ISSN : 2576-3911
Integrative Journal of Global Health
Hemolytic Uremic Syndrome in a Young Adult
Department of Medicine, Tata Main Hospital Jamshedpur, Jharkhand, India
- *Corresponding Author:
- Bijaya M,
Department of Medicine
Tata Main Hospital Jamshedpur, Jharkhand, India
Tel: +91-7763807140
E-mail: bijayamohantytmh@gmail.com
Received date: March 01, 2018; Accepted date: April 16, 2018; Published date: April 18, 2018
Citation: Sunder A, Bijaya M (2018) Hemolytic Uremic Syndrome in a Young Adult. Integr J Glob Health. Vol.2 No.1:3. doi:10.4172/2576-3911.1000003
Copyright: © 2018 Sunder A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Hemolytic uremic syndrome is a disease characterized by hemolytic anemia, acute kidney failure (uremia) and a low platelet count. It is acquired as a foodborne illness or from contaminated water supply and is a medical emergency carrying a risk of 5-10% mortality. Here we report a case of HUS who presented to us with acute gastroenteritis, developed acute renal failure with thrombocytopenia and gradually recovered with subsequent dialysis.
Keywords
Hemolytic uremic syndrome; Low platelet count; Acute kidney failure
Introduction
Hemolytic uremic syndrome was first described in 1955 by Gasser and colleagues [1]. The overall incidence is estimated to be 2.1 cases per 100,000 persons/year with a peak incidence between 6 months and 4 years of age. Most cases are preceded by an episode of infectious sometimes bloody diarrhea caused by E. coli O157H7. It is a kind of thrombotic micro-angiopathy which also includes atypical HUS and TTP (thrombotic thrombocytopenic purpura). The association between Enterohaemorrhagic E. coli serotype O157H7 and HUS was first reported in early 1980s.
Case Report
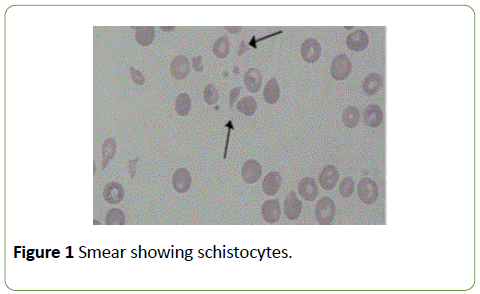
A 30 years old tribal male presented to us with the complaints of bloody diarrhoea, fever and recurrent vomiting. There was no history of pain abdomen, blood in vomitus, joint pains, rashes, altered sensorium, cough or difficulty in breathing. He was doing apparently well three days prior to admission. There was no history of any chronic illness or exposure to any drugs or toxins or any family history of similar complaints. On admission the young man was afebrile with stable parameters and adequate urine output. He was diagnosed to be a case of bacillary dysentery and was started on intravenous antibiotics and fluids. Routine blood tests were grossly abnormal. The haemoglobin was-8.9 gm%, platelets were 80,000 and blood urea was elevated to 68 mg% whilst the initial S. Creatinine was-1.4. Seventy-two hours after admission the patient developed petechial rashes all over the body and there was a decreased urine output. Blood reports revealed thrombocytopenia with the platelet count dropping to 50000 and then to 30,000. The S. Creatinine was showing a rising trend from 1.4-2.6, 2.6-3.5 and finally to 6 with a marked decrease in urine output. Initially the patient was given Platelet concentrates and was started on hemodialysis but there was no improvement in the platelet count. There was a further fall in haemoglobin and platelet levels to 6 gm% and 20,000 respectively. Stool examination for ova and cysts revealed nothing significant. Examination of the peripheral blood smear showed presence of schistocytes (Figure 1) and crenated RBCs which prompted us to think otherwise. Now with all the features and the blood reports mentioned above a presumptive diagnosis of E. coli associated hemolytic uraemic syndrome was made. Hemodialysis was continued, no further platelet concentrate was given because it has been noted to further worsen the condition. Two units of packed cells were transfused along with other supportive care. Unfortunately, our haemodialysis unit has no facilities for plasmapheresis at that time which would have been the ideal treatment in this case. But there was marked improvement after starting steroids. The platelet count improved, normal levels of S. Creatinine were attained after serial haemodialysis and the patient was discharged in a stable condition. His haemoglobin was a 12 gms and the platelet count was more than 200,000/cu mm. He went home afebrile. The bouts of diarrhea and vomiting had abated.
Discussion
Diagnosis of HUS requires a high index of suspicion because an incubation period of approximately one week occurs between the start of diarrhoea and its onset. It is a syndrome complex first described in 1955 and is characterized by hemolytic anemia, acute kidney failure and thrombocytopenia. It is one of the thrombotic micro-angiopathies a category of disorders that includes STEC-HUS, a HUS and thrombotic thrombocytopenic purpura (TTP). The exact mechanism and entire pathogenesis is not well understood but it involves binding of shiga toxin to globotriosyl ceramide receptor on the surface of glomerular endothelial cells. This results in complement, leukocyte and platelet activation resulting in release of cytokines, widespread inflammation and multiple thrombosis in small blood vessels resulting in consumption of platelets, however the coagulation factors are not involved. In closely related TTP the binding of shiga toxin activates a metalloproteinase ADAMTS13 which results in formation of multimers of vWF causing platelet activation and formation of micro thrombi. The formation of micro thrombi in small blood vessels leads to microangiopathic hemolytic anemia and reduced blood flow to vital organs leading to ischemia and organ failure like renal failure and central nervous system manifestations. In the rare and chronic form of a HUS (atypical hemolytic uremic syndrome) there is a genetic defect which results in uncontrolled complement activation. It is most commonly caused by E. coli O157:H7, but in 5-10% of cases it is caused by Shigella dysenteriae, Streptococcus pneumonia, as a sequelae of HIV infection, SLE, postpartum, scleroderma, familial predisposition with factor H deficiency, exposure to drugs and toxins like cyclosporine, mitomycin, bleomycin, cisplatin. The main features noticed are bloody diarrhea, vomiting, abdominal pain, Symptoms of renal failure like oliguria, hematuria, confusion, fatigue and edema. In patients with a HUS there is very rapid progression of signs and symptoms resulting in multiple organ failure, coma and death can occur at any time. The laboratory features seen are decrease in haemoglobin levels, platelet count, haptoglobin, rise in lactate dehydrogenase, S. creatinine, presence of schistocytes. This disease requires aggressive management in the form of plasmapheresis or plasma infusion and subsequent dialysis. Use of monoclonal antibodies is also being recommended. In our case report the patient was managed with steroids and subsequent dialysis till the renal function was restored. Acute renal failure is seen in about 55-70% of cases with an overall mortality rate of 5-15%. Generally, these patients have poor outcome and require aggressive management in acute stage.
References
- Gasser C (1955) Haemolytic Uremic syndrome. Schweiz Med Wochenschr 85: 905-909.
- Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, et al. (1983) Haemorrhagic colitis associated with a rare E. coli serotype. N Engl J Med 308: 681-685.
- Andreoli SP (1999) The pathophysiology of the HUS. Curropin Nephrol Hypertens. 8: 459-464.
- Walker WA (2004) Paediatric Gastrointestinal disease: Pathophysiology, Diagnosis, Management. 4th edn. Hamilton, Ont: BC Decker.
- Green DA, Murphy WG, Uttley WS (2000) Hemolytic Uremic Syndrome: Prognostic factors. Clin Lab Haematol. 22: 11-14.
- Andreoli SP (2002) Acute renal failure. Curr Opin Paediatr 14: 183-188.
- Bae WK, Lee YK, Cho MS, Ma SK, Kim SW, et al. (2006) A case of hemolytic uremic syndrome caused by Escherichia coli O104: H4. Yonsei Med J 47: 437-439.
- Banatvala N, Griffin PM, Greene KD, Barrett TJ, Bibb WF. et al (2001) Hemolytic Uremic Syndrome Study Collaborators. The United States national prospective hemolytic uremic syndrome study: microbiologic, serologic, clinical and epidemiologic findings. J Infect Dis 183: 1063-1070.
- Ramegowda B, Samuel JE, Tesh VL (1999) Interaction of Shiga toxins with human brain microvascular endothelial cells: cytokines as sensitizing agents. J Infect Dis 180: 1205-1213.
- Safdar N, Said A, Gangnon RE (2002) Risk of hemolytic uremic syndrome after antibiotic treatment of Escherichia coli O157: H7 enteritis: a meta-analysis. JAMA 288: 996-1001.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences