], [Indexed]
- Ghazipura M, Mammen MJ, Bissell BD, Macrea M, Herman D, et al. (2022) Pirfenidone in progressive pulmonary fibrosis: A systematic review and meta-analysis. Ann Am Thorac Soc 19: 1030-1039.
[Crossref], [Google Scholar], [Indexed]
- Ghazipura M, Mammen MJ, Herman DD, Hon S, Bissell B, et al. (2022) Nintedanib in progressive pulmonary fibrosis: A systematic review and meta-analysis. Ann Am Thorac Soc 19: 1040-1049.
[Crossref], [Google Scholar], [Indexed]
- Yang M, Wu Y, Liu X, Zhao C, Li T, et al. (2023) Efficacy and safety of antifibrotic agents in the treatment of CTD-ILD and RA-ILD: A systematic review and meta-analysis. Respir Med 216: 107329.
[Crossref], [Google Scholar], [Indexed]
- Cottin V (2019) Treatment of progressive fibrosing interstitial lung diseases: A milestone in the management of interstitial lung diseases. Eur Respir Rev 28: 190109.
[Crossref], [Google Scholar], [Indexed]
- Liu GY, Budinger GRS, Dematte JE (2022) Advances in the management of idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. BMJ 377: e066354.
[Crossref], [Google Scholar], [Indexed]
- Juge PA, Borie R, Kannengiesser C, Gazal S, Revy P, et al. (2017) Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J 49: 1602314.
[Crossref], [Google Scholar], [Indexed]
- Ley B, Newton CA, Arnould I, Henry T, Vittinghoff E, et al. (2017) The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: An observational cohort-control study. Lancet Respir Med 5: 639-647.
[Crossref], [Google Scholar], [Indexed]
- Oldham JM, Adegunsoye A, Valenzi E, Lee C, Witt L, et al. (2016) Characterisation of patients with interstitial pneumonia with autoimmune features. Eur Respir J 47: 1767-1775.
[Crossref], [Google Scholar], [Indexed]
- Khanna D, Tashkin DP, Denton CP, Renzoni EA, Desai SR, et al. (2020) Etiology, risk factors, and biomarkers in systemic sclerosis with interstitial lung disease. Am J Respir Crit Care Med 201: 650-660.
[Crossref], [Google Scholar], [Indexed]
- Ko UW, Cho EJ, Oh HB, Koo HJ, Do KH, et al. (2020) Serum Krebs von den lungen-6 level predicts disease progression in interstitial lung disease. PLoS One 15: e0244114.
[Crossref], [Google Scholar], [Indexed]
- Birch J, Barnes PJ, Passos JF (2018) Mitochondria, telomeres and cell senescence: Implications for lung ageing and disease. Pharmacol Ther 183: 34-49.
[Crossref], [Google Scholar], [Indexed]
- Lee JS, La J, Aziz S, Dobrinskikh E, Brownel R, et al. (2021) Molecular markers of telomere dysfunction and senescence are common findings in the usual interstitial pneumonia pattern of lung fibrosis. Histopathology 79: 67-76.
[Crossref], [Google Scholar], [Indexed]
- Lee KS, Han J, Wada N, Hata A, Lee H, et al. (2024) Imaging of pulmonary fibrosis: An update, from the AJR special series on imaging of fibrosis. AJR Am J Roentgenol 222: 1-17.
[Crossref], [Google Scholar], [Indexed]
- Tzilas V, Tzouvelekis A, Ryu JH, Bouros D (2022) 2022 update on clinical practice guidelines for idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. Lancet Respir Med 10: 729-731.
[Crossref], [Google Scholar], [Indexed]
- Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, et al. (2010) Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 35: 1322-1328.
[Crossref], [Google Scholar], [Indexed]
- (2023) European Public Assessment Report (EPAR) Ofev. European Medicines Agency.
- (2023) European Public Assessment Report (EPAR) Esbriet. European Medicines Agency.
- Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SL, et al. (2019) Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 381: 1718-1727.
[Crossref], [Google Scholar], [Indexed]
- Wells AU, Flaherty KR, Brown KK, Inoue Y, Devaraj A, et al. (2020) Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: A randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 8: 453-460.
[Crossref], [Google Scholar], [Indexed]
- Brown KK, Martinez FJ, Walsh SLF, Thannickal VJ, Prasse A, et al. (2020) The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J 55: 2000085.
[Crossref], [Google Scholar], [Indexed]
- Behr J, Prasse A, Kreuter M, Johow J, Rabe KF, et al. (2021) Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med 9: 476-486.
[Crossref], [Google Scholar], [Indexed]
- Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, et al. (2019) Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 380: 2518-2528.
[Crossref], [Google Scholar], [Indexed]
- Jo HE, Glaspole I, Grainge C, Goh N, Hopkins PM, et al. (2017) Baseline characteristics of idiopathic pulmonary fibrosis: Analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Respir J 49: 1601592.
[Crossref], [Google Scholar], [Indexed]
- Khor YH, Farooqi M, Hambly N, Kolb M, Ryersone CJ, et al. (2023) Patient characteristics and survival for progressive pulmonary fibrosis using different definitions. Am J Respir Crit Care Med 207: 102-105.
[Crossref], [Google Scholar], [Indexed]
- Takei R, Brown KK, Yamano Y, Kataoka K, Yokoyama T, et al. (2022) Prevalence and prognosis of chronic fibrosing interstitial lung diseases with a progressive phenotype. Respirology 27: 333-340.
[Crossref], [Google Scholar], [Indexed]
- Munker D, Arnold P, Leuschner G, Irlbeck M, Michel S, et al. (2023) Impact of ILD-specific therapies on perioperative course in patients with progressive interstitial lung disease undergoing lung transplantation. J Clin Med 12: 4996.
[Crossref], [Google Scholar], [Indexed]
- Rajan SK, Cottin V, Dhar R, Danoff S, Flahertye KR, et al. (2023) Progressive pulmonary fibrosis: An expert group consensus statement. Eur Respir J 61: 2103187.
[Crossref], [Google Scholar], [Indexed]
- Herman DD, Ghazipura M, Raghu G, Richeldi L, Jardin MR, et al. (2023) Summary for clinicians: Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults. Ann Am Thorac Soc 20: 632-637.
[Crossref], [Google Scholar], [Indexed]
- Jones S, Flewett M, Flewett R, Lee S, Vick B, et al. (2023) Clinical trial simulations in pulmonary fibrosis: Patient-focused insights and adaptations. ERJ Open Res 9: 00602-2022.
[Crossref], [Google Scholar], [Indexed]
- Bonella F, Spagnolo P, Ryerson C (2023) Current and future treatment landscape for idiopathic pulmonary fibrosis. Drugs 83: 1581-1593.
[Crossref], [Google Scholar], [Indexed]
- Mackintosh JA, Keir G, Troy LK, Holland AE, Grainge C, et al. (2024) Treatment of idiopathic pulmonary fibrosis and progressive pulmonary fibrosis: A position statement from the Thoracic Society of Australia and New Zealand 2023 revision. Respirology 29: 105-35.
[Crossref], [Google Scholar], [Indexed]
- Piotrowski WJ, Martusewicz-Boros MM, BiaÅ?as AJ, Barczyk A, Batko B, et al. (2022) Guidelines of the polish respiratory society on the diagnosis and treatment of progressive fibrosing interstitial lung diseases other than idiopathic pulmonary fibrosis. Adv Respir Med 90: 425-450.
[Crossref], [Google Scholar], [Indexed]
- White ES, Thomas M, Stowasser S, Tetzlaff K (2022) Challenges for clinical drug development in pulmonary fibrosis. Front Pharmacol 13: 823085.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
 Placebo;
Placebo;  Nintedanib 150 bid, FVC: Forced Vital Capacity; bid: Twice daily.
Nintedanib 150 bid, FVC: Forced Vital Capacity; bid: Twice daily.