Groundwater Quality Assessment and Suitability for Different Uses in the White Nile State, Sudan
Osman AE Abdalla1*, Mannah Altaher Abdulrahman Moahamda2, Mustafa Mohammed Osmana2, Mohammed Babo El Awad Hassaballa3 and Salah Mahgoub3
1Department of Earth Sciences, Sultan Qaboos University, Muscat, Oman
2Department of Atomic Energy, University of Khartoum, Khartoum, Sudan
3Department of Irrigation and Water Resources, Sudan University of Science and Technology, Khartoum, Sudan
- *Corresponding Author:
- Osman AE Abdalla Department of Earth Sciences, Sultan Qaboos University, Muscat, Oman, Tel: 96892124460; E-mail: osman@squ.edu.om
Received date: June 14, 2022, Manuscript No. IPWPC-22-13806; Editor assigned date: June 17, 2022, PreQC No. IPWPC-22-13806 (PQ); Reviewed date: July 01, 2022, QC No. IPWPC-22-13806; Revised date: August 14, 2022, Manuscript No. IPWPC-22-13806 (R); Published date August 22, 2022, DOI: 10.36648/ IPWPC.4.1.30
Citation: Abdalla OAE, Moahamda MAA, Osmana MM, Hassaballab MBEA, Mahgoub S (2022) Ground Water Quality Assessment and Suitability for Different uses in the White Nile State, Sudan. J Water Pollut Control Vol:4 No:1
Abstract
Ground water is the most important natural source of water in the eastern to Bara basin, White Nile state, Sudan. In order to evaluate their suitability for drinking, domestic and irrigation purposes, chemical characteristics of groundwater have been investigated and evaluated. The samples have been analyzed to determine physical parameters like pH, EC, and TDS, the chemical parameters like Na, K, Ca, Mg, HCO3, SO4 and Cl. These parameters are used to determine the quality of groundwater by comparing the quality of drinking water standards published by WHO. From the analyzed data, some parameters like Sodium Absorption Ratio (SAR), Residual Sodium Carbonate (RSC), percent sodium (Na%), Magnesium Hazards (MH) and Permeability Index (PI) have also been determined. A total of 25 ground water samples were collected from different sources shallow and deep wells and analyzed for major cations and anions. The domination of cations and anions was in the order of Na+ >Ca2+>Mg2+>K+ and HCO3 ->Cl->SO4 2->CO3 2-. The calculated parameters show that the majority of the groundwater samples were suitable for drinking and irrigation uses. However, high salinity values at few wells restrict the suitability of the water for irrigation uses.
Keywords
Groundwater; Water quality; Irrigation; Sudan
Introduction
Ground water is still the best and most reliable source of fresh water. For both human needs and agriculture production, the aquifers are the principal reservoir against drought.
Furthermore, groundwater is more resistant to pollution than surface water [1]. In the view of growing population, rising of living standards, anthropogenic pollution and climatic changes, water resources are stressed and the demand for food is growing. In times of drought, groundwater acts as a key strategic reserve. Agriculture in Sudan continues to be the primary source of income and livelihood for 60% to 80% of the people, as well as the engine of growth for other economic sectors such as trade, industry, and transportation. Agricultural development is predicted to result in the establishment of more job opportunities. This improves rural development prospects while reducing internal migration to big cities, resulting in food security stabilization and poverty reduction. Availability of renewable resources, as well as their sustainable management and exploitation, are some of the key future problems that may influence groundwater [2]. In the management and optimization of groundwater resources, groundwater studies, understanding, and assessing resources in dry and semi-arid environments are critical. Water quality concerns have become a significant concern in recent years as a result of population increase, urban expansion, and technological. Recently, rapid urbanization, industrialization, and agricultural expansion have resulted in the release of a variety of contaminants into the environment, including water sources. Groundwater quality, which is influence by recharge and pollution, can improve or deteriorate over time.
One of the most important sources of water in the White Nile State is groundwater. Agriculture is the main source of income in the area [3]. For individuals living in this area, ground water is used for drinking and agricultural reasons by a large majority of the population. Economic development and a variety of human activities are heavily reliant on the availability of sufficient, high quality water. Several hydrogeological and hydro chemical studies were conducted around the study area. However, the ability of groundwater to meet household needs and irrigation water needs further study and evaluation in that area. In the context of the effective management of this vital resource in the region, it has become necessary to assess its suitability for these uses. Since there are no industries in this area, the main objective of this work is primarily to assess the suitability of groundwater resources for use in drinking, irrigation and for domestic life. In general, it is widely known that the uses of ground water resources are closely related to their geochemical characteristics. Ground water quality is determined by a variety of chemical constituents and their concentrations, which are primarily based on the geological data of a specific region [4]. The chemistry of groundwater is influenced by a number of factors, including soil/rock water interaction during recharge and groundwater flow, aquifer storage, and mineral species dissolution [5]. These processes are linked to mineral weathering, which has a significant impact on groundwater chemistry. The main objective of this research is to establish whether groundwater resources are suitable for irrigation and domestic usage. The rural population in the study area relies on groundwater for agriculture and domestic purposes for the majority of the year. As a result, the focus of this study was on the geochemical and qualitative evaluation of groundwater samples [6-9].
Materials and Methods
Study area
The study area is located in west central Sudan, west of the White Nile State. It is situated between longitudes 32.00 and 32.400 E and latitudes 13.00 and 13.400 N in Figure 1 and covers an area of about 32 square kilometers (30,411 Km2). The climate was semi arid throughout the area. Precipitation rates range from 200 mm per year in the north to 450 mm per year in the south [10]. The average temperature is 27 degrees celsius, with lowest temperatures of 10 degrees celsius and high temperatures of 46 degrees celsius. The natural vegetation in the study area as characterized by a mixture of grasses and herbs with scattered bushes (Figure 1). The geology of the study area was summarized from top to bottom a superficial deposits, Umm Ruwaba formation and basement complex rocks. There are two types of aquifer system in the study area shallow and deep aquifers [11]. The water table is relatively shallow (5–10 m), Kosti Basin complexes make the main basin in the study area and are generally trending northwest to southeast, whereas the Kosti Basin is in the western extension of the Blue Nile Rift System (BNRS) [12].
Methodology
During January 2018, 23 ground water samples were collected from wells. The most of these shallow wells have been used for agricultural and domestic reasons. Several water quality parameters, including Electrical Conductivity (EC), pH, and Total Dissolved Solid (TDS), were measured in the field with digital meters. The pH, electrical conductivity, total dissolved solids, as well as major cations Na+, K+, Ca2+, Mg2+ and anions Cl-, SO4 2-, CO3 2-, HCO3 -, and NO3 - in the laboratory were analyzed using Standard Methods for the Examination of Water and Wastewater 25th Edition 2005 (SMEWW) in Khartoum, UNESCO, water quality lab, and University of Omdurman Islamic [13].
The suitability of groundwater for drinking, domestic purpose and agricultural were assessed by comparing the parameter value with WHO and SSMO [14]. Parameters such as Sodium Adsorption Ratio (SAR), Soluble Sodium Percentage (SSP), Magnesium Adsorption Ratio (MAR), Kellys Ratio (KR) were also calculated from the result to determine the suitability of the water for irrigation purpose [15].
Results and Discussion
pH, EC and TDS
The results of the analysis were compared to World Health Organization criteria for significant elements [16]. pH of the water samples ranged from 7.2 to 8.3, with an average pH of 7.5, suggesting that the groundwater samples were slightly alkaline nature. The measured EC of the ground water in the study area varies from 336 to 5103 μS cm-1, with an average 1280 μS cm-1. The TDS in the area were varies between 201 and 2806 mg/l with an average of 743 mg/l [17].
Major ion chemistry
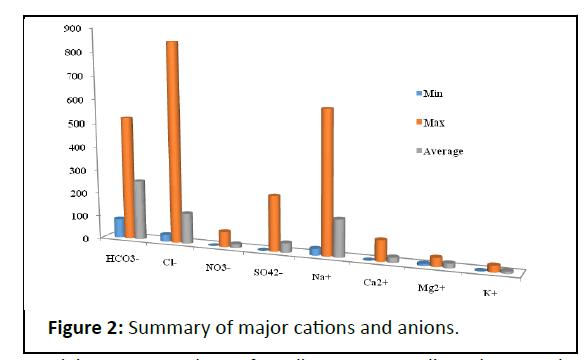
Table 1 provides the analytical data, calculated values, and statistical characteristics such as the lowest and maximum average of groundwater samples. The order of anion and cation abundance was HCO3 ->Cl->SO4 2- and Na+>Ca2+>Mg2+>K+ in mg/ L respectively showed in Figure 2 [18].
| Parameters | Min | Max | Average | WHO (2008) |
|---|---|---|---|---|
| pH | 7 | 8 | 7.5 | 6.5-8.5 |
| TDS | 201 | 2806 | 743 | 1000 |
| EC | 336 | 5103 | 1280 | 1500 |
| TH | 40 | 380 | 138 | 500 |
| HCO3- | 81 | 521 | 254 | 350 |
| Cl- | 30 | 850 | 129 | 250 |
| NO3- | 0.4 | 65 | 17 | 50 |
| SO42- | 0 | 235 | 41 | 250 |
| Na+ | 28 | 600 | 159 | 200 |
| Ca2+ | 0 | 89 | 22 | 100 |
| Mg2+ | 8 | 37 | 19 | 50 |
| K+ | 1 | 27 | 10 | - |
| All major elements in mg/l | ||||
Table 1: Summary statistics of parameters in groundwater compared with WHO (2008).
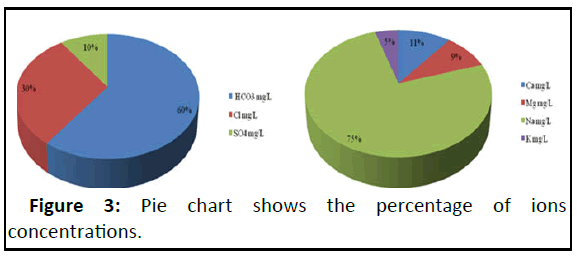
Bicarbonate was the most common anion, accounting for around 60% of the total anions. The high concentration of HCO3 - can be attributed to the contribution of carbonate rocks which may be derived from carbon dioxide in the atmosphere, soils, and carbonate rocks [19]. Chloride is the second most abundant anion, accounting for around 30% of all anions. The high Clconcentration attributed to leaching from upper soil layers, direct infiltration and the processes associated with the evaporation of water during the movement in the direction of the flow path. Sulphate was the least prominent ions, accounting for just 10% [20]. Sodium was the most common major cation, accounting for 75% of total cations on average.
Calcium and magnesium ions were second and third in significance, accounting for 11% and 9% of total cations, respectively. Potassium was the least prominent cation, accounting for only 5% of all cations. Concentration of Ca2+, Mg2+ and K+ are not exceeding the desirable limits. Some sample give high concentration of Na+ caparison with the recommended permissible limit [21].
High concentration of sodium was attributed to cation exchange among minerals and dissolution from rocks [22].
Sodium and potassium contributed more than to the magnesium and calcium ions in the groundwater taken from study area.
Ground water is suitable for drinking in the study area based on the concentrations of major ions and quality parameters like pH, TDS, TH (Figure 3).
The physical and chemical characteristics that impact groundwater quality in a given location are substantially influenced by geological formations and anthropogenic activity.
The World Health Organization's standard recommendation values for drinking were compared to the analytical results of physical and chemical properties of groundwater [23]. More than 90% of the samples in these areas are of excellent quality and appropriate for drinking. Natural processes such as interaction between water and rocks, ion exchange, sedimentation, and evaporation have caused certain samples to show aqueous with NaCl.
Suitability of ground water for irriga ion and domestic uses
The results of the water analysis performed in this study were used to determine the appropriateness of water for agricultural use [24]. SAR, Na percent, Magnesium Hazard (MH), PI, and RSC are some of the metrics that may be used to determine the quality of irrigation water.
The alkali hazard
The absolute and relative concentrations of cations determine the sodium or alkali danger in the use of water for irrigation, which is stated in terms of Sodium Adsorption Ratio (SAR) [25].
The Sodium Adsorption Ratio (SAR) is a measurement of water’s appropriateness for agricultural irrigation based on the quantities of solids dissolved in it. Irrigation with Na+ rich water can diminish soil permeability, limiting air and water movement. This is owing to the established exchange mechanisms between water and soil. The clay particles absorb the Na+ ions, which replace the Mg2+ and Ca2+ ions.
The Sodium Adsorption Ratio SAR Equation is used to determine whether there is too much sodium or too little calcium and magnesium [26].
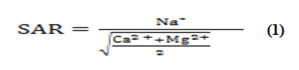
All concentrations were reported in meq/L
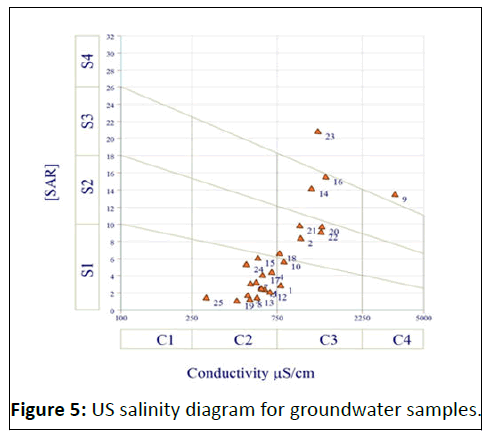
SAR values in ground water samples varied from 1 to 20.8 with an average of 6.2 in two ranges based on sodium adsorption value [27]. The sodium hazard in the analyzed water is classi ied as low (64%), medium (20%), and (6%) in high and very high based on the SAR values projected on the salinity diagram as alkalinity hazard (Figure 4). Table 2 depicted the SAR categorization of groundwater samples from the research
| SAR | Suitability for irrigation |
|---|---|
| 1–10 (71.42%) | Suitable for all types of crops and soil except for those crops sensitive to sodium |
| 10–18 (14.82%) | Suitable for coursed textured or organic soil with permeability |
| 18–26 (3.57%) | Harmful for almost all soil |
| >26 (0.0%) | Unsuitable for irrigation |
Table 2: Suitability of water for irrigation with different value of SAR
Sodium content
Sodium concentration in the irrigation water is also expressed by percent sodium or soluble sodium percentage (Na%), which can be using calculated the formula.
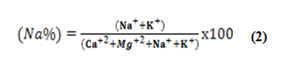
All concentrations were reported in meq/L
In this work, the values obtained for the percent of sodium parameter in the study area ranged from 31% to 94% with average 65%. 52% of groundwater samples are below the threshold of 50 percent, that samples has no sodium hazard and fit for irrigation as far as sodium percent is concerned.
Magnesium hazard
Magnesium Hazard (MH) can be calculated using this formula.

All concentrations were reported in meq/L
In most cases, calcium and magnesium in water are "in a condition of balance." The magnesium concentration of water is one of the most significant qualitative factors for assessing irrigation water quality [28]. As soils grow more salty, more magnesium in water will have a negative impact on agricultural production. The presence of high levels of magnesium in water has a negative impact on soil quality. It causes the soil to become alkaline in nature, lowering agricultural output. Ground water appropriate for agriculture may be found using MH. Water is deemed good for agriculture if the MH is less than 50 and unsuitable if the MH is greater than 50. From the calculated values in the study area, the magnesium hazard ranged between 22.9% and 100%.Some samples had a MH above 50 and were thus not suitable for irrigation [29].
Permeability index
The Permeability Index (PI) is another metric for determining whether or not water is suitable for irrigation. The Permeability Index was used by Doneen to classify irrigation fluids (PI). The formula for calculating PI might well be found in equation 4 [30].
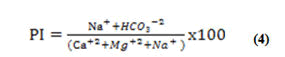
All concentrations were reported in meq/L
Agricultural techniques such as irrigation have a long-term impact on the soil and its permeability [31]. The permeability processes in the soil are influenced by sodium, calcium, magnesium, and bicarbonate ions. Consistent irrigation water usage reduces soil permeability by increasing the presence of salt, calcium, magnesium, and bicarbonate in the soil. Doneen classi ied irrigation water in three PI classes [32]. Class-I and class-II water types are suitable for irrigation with 75% or more of maximum permeability, while class-III type of water, with 25% of maximum permeability, are unsuitable for irrigation. In the present study, the P.I of the groundwater samples ranged from 33 % to 74 % with a mean value of 56%. The ground water samples in the study area were suitable for irrigation category of Doneen chart [33].
Residual sodium carbonate
The amount of HCO3 - and CO3 - in excess of alkaline earths (Ca2++Mg2+) represented as RSC also affects the irrigation appropriateness of water. The influence of carbonate and bicarbonate, as well as the suitability of water for irrigation, may be determined by using the following method to calculate Residual Sodium Carbonate (RSC) values.
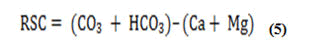
All concentrations were reported in meq/L
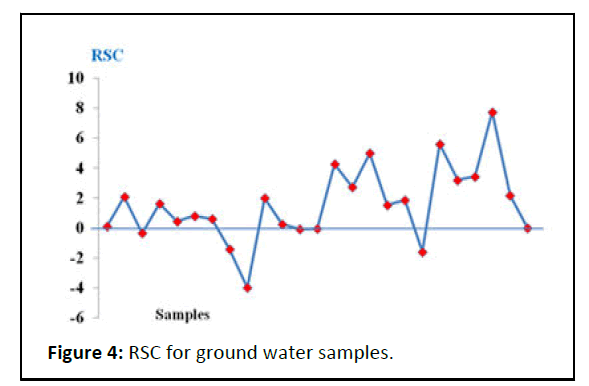
The water suitability for irrigation according to the RSC is based on three classes: water is not suitable for irrigation if the water RSC exceeds 2.5 meq/L, slightly adapted to irrigation if RSC ranges between 1.25 and 2.5 and acceptable for irrigation if the RSC values are less than or equal to 1.25. The classi ication of groundwater for irrigation according to the RSC values is presented in Table 3 and Figure 5, 48% of samples RSC is <1.25 indicating that those samples are suitable for agriculture and 24% within 1.25-2.5. 28% whose RSC are of >2.5 indicating alkaline hazard and non-suitability of water for agriculture (Table 3) [34].
| RSC | Quality | Percentage |
|---|---|---|
| <1.25 | Low (Good) | 48% |
| 1.25-2.5 | Medium | 24% |
| >2.5 | High (Unsuitable) | 28% |
Table 3: Quality of groundwater based on RSC.
Water classification
The plot of data in Figure 5 by US salinity diagram proposed by US Salinity Laboratory Staff, Richard, in which the EC is taken as salinity hazard and SAR as alkalinity hazard, shows that majority of the water samples fall in the category C2-S1 indicating medium salinity and low alkali water which were suitable for irrigation [35]. The majority of the data occurred in these category, this water can be used for irrigation without any problem.
The water samples were divided into five groups based on the data plotted on the salinity map. Most groundwater samples fall under C2-S1 which was suitable for irrigation. This water can be used for irrigation without any problem. Comparatively few samples were grouped under C3-S2 should only be used on those soils which have good drainage and only plants having a good salt tolerance should be grown. Some samples considered to show high salinity and high sodium hazard within the water category class C3-S3. One sample in categories C3-S4 and C4-S4 distinguished by very high salinity and high to very high levels of sodium is not suitable for irrigating in any type of soil [36].
Conclusion
Field and laboratory researches of groundwater in the White Nile State, Sudan to analysis twenty five groundwater samples from deep and shallow wells to determine the quality for both drinking and irrigation purposes. The pH and TDS of ground water showed that the water were slightly alkaline nature. From the above study, it is inferred that the abundance of anions and cations was in the order of HCO3 ->Cl->SO4 2->CO3 2-and Na+> Ca2 +>Mg2+>K+. The ions such as Na, Cl and HCO3 in very few samples were higher than the permissible limit due to mineral dissolution. The groundwater is largely safe and suitable for drinking and domestic purposes except in few locations. The quality of groundwater for irrigation is analyzed by calculating parameters such as SAR, Na%, PI, MH and RCS. SAR in water samples ranged from 1 to 20.8 an average value of 6.2 and can be classified as excellent and good for irrigation. The values obtained for the percent of sodium parameter in the study area ranged from 31% to 94% with average 65%. Some samples had a MH above 50 and were thus not suitable for irrigation. Based on PI, it is found that groundwater is good to moderately suitable for agriculture. The results shows that almost of the groundwater samples fell in the field of C2-S1, indicating water of medium salinity and low sodium, which can be used for irrigation in almost all types of soil with little danger of exchangeable Na+. Generally, groundwater in the study area is suitable for both domestic and irrigation uses. The study's conclusion is that groundwater in this region is a big and crucial resource for growth in both the social and economic sectors, and that sustainable management of this vital resource is desperately required.
Acknowledgments
This work is financially supported by the ministry of higher education and scientific research, Sudan. I would also like to extend my sincere thanks to the research group participating in this work from the Sudan atomic energy commission, and especially the colleagues in the radiation environmental monitoring department. The authors gratefully acknowledge the assistance for the water quality analysis, by the staff of UNISCO laboratory of Omdurman Islamic university. Also we would extend our sincere thanks to department of groundwater and Wadies ministry of irrigation.
References
- Siebert S, Burke J, Faures JM, Frenken K, Hoogeveen J, et al. (2010) Ground water use for irrigation a global inventory. Hydrol Earth Syst Sci 14:1863-1880
- Mahgoub F (2014) Current status of agriculture and future challenges in Sudan. (57th edition). Nordiska Afrikainstitutet, Uppsala, 1-98
- Burri NM, Weatherl R, Moeck C, Schirmer M (2019) A review of threats to groundwater quality in the anthropocene. Sci Total Environ 684:136-154
- Abdalla OA (2008) Ground water discharge mechanism in semiarid regions and the role of evapotranspiration. Hydrol Process: An Int J 22:2993-3009
- Elfaki T (2010) Determination, source identification and GIS mapping for nitrate concentration in ground water from Bara aquifer. Thesis (MSc). Sudan Academy of Sciences, Atomic Energy Council, Khartoum, Sudan, 107
- Abdo G, Salih A (2012) Challenges facing ground water management in Sudan. Glob Adv Res J Phys Appl Sci 2012:001-011
- Abdalla OA (2006) Aquifer systems in Kordofan, Sudan: subsurface lithological model. S Afr J Geol 109:585-598
- Abdalla OA (2009) Groundwater modeling in semiarid Central Sudan: adequacy and long term abstraction. Arab J Geosci 2:321-335
- Singh P, Tiwari AK, Singh PK (2014) Hydro chemical characteristic and quality assessment of ground water of Ranchi township area, Jharkhand, India. Curr World Environ 9:804
- Odukoya AM (2015) Geochemical and quality assessment of groundwater in some Nigerian basement complex. Int J Environ Sci Technol 12:3643-3656
[Crossref] [Googlescholar] [Indexed]
- Jyrkama MI, Sykes JF (2007) The impact of climate change on spatially varying groundwater recharge in the grand river watershed (Ontario). J Hydrol 338:237-250
[Crossref] [Googlescholar][Indexed]
- Abdalla OA (2009b) Groundwater recharge/discharge in semi-arid regions interpreted from isotope and chloride concentrations in north White Nile Rift, Sudan. Hydrogeol J 17:679-692
- Elhaja ME, Csaplovics E, Abdelkareem OE, Adam HE, Ibrahim KA, et al. (2017) Land use land cover changes detection in White Nile State, Sudan using remote sensing and GIS techniques. Int J Environ Monit Prot 4:14-19
- APHA (2005) Standard Methods for the Examination of Water and Wastewater. (25th Edition). American Public Health Association, American Water Works Association/Water Environment Federation, Washington DC.
- WHO (2011) Guidelines for drinking water quality. (4th Edition) World Health Organization Geneva, Switzerland, 1-564.
[Indexed]
- Kumar M, Kumari K, Ramanathan AL, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ Geol 53:553-574
- Kudoda AM, Abdalla OA (2015) Hydro chemical characterization of the main aquifers in Khartoum, the capital city of Sudan. Environ Earth Sci 74:4771-4786
[Crossref] [Googlescholar][Indexed]
- Sridharan M, Nathan DS (2017) Ground water quality assessment for domestic and agriculture purposes in Pondicherry region. Appl Water Sci 7:4037-4053
- Singh S, Raju NJ, Ramakrishna C (2015) Evaluation of ground water quality and its suitability for domestic and irrigation use in parts of the Chandauli-Varanasi region, Uttar Pradesh, India. J Water Resour Prot 7:572
- Batayneh A, Elawadi E, Mogren S, Ibrahim E, Qaisy S (2012) Ground water quality of the shallow alluvial aquifer of wadi Jazan (southwest Saudi Arabia) and its suitability for domestic and irrigation purpose. Scientifc Res Essays 7:352-364
- Ndoye S, Fontaine C, Gaye CB, Razack M (2018) Ground water quality and suitability for different uses in the Saloum Area of Senegal. Water 10:1837
[Crossref] [Googlescholar] [Indexed]
- Richard L (1954) Diagnosis and improvement of Saline and Alkali Soils. Agricultural Handbook 60 Washington, DC 78:154
- Karanth KR (1987) Groundwater Assessment, Development and Management. Tata McGraw Hill Publishing Company Limited, New Delhi, India, 576-638
- Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 69:123-134
- Szaboles I, Darab C (1964) The influence of irrigation water of high sodium carbonate content on soils. Agro Soil Sci 13:803-812
- Doneen LD (1962) The influence of crop and soil on percolating water. In Proceedings of the 1961 Biennial Conference on Groundwater Recharge, California, Davis, 156-163
- Al-Ruwaih F, Shafiullah G (2017) Geochemical processes and assessment of water quality for irrigation of Al-Shagaya field-C, Kuwait. Int J Environ Agric Biotechnol 2
- Doneen LD (1964) Notes on water quality in agriculture. Water science and engineering. Department of Water Sciences and Engineering, University of California, Davis
- Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099-1110
- Yidana SM (2010) Ground water classification using multivariate statistical methods: Southern Ghana. J Afr Earth Sci 57:455-469
- US Salinity Laboratory. Staff 1954. Diagnosis and improvement of saline and alkali soils. US Agric Handbook, No. 60, US Dept Agric, Washington DC, 160
- Laze P, Rizani S, Ibraliu A (2016) Assessment of irrigation water quality of Dukagjin basin in Kosovo. J Int Sci Pub Agric Food 4:544-551
- Akram W, Tariq JA, Ahmad M (2011) Major ion chemistry and quality assessment of groundwater in Haripur area. Pakistan Institute of Nuclear Science and Technology, Pakistan, 29.
- Aza-Gnandji CDR, Xu Y, Raitt L, Levy J (2013) Salinity of irrigation water in the Philippi farming area of the Cape Flats, Cape Town, South Africa. Water SA 39:199-210
[Crossref] [Googlescholar][Indexed]
- Idriss H, Salih I, Sam A (2011) Study of radon in ground water and physicochemical parameters in Khartoum state. J Radioanal Nucl Chem 290: 333-338
[Crossref] [Googlescholar][Indexed]
- Kim K (2002) Plagioclase weathering in the groundwater system of a sandy, silicate aquifer. Hydrol Process 16:1793-1806
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences