ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Graphene Quantum Dot-Modified Lipase for Synthesis of L-menthyl Acetate with Improved Activity, Stability and Thermostability
Zhou Xiaoyan1, Bei Hongxia1, Li Zaijun1,2* and Liu Junkang1
1School of Chemical and Material Engineering, Jiangnan University, Wuxi, China
2Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, Wuxi 214122, China
- *Corresponding Author:
- Zaijun Li
Department of Materials Chemistry
Jiangnan University, China
Tel: +8613912371144
E-mail: zaijunli@jiangnan.edu.cn
Received date: October 12, 2016; Accepted date: November 04, 2016; Published date: November 08, 2016
Citation: Xiaoyan Z, Hongxia B, Zaijun L, et al. Graphene Quantum Dot-Modified Lipase for Synthesis of L-menthyl Acetate with Improved Activity, Stability and Thermostability. Synth Catal. 2016, 1:1.
Copyright: © 2016 Xiaoyan Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Title: Pseudomonas cepacia lipase modified with Graphene Quantum Dots (GQD) offers a higher activity, stability and thermostability compared to the lipase modified with graphene oxide.
Background: GQD presents extraordinary properties attracting extensive attention from scientists in chemistry, physics, materials, biology and other interdisciplinary sciences. However, no research involves the application in biocatalysts in non-aqueous media up to now. In the study, we for the first time reported a promising GQDmodified lipase for the synthesis of l-menthyl acetate.
Methods and findings: Pseudomonas capaci lipase was modified with GQD and then used as a biocatalyst for the synthesis of l-menthyl acetate in 1-isobutyl-3- methylimidazolium hexafluorophosphate medium. As contrasts, the reaction was also carried out using bare lipase and graphene oxide-modified lipase as the catalyst. Besides, the modification method of enzyme, the amounts of GQD, the reaction temperature, molar ratio of the two substrates, and reuse of the enzyme were investigated. The result shows that the GQD-modified lipase as a biocatalyst was best among the three enzymes. Under optimal reaction conditions, the reaction reaches the equilibrium within 8 h with a high conversion of lmenthol (97.3%). Its initial enzyme activity and halflifetime were more than 1.08 and 2.07-fold that of the bare lipase, and 1.04 and 1.66-fold that of the graphene oxide-modified lipase, respectively. The lipase was recycled 10 times without substantial diminution in activity. In addition, the GQD-modified lipase also offers a better thermostability compared with bare lipase. These improvements could be attributed to the unique small size and edge effect of GQD.
Conclusion: GQD-modified lipase showed a higher activity, stability and thermostability compared to GO- modified lipase. GQD was widely used as a promising material for enzyme modification due to its good biocompatibility and small size.
Keywords
Graphene Quantum Dot (GQD); Lipase; Biocatalyst; l-menthyl acetate; Synthesis
Introduction
Enzyme is a biological molecule that can regulate chemical reactions in biological process [1,2]. Lipase as a biocatalyst has been widely applied in various organic reactions, including hydrolysis, transesterification, resolution of racemic mixtures and stereoselective oligomerization [3-6]. The significant value of lipases mainly lies on their wide industrial applications in pharmaceutical synthesis, detergent formulation, cosmetics production and oil/fat degradation [7-10]. However, lipase-based catalysts are not always sufficient for industrial application due to their poor catalytic activity and stability, which largely limits their application [11]. To overcome the above limitations, many efforts have been made to improve enzyme catalytic performance to meet the need of practical applications, including the use of additives with stable effect, modification of structure by chemical methods or protein engineering methods and various immobilization strategies [12-15]. The enzyme immobilization or modifications have gained widespread attentions due to their advantages of enzyme reutilization, industrial-scale application and simplification of operation Process [16].
The performance of immobilized enzyme greatly depends on the support materials. Recently, nanostructured materials evolve as promising alternatives for enzyme modification. The advantages for nanomaterials standing out among other supporters are their ultrahigh surface area for biomolecule adsorption and access to functionalization of surface functional groups for interacting with biomolecules. Enzyme immobilized on nanostructured materials can enhance catalytic activity, stability. Besides, they have the advantages of repeated or continuous use, easy separation from the reaction mixture, prevention of protein contamination in the product. Accompanying the rapid development of nanotechnology, a great number of nanomaterials have been utilized for enzyme immobilization or modification, such as gold nanoparticles, magnetic nanoparticles and porous silica structures [17-20]. However, no reports refer on the modification of industrial enzyme by Graphene Quantum Dots (GQD) to improve the enzyme performance of nonaqueous catalysts.
GQD, one type of D graphene sheets with lateral size less than 100 nm, has attracted increasing attention owing to their robust chemical inertness, low cytotoxicity, excellent biocompatibility, high photostability and ease of preparation [21-23]. Great potential of GQD has been shown in various applications including biosensing, cellular imaging, photovoltatic devices and biomedicine [24-26]. Similar to Graphene Oxide (GO), GQD contains rich of oxygen-containing groups such as hydroxyl, carboxyl and epoxy groups. The characteristic makes it possible to modify enzymes without any surface modification or coupling reagents. Particularly, small size and controllable polarity make it more suitable for enzyme modification compared with GO. The physicochemical properties and structure suggest GQD has great potential for applications in nonaqueous reaction media.
In this study, we reported synthesis of nitrogen and sulfur co-doped GQD via pyrolyzing the mixture of citric acid and L-cysteine and its application in the modification of Pseudomonas cepacia lipase. The modified lipase was used as a biocatalyst for synthesis of l-menthyl acetate in the hydrophobic ionic liquid 1-isobutyl-3-methylimidazolium hexafluorophosphate. The modified lipase exhibits a better catalytic efficiency and reproducibility and operational stability compared to bare lipase. It can be widely used in various bio-catalysis reactions for practical applications to improve the activity and stability of enzymes.
Methods
Materials and reagents
Pseudomonas cepacia lipase (PCL, 30 U mg-1, solid) and lmenthol were obtained from Sigma-Aldrich Chemical Company (Mainland, China). The ionic liquid, 1-isobutyl-3- methylimidazolium hexafluorophosphate ([i-C4min][PF6]) was prepared according to the literature [4]. GO was synthesized from natural graphite powder by a modified Hummers method [27]. All other reagents employed were purchased from Shanghai Chemical Company (Shanghai, China) with analytical reagent grade. Ultrapure water (18.2 MΩ cm) purified from a Milli-Q purification system was used throughout the experiment.
Apparatus
Transmission electron microscope (TEM) image was obtained by a JEOL 2010 FEG microscope at 200 KeV. The sample was prepared by dispensing a small amount of dry Val-GQDs in ultrapure water and followed by drop one drop of the suspension on 200 mesh copper TEM grids covered with thin amorphous carbon films. Scanning electron microscope (SEM) analysis was carried out using a S4800 field emission scanning electron microscope (Hitachi, Japan). X-ray photoelectron spectroscopy (XPS) measurement was carried out on a PHI 5700 ESCA spectrometer using Al HR radiation (hν=1486.6 eV). Infrared spectrum (IR) was recorded on a Nicolet FT-IR 6700 spectrometer. GC analysis was performed with a FULI 9790 instrument equipped with FID detector (Fuli Analytical Instrument Co. Ltd, China) and a chiral β-DEX 120 capillary column (30 m × 0.25 mm × 0.25 μm). Fluorescence images were obtained digitally on a Nikon-80i upright fluorescence microscope. GC analysis was performed with a FULI 9790 instrument equipped with FID detector, using benzyl acetate as internal standard. The chromatographic conditions were as follows: Column temperature, 170; injection temperature and detector temperature, 300; Carrier gas, nitrogen; N2 stream, 30 mL min-1; spilt ration, 1:50. The peak retention times were as follows: l-menthyl acetate, 2.89 min; l-menthol, 3.24 min; benzyl acetate, 3.81 min. The yield was calculated from the amount of the easter peak. Substrate and product concentrations were calculated from calibration curves using stock solutions of pure compounds.
Synthesis of GQD
GQD was prepared by pyrolyzing the mixture of citric acid and L-cysteine following the previous report, with some modifications [28]. Briefly, a 4 g of citric acid and 2 g of L-cysteine were dissolved in 10 mL of ultrapure water. Followed by heated at 200 for 3 h with evaporation until dry. The resulting black product was dissolved by dropwise addition of 1 M NaOH until pH of the solution was neutral. The solution was subsequently purified by dialyzing and a powdery product was obtained by lyophilisation.
Modification of PCL
First, GQD (500 μg) was dispersed in water to form a concentrated GQD dispersion. The dispersion was subsequently transferred to a sprayer and sprayed onto the surface of dry PCL (150 mg) under vigorously vortex shaking. Finally, the hybrid was dried by freeze drying to enhance longterm stability of GQD-PCL.
General procedure for the synthesis of l-menthyl acetate
A 0.5 g of l-menthol (3.2 mmol), 300 μL of acetic anhydride, 1 ml of ionic liquid and 30 mg lipase PCL were added into a 25 mL conical flask with stopper in sequence, followed by incubation the above mixture at 40 in an air bath constant temperature oscillator with a stirring speed of 180 r min-1. At different times, 10 μL aliquots were withdrawn and dispersed in 400 μL of hexane. After being precipitated with centrifugation, 10 μL of benzyl acetate (internal standard) was added to the supernatant (100 μL) and then the solution was analyzed by GC. All experiments were repeated for five times, and mean values were reported.
Half-lifetime measurement
Mixtures containing 1.0 mL of ionic liquid and 30 mg of lipase PCL or modified PCL were incubated at 40. After a certain time, 0.5 g of l-menthol and 300 μL of acetic anhydride were added and the initial activity of biocatalyst was measured. Then the specific activity A was calculated based on the measured results. Specific activity is defined as the enzyme amount needed for synthesis of 1 μmol of products under optimal conditions. All the experiments were carried out in duplicate. Half-lives were calculated from a first-order exponential decay of the activity:
A=A0 × e-kt
where k is the first-order deactivation rate constant, and A0 and A are the initial and residual enzyme activity, respectively. The half-life t1/2 is defined as the t at A=A0/2 or t1/2=ln2/k.
Results
Materials characterization
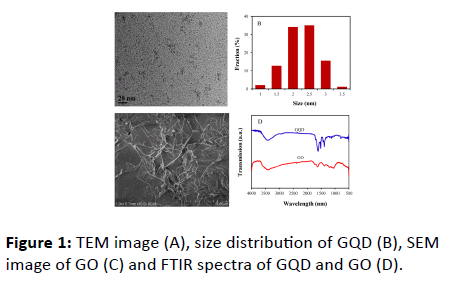
SEM image of GO and TEM image of GQD as well as their FTIR spectra are shown in Figure 1. It can be seen that GQD offers a narrow particle size distribution with the average size of 2.3 ± 0.4 nm. While GO is composed of relatively large graphene sheets of a few micrometer. On the FTIR spectrum in Figure 1D there are strong absorption peaks at 3420 cm-1, 1701 cm-1 and 1400 cm-1. They could be attributed to stretching vibration of -OH, -C=O and -COOH, respectively. The results also show that GQD contains the rich of oxygen-containing groups, which renders GQD strongly hydrophilic and water soluble. The presence of peak at 1296 cm-1 suggests the existence of C-N and C-S groups.
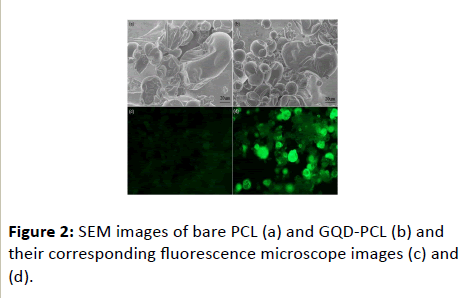
The proposed modification by spraying method makes the GQD drops fully contact with PCL. This can promote the dispersion of GQD compared with solid direct mixing. Moreover, the lipase immobilized on carriers easily collapse when the solution mixing was used. This is because the solution mixing may lead to destroying the special structure of lipase during the dissolving process. The damaged structure is no favor for PCL to exert their bioactivities. Figure 2 presents the SEM images of lipase before and after the modification. It can be seen from Figure 2 that the PCL lipase structure can be well maintained after the introduction of GQD. The result verifies that small amount of GQD renders PCL displaying an undamaged structure with uniformly dispersed GQD. Besides, PCL sample after modified GQD exhibits a strong and uniform fluorescence, indicating that the GQD has been successfully modified on the surface of PCL again.
Optimization of reaction conditions
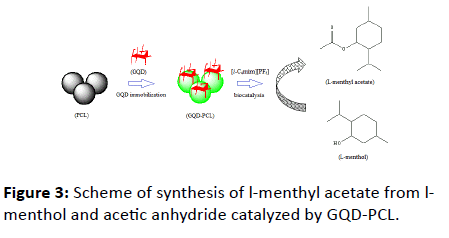
The GQD-modified lipase (GQD-PCL) was investigated as biocatalyst for the model reaction of esterification of l-menthol with acetic anhydride in [i-C4min][PF6] ionic liquid medium (schemed in Figure 3). Herein, the conversion of l-menthol can well present the catalytic activity of the biocatalyst. To optimize the experimental conditions for this esterification reaction, effects of amounts of GQD, reaction time, reaction temperature and alcohol to anhydride molar ratio on activity of modified lipase were tested in the laboratory.
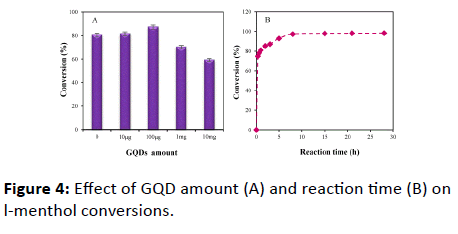
An appropriate amount of GQD should be considered to obtain high catalytic efficiency. Figure 4A shows that the amounts of GQD obviously effects on the conversion of lmenthol. The conversion increased with increasing the GQD content, and reaches the maximum value when the amounts of GQD are about 100 μg. However, the l-menthol conversion will rapidly decrease with the amount of GQD is more than 100 μg. This is because the excessive GQD for the modification of lipase could cause conformational hindrance and structural damage of PCL. This may reduce the interfacial area, leading to a low conversion. Thus, the GQD amount of 100 μg was selected for the modification of PCL.
The effect of reaction time on the l-menthol conversion was investigated. Figure 4B shows that the conversion was rapidly increased with the increase of the reaction time from 0 to 1 h. However, the conversion doesn’t significantly increase for the reaction times in excess of 8 h. This indicates that the reaction reaches the equilibrium within 8 h. Thus, the reaction time of 8 h was selected for the esterification reaction of l-menthol.
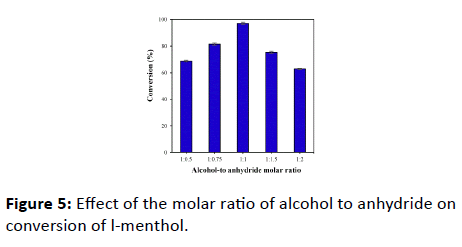
Influence of the molar ratio of l-menthol to acetic anhydride on the synthesis of l-menthyl acetate catalyzed by GQD-PCL was examined, in which the amounts of GQD was fixed at 100 μg. As shown in Figure 5, the l-menthol conversion increased with the increase of acetic anhydride content. The highest conversion were observed when the alcohol-to anhydride molar ratio was 1:1. However, the conversion of l-menthol decreased with the addition of anhydride. The result is similar to that of a well-established method [29]. This may be because a low concentration of anhydride leads to a low reaction rate. Meanwhile, the progressively produced acetic acid may be acted as main acyl donors along with the decrease of anhydride. However, the lower ability of acetic acid acylation leads to a decreased conversion. Besides, too much acetic anhydride will dehydrate into acetic acid, decreasing the activity of biocatalyst. Therefore, a best alcohol-to anhydride molar ratio of 1:1 was selected in the subsequent studies.
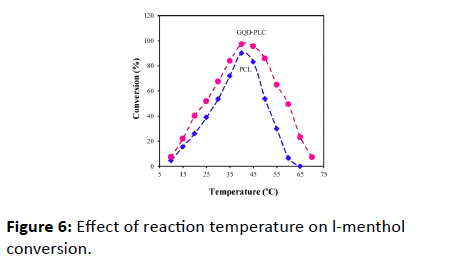
The temperature mostly has the significant influence on the activity of lipase. High temperatures offer an important advantage of accelerating the reaction rates due to the reduced medium viscosity and increased substrate diffusion coefficient. However, high temperatures lead to promote protein denaturation [4]. Thus, it is of great importance to investigate the thermo-stability of lipase, especially for industrial applications. The effects of temperature in the range of 10 to 70 on the activity of the bare lipase and the modified one were investigated. With GQD amount being kept constant at 100 μg, pre-treatment of GQD-PCL in different temperatures and each l-menthol conversion was measured, respectively. It can be seen from Figure 6, the conversion of l-menthol has the same change trend when the GQD-PCL and bare PCL were used as the biocatalyst. At the temperatures of lower than 40, the conversion increased rapidly with the increase of reaction temperature. However, a gradual decrease in the conversion was observed with the further increase in temperature due to the gradual denaturation of lipase at high temperature. The optimum temperature was 40 for both bare PCL and GQD-PCL. More importantly, the conversion of l-menthol achieved by GQD-PCL is much higher than that by bare lipase under the same temperature. For the temperature at 50, the modified PCL retained nearly 85% of its relatively activity for an incubation of 8 h, whereas that of the bare PCL only left 50%. However, at 60, the bare nearly lost all its initial activity, whereas the modified PCL still retained 50% of its activity. Such significant resistance to heat of GQD-PCL could be due to attachment of GQD on PCL, which may cause robust conformation limitations in preventing lipase conformational transitions at high temperature.
According to the above studies, the optimal conditions for the esterification reaction of l-menthol catalyzed by the GQD-PCL were as follows: A GQD amount of 100 μg, reaction temperature of 40 and alcohol-to anhydride molar ratio of 1:1. Under the above conditions, initial reaction rate, the conversion of l-menthol was 97.3%, which was much higher than that of bare PCL (90.2%).
Possible Mechanism of the Improved Activity
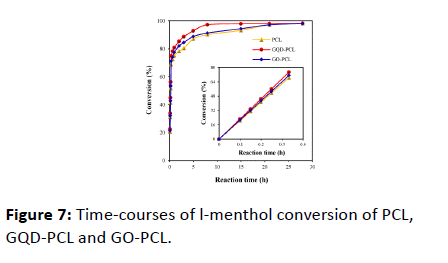
To further understand the improved catalysis efficiency of the GQD-PCL, another additive, GO was used to replace GQD for the modification of PCL. shows the change of l-menthol conversion with increasing reaction time, in which bare PCL, GO-PCL or GQD-PCL was employed as the catalyst. It can be seen the initial reaction rates and l-menthol conversions for GO-PCL and GQD-PCL were higher than that of bare PCL, indicating both GO and GQD can accelerate esterification reaction. This may be due to the hydrophilic groups of both GO and GQD provide a benign microenvironment for enzyme’s biological function. However, an obvious difference was unexpectedly observed when using these two biocatalysts. The reaction catalyzed by GQD-PCL reaches equilibrium within 8 h with a high conversion (97.3%), which is 1.04-fold greater than that of the GO-PCL and 1.08-fold greater than that of bare lipase, respectively.
The key criterion for selecting an appropriate additive for the enzyme modification is the stability within the reaction medium. In this experiment, the stability of GO-PCL, GQD-PCL as well as bare lipase were investigated by incubating bare lipase and modified lipases in ionic liquids without substrates, followed by measurement of the residual activity using the method described in the general procedure.
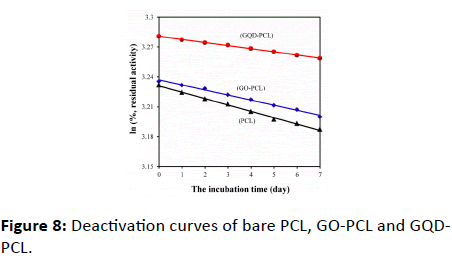
Figure 8 shows the deactivation curves of the different biocatalysts. The half-life time of bare lipase, GO-PCL and GQDPCL were found to be 108, 135 and 224 h, respectively, which were calculated using the formula (t1/2=ln2/k). The data confirm that the stability of GQD-PCL is obviously higher than that of the other two catalysts. Its half-life time is more than 2.07-fold that of bare lipase and 1.66-fold that of GO-PCL. The result demonstrates that the addition of GQD improve the stability of lipase. On the one hand, ionic liquid [i-C4min][PF6] provides a benign microenvironment for the lipase, which helps to retain the activity [5]. The large number of carboxylic groups existing at the GQD sheets’ periphery provide a hydrophilic microenvironment that enables lipase to maintain the ‘essential water’ for its biological function [30,31] The above two factors improve the enzyme stability, which is necessary for the enzyme to display its biological function.
Enzyme recycling
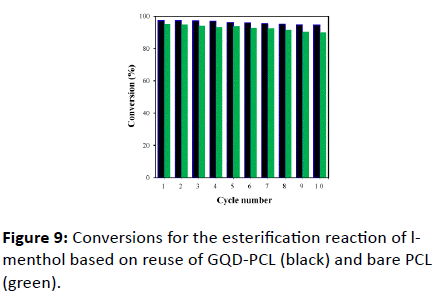
To evaluate the possibility of the reuse of GQD-PCL, it was applied as a biocatalyst for esterification reaction of l-menthol repeatedly. The mixed solution was completely removed after l-menthol reacts with acetic anhydride for 8 h, and the collected GQD-PCL was directly used for the next esterification reaction with new raw materials under the same reaction conditions. After each reaction was completed, the conversion of l-menthol was measured by GC and the results are shown in . It can be seen that the conversion of l-menthol decreased slightly after 10 cycles from 97.3% to 94.3%, indicating no substantial diminution of enzyme activity. Figure 9 also indicates that the cycling stability of GQD-PCL is much better than that of the bare PCL. This demonstrates that the introduction of GQD can improve the stability of lipase.
Discussion
The study demonstrates the preparation of lipases-nitrogen and sulfur co-doped GQD composite and its application as the biocatalyst for esterification reaction of l-menthol with acetic anhydride in [i-C4min][PF6] ionic liquid. The result shows that the GQD are composed of tiny graphene sheets with the rich of oxygen-containing groups. The small size of graphene sheets reduces the steric hindrance for the substrates entrance. The oxygen-containing groups increase with the polarity of lipase. The doped nitrogen and sulfur groups further improve the affinity of lipase with the substrate due to their relatively big electronegativity. The association of GQD with the lipase causes a special structural change that leads to exposure of the active site and results in an improvement in the enzyme activity. The lipase modified with the GQD exhibits an obviously enhanced activity, stability and thermo-stability. The study also provides a promising approach for enzyme modification in industrial catalysis to improve the enzyme activity and stability using GQD with good biocompatibility.
References
- Viloca MG, Gao JL, Karplus M, Truhlar DG (2004) How Enzymes Work: Analysis by Modern Rate Theory and Computer Simulations. Science 303: 186-195.
- Huber PC, Almeida WP, De Fatima A (2008) Glutathione and related enzymes: Biological roles and importance in pathological processes. Quimica Nova 31: 1170-1179.
- Mahmood I, Guo C, Xia HS, Ma JH, Jiang YY, et al. (2008) Lipase Immobilization on Oleic Acid-Pluronic (L-64) Block Copolymer Coated Magnetic Nanoparticles, for Hydrolysis at the Oil/Water Interface. IndEngChem Res 47: 6379-6385.
- Shan HX, Li ZJ, Li M, Ren GX, Fang YJ (2008) Improved activity and stability of pseudomonas capaci lipase in a novel biocompatible ionic liquid, 1-isobutyl-3-methylimidazolium hexafluorophosphate. J Chem Technol Biotechnol 83: 886-891.
- Li M, Shan HX, Zhou LT, Yin YY, Li ZJ (2013) Novel bioreactor for resolution of (R,S)-1-phenylethanol using the functional conducting polymer and ionic liquid with excellent catalytic activity and stability. J Chem Technol Biotechnol 88: 2091-2097.
- Attya M, Russo Anna, Perri E, Sindona G (2012) Endogenous lipase catalyzed transesterification of olive oil fats. The formation of isomeric and oligomerictriacyleglycerols. J Mass Spectrom 47: 1247-1253.
- Vicente GF, Rosario B, Vicente G (2006) Lipases: Useful biocatalysts for the preparation of pharmaceuticals. J Mol Catal B: Enzym 40: 111-120.
- Pundir CS, Chauhan N (2012) Coimmobilization of Detergent Enzymes onto a Plastic Bucket and Brush for Their Application in Cloth Washing. IndEngChem Res 51: 3556-3563.
- Ansorge-Schumacher MB, Thum O (2013) Immobilised lipases in the cosmetics industry. ChemSoc Rev 42: 6475--6490 6475.
- Lai CC, Zullaikah S, Vali SR, Ju YH (2005), Lipase-catalyzed production of biodiesel from rice bran oil. J Chem Technol Biotechnol 80:331-337.
- Bornscheuer UT, Bessler C, Srinivas R, Krishna SH (2002) Optimizing lipases and related enzymes for efficient application. Trends Biotechnol 20: 433-437.
- Kumar R, Singh R, Kaur J (2014) Combinatorial reshaping of a lipase structure for thermostability: Additive role of surface stabilizing single point mutations. BiochemBiophys Res Commun 447: 626-632.
- Sáez RT, López CO, Barbosa O, Fernández-Lafuente R (2014) Chemical modification of lipase B from Candida antarctica for improving biochemical properties of activity, stability and selectivity. New Biotechnol 31: S85.
- Cui CX, Tao YF, Ge CL, Zhen YJ, Chen BQ, et al. (2015) Synergistic effects of amine and protein modified epoxy-support on immobilized lipase activity. Colloid Surfaces B 133: 51-57.
- Manoela EA, dos Santosb JCS, Freired DMG, Ruedae N, Fernandez-Lafuenteb R (2015) Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb Tech 71: 53-57.
- Verger R (1997) Interfacial activation of lipases: facts and artifacts. Trends Biotechnol 15: 32-38.
- Venditti I, Palocci C, Chronopoulou L, Fratoddi I, Fontana L, et al. (2015) Candida rugosa lipase immobilization on hydrophilic charged gold nanoparticles as promising biocatalysts: Activity and stability investigations. Colloid Surfaces B 131: 93-101.
- Lee DG, Ponvel KM, Kim M, Hwang S, Ahn IS, et al. (2009) Immobilization of lipase on hydrophobic nano-sized magnetite particles. J Mol Catal B: Enzym 57: 62-66.
- Dyal A, Loos K, Noto M, Chang SW, Spagnoli C, et al. (2003) Activity of Candida rugosa Lipase Immobilized on γ-Fe2O3 Magnetic Nanoparticles. J Am ChemSoc 125: 1684-1685.
- Blanco RM, Terreros P, Fernández-Pérez M, Otero C, DıÌÂÂaz-González G (2004) Functionalization of mesoporous silica for lipase immobilization: Characterization of the support and the catalysts. J Mol Catal B: Enzym 30: 83-93.
- Zheng XT, Ananthanarayanan A, Luo KQ, Chen P (2015) Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11: 1620-1636.
- Sun HJ, Wu L, Wei WL, Qu XG (2013) Recent advances in graphene quantum dots for sensing. Mater Today 16: 433-442.
- Lin LP, Rong MC, Luo F, Chen DM, Wang YR, et al. (2014) Luminescent graphene quantum dots as new fluorescent materials for environmental and biological applications. Trends Anal Chem 54: 83-102.
- Wang D, Chen JF, Dai L (2014) Recent Advances in Graphene Quantum Dots for Fluorescence Bioimaging from Cells through Tissues to Animals. Part PartSyst Char 16: 433-442.
- Routh P, Das S, Shit A, Bairi P, Das P, et al. (2013) Graphene quantum dots from a facile sono-Fenton reaction and its hybrid with a polythiophene graft copolymer toward photovoltaic application. ACS Appl Mater Inter 5: 12672-12680.
- Yang Y, Asiri AM, Tang Z, Du D, Lin Y (2013) Graphene based materials for biomedical applications. Mater Today 16: 365-373.
- Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am ChemSoc 80: 1339-1339.
- Dong YQ, Pang HC, Yang HB, Guo CX, Shao JW, et al. (2013) Carbon-Based Dots Co-doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission. AngewChemInt Edit 52: 7800-7804.
- Yuan, Y, Bai, S, Sun, Y (2006) Comparison of lipase-catalyzed enantioselective esterification of (±)-menthol in ionic liquids and organic solvents. Food Chem 97: 324-330.
- Ge J, Yan M, Lu D, Zhang M, Liu Z (2007) Hyperbranched polymer conjugated lipase with enhanced activity and stability. BiochemEng J 36: 93-99.
- Sheldon RA, Lau RM, Sorgedrager MJ, Rantwijk F, Seddon KR (2002) Biocatalysis in ionic liquids. Green Chem 4:147-151.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences