Frequency of Factor V Leiden, Prothrombin G20210A and MTHFR C667T Mutations in Beta-Thalassemia Patients Compare with Normal Individuals from North of Iran
Seyed Mohammad Bagher Hashemi-Soteh1*, Aily Aliasgharian2, Alireza Tafazoli3,4, Hossein Karami5 and Mehrnosh Kowsaryan2
1Immunogenetic Research Center, Molecular and Cell Biology Research Center, Medical Faculty, Mazandaran University of Medical Sciences, Sari,Iran
2Hemoglobinopathy Institute, Thalassemia Research Center, Mazandaran University of Medical Sciences, Sari, Iran
3Department of Biochemistry, Biophysics and Genetics, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
4Medical Genetics Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
5Departments of Pediatrics, Hematology and Oncology, Thalassemia Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
- *Corresponding Author:
- Mohammad Bagher Hashemi-Soteh Ph.D.
Sari Medical Faculty
Mazandaran University of Medical Sciences
Mazandaran, Iran
Tel: +9811-3543081-3
Fax: +9811-3543087
E-mail: Hashemisoteh@mazums.ac.ir
Received Date: October 18, 2017; Accepted Date: October 24, 2017; Published Date: November 12, 2017
Citation: Hashemi-Soteh SMB, Aliasgharian A, Tafazoli A, Karami H, Kowsaryan M (2017) Frequency of Factor V Leiden, Prothrombin G20210A and MTHFR C667T mutations in beta-thalassemia patients compare with normal individuals from north of Iran. J Genom Gene Study Vol.1 No.1:1
Abstract
Background: Thrombophilia is an abnormality of blood clotting due to both genetic and non genetic factors. Among genetic causes, Factor V Leiden (R506Q), G20210A in prothrombin, and C667T in methylenetetrahydrofolate reductase (MTFHR) mutations are more important. The purpose of this study was to define the frequency of these three risk factors among normal individuals and betathalassemia from Mazandaran, a province in north of Iran.
Methods and findings: In this study, 105 normal individuals (as control) and 158 patients with beta-thalassemia were investigated in Sari, Mazandaran province. After DNA extraction, genotyping for three genes was determined using PCRRFLP methods. Frequency of the FV Leiden was 4.8% in normal control and 4.43% in beta-thalassemia cases, respectively. C667T allele frequency (allele T) in the MTHFR gene was 45.19% and 36.70% in normal and patients, respectively. Frequency of G20210A variant in normal control was 0.96 percent, but it was not seen in patients.
Conclusions: The results of this study showed no significant rates among the beta-thalassemia patients and normal individuals regarding the three genetic thrombophilia risk factors in this population. So these three genetic variants are not the cause of higher thrombotic risk among the beta-thalassemia patients from the Mazandaran province in Iran.
Keywords
Factor V Leiden; Prothrombin G20210A; MTFHR C667T; Thrombosis; Thalassemia
Introduction
Thrombosis is one of the most common causes of mortality and morbidity, in which multiple interactions between genetic and environmental factors contribute to the development of the disease [1]. Age, obesity, long immobility; surgery, cancer, and pregnancy are the most environmental factors that make a person susceptible to thrombosis. There are two sets of genetic mutations which are involved in thrombosis. First group includes the mutations that result in reduction at the level of anti-thrombotic proteins (like the mutations at Antithrombin3, S and C proteins) [1,2]. 5-10% of patients with venous thrombosis hold mutations in these genes [3-5]. Second group consists of the mutations which increase the level of prothrombotic proteins, like mutation at Factor V Leiden (G1691A), prothrombin G20210A, and MTHFR C677T variants [6-9].
Individuals with the Factor V Leiden mutation (A1691G; R506Q) are resistant to activated protein C and more susceptible to venous thrombosis. Factor V Leiden is the most prevalent inherited factor causing thrombosis with autosomal dominant segregation pattern. Compared with normal people, patients who are heterozygous for this mutation are almost 3% to 5% more susceptible to thrombosis, but those with homozygous form of the mutation, are significantly higher prone to the disease (50% to 100%) [9-13]. The second common polymorphism among patients with thrombosis is a mutation in the 3’UTR of the prothrombin gene (G20210A) which was described for the first time in 1996. Individuals with this mutation have the risk of developing thrombosis three times more than normal ones [8-14]. Another factor which can cause thrombotic is hyper homocysteinemia. Decreasing the function of methylenetetrahydrofolate reductase (MTHFR), an enzyme that converts homocysteine to methionine, causes hyper homocysteinemia. C667T mutation reduces the MTHFR enzyme’s function and increases the risk of thrombosis [15].
Many studies have shown that patients with beta-thalassemia, have a higher risk for thrombosis than normal individuals. Also thrombosis is more prevalent among splenectomized patients [16,17]. Thrombotic repeatedly have been reported among betathalassemia patients with diabetes, heart and lung Abnormalities, hypothyroidism, liver abnormalities, and splenectomy. Some thalassemia patients are susceptible to splenectomy, and suffer more from morphological abnormalities in platelets, which lead to increased risk for thrombosis [18,19]. A research was done in seven countries and the risk of thrombosis was investigated among thalassemia patients. The result indicated that 4.9% of beta-thalassemia patients had thrombosis experiences [20]. In this study, we have investigated the prevalence of three genetic risk factors of thrombophilia among beta-thalassemia patients, the most prevalent genetic diseases in Iran and in Mazandaran compared with normal individuals.
Material and Methods
Patients
This is a descriptive cross sectional study. The study population consisted of 158 individuals whose diagnosis of thalassemia and was registered in the department of thalassemia, Bali Sina hospital, in Mazandaran province, north of Iran. Also 104 healthy blood donors and university staffs as a control group with the age between 20 to 40 years were recruited. Among the cases, 84 were men with the mean age of 28.5 ± 9 (ranging from 12 to 49), and 74 women with the mean age of 28.1 ± 10.7 (ranging from 13 to 52). Some of the patients were spleenctomized, which were at higher risk for thrombosis. Some other patients came to department for blood transfusion regularly. This study was approved by the Institutional Review Board and Ethics Committee of Mazandaran University of Medical Sciences, Iran.
DNA extraction
Blood samples were collected in EDTA for all 262 subjects and Genomic DNA was isolated from whole blood by a modified Nucleon BACC II method from whole blood (Tepnel Life Sciences, Manchester, UK). Concentration and purification of extracting DNA were measured using a UV spectrophotometer and samples were stored at -20°C.
Genotyping and molecular studies
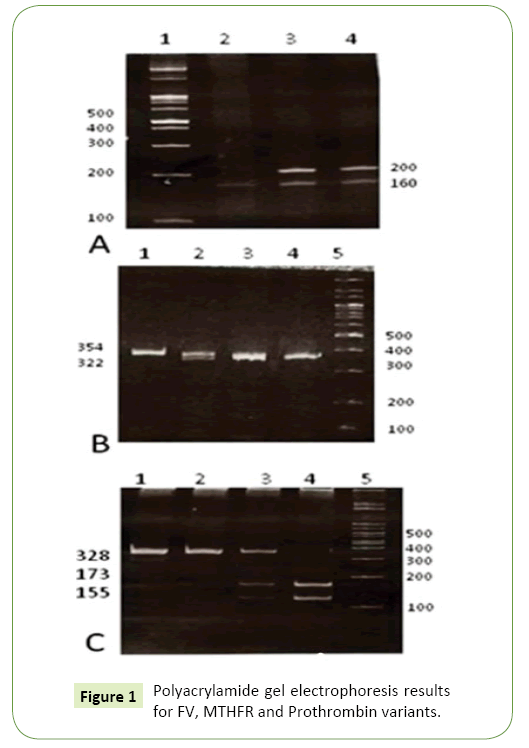
All samples were tested for Factor V Leiden, G20210A in prothrombin and C667T in MTHFR gene using PCR-RFLP method. 1-2 μl of extracting genomic DNA was amplified by Taq DNA polymerase, and each PCR reaction contained 10 mm Tris (PH=8.3), 50 mm KCL, 1.5 mm MgCL2, 200 μM each deoxynucleoside triphosphates (dATP, dCTP, dTTP, and dGTP), 0.25 mg of forward and reverse primers, and 1U Taq DNA polymerase (Cinnagen, Iran) in a total volume of 25 μL. PCR products were treated by digestion with restriction enzymes. For the detection of each mutation, we used PCR reaction with specific primers and the unique restriction enzyme. In brief, 7 to 10 μL of each PCR product was digested with restriction enzyme overnight. Finally, the digestion products were loaded on 10% polyacrylamid gel and stained with Ethidium bromide. A. PCR products of Factor V Leiden run on 10% polyacrylamide gel after digesting with Mnl I restriction enzyme. Samples 3 and 4 are heterozygous and showing two bonds for 160 and 200 bp. Sample 2 display a normal individual with three bonds for 37, 53 and 160 bp. Bonds 37 and 53 bp are not seen in the picture. PCR products of prothrombin (G20210A) run on 10% polyacrylamide gel after digesting with Hind III restriction enzyme. Sample 2 is a heterozygous with three bonds of 23, 322 and 345 bp. Other samples are normal showing just one bond in 345 bp. Bond 23 bp is not seen in the picture. PCR products of MTFHR (C667T) run on 10% polyacrylamide gel after digesting with Hinf I restriction enzyme. Samples 1 and 2 are from normal individuals, showing a 328 bp only. Sample 3 is a heterozygous with three bonds of 155, 173 and 328 bp. Sample 4 demonstrates a mutant homozygous with two bonds in 155 and 173 bp (Figure 1A-1C).
FV Leiden genotyping: Amplification was carried out for 35 cycles of 60 seconds at 95°C, 1 minute at 62°C, and 1 minute at 72°C, with a final extension for 10 minutes at 72°C. A denaturation step at 94°C for 5 minutes at the beginning was applied to all DNA samples. PCR products were run on 10% polyacrylamide gel followed by ethidium bromide staining. A DNA fragment of 250 bp was produced. The amplified fragments were digested with Mnl I restriction enzymes.
G20210A genotyping: To detect G20210A mutation, amplification was carried out for 35 cycles of 60 seconds at 95°C, 1 minute at 60°C, and 1 minute at 72°C, with a final extension for 5 minutes at 72°C. A denaturation step at 94°C for 5 minutes at the beginning was applied to all DNA samples. A DNA fragment of 345 bp was produced as the PCR product. The amplified fragments for detection of mutation were digested with HindIII restriction enzyme. PCR products were run on 10% polyacrylamide gel electrophoresis followed by ethidium bromide staining.
C667T MTHFR genotyping: In order to detect C667T mutations, amplification was carried out for 35 cycles of 60 seconds at 95°C, 1 minute at 60°C, and 1 minute at 72°C, with a final extension for 5 minutes at 72°C. A denaturation step at 94°C for 5 minutes at the beginning was applied to all DNA samples. PCR products were run on 8% polyacrylamide gel followed by ethidium bromide staining. A DNA fragment of 328 bp was produced by the relevant primers. Like two previous factors, the amplified fragments for the detection of mutation were digested with Hinf I restriction enzyme. Table 1 indicates the genotyping details for all three investigated variants.
Table 1 List of primers, PCR products length and restriction enzymes used in genotyping of FV, MTHFR and Prothrombin variants. Also, PCR fragment sizes after enzyme digestion are shown in the table for each genotype.
| Gene/variant | Specific primers | PCR product length (bp) | Restriction enzyme | Fragment lengths (bp) |
|---|---|---|---|---|
| FVL-F | 5'-CCATACTACAGTGACGTGGA-3' | 250 | MnlI | Normal: 160, 53, 37 |
| FVL-R | 5'-AATGTTATCACACTGGTGCTA-3' | Mutant: 200, 50 | ||
| MTHFR-F | 5'-AGGACGGTGCGGTGAGAGTG-3' | 328 | Hinf I | Normal: 328 |
| MTHFR-R | 5'-AGTCCCTGTGGTCTCTTCAT-3' | Mutant: 173, 155 | ||
| Prothrombin-F | 5'-TCTAGAAACAGTTGCCTGGC-3' | 345 | Hind III | Normal: 345 |
| Prothrombin-R | 5'-ATAGCACTGGGAGCATTGAAGC-3' | Mutant: 322, 23 |
Statistical Analysis
The allelic frequencies and the genotype frequencies were studied using Hardy-Weinberg principle. For the investigation of any correlation between patients and control group, a chi-square (χ2) statistical analysis was used and statistical significance was assumed at the P<0.05 level.
Results
158 thalassemia patients and 104 healthy individuals were studied for three genetic risk factors. Table 2 represent the genotype distribution achieved in this study. Seven thalassemia patients were heterozygous for factor V Leiden, as well as 3 heterozygous and 2 homozygous in control individuals. There was no thalassemia patient, with G20210A mutation, but one person among 104 control individuals has been heterozygous for this mutation. For C667T MTHFR, from 104 normal individuals, 44 were heterozygous and 3 were homozygous for TT, while in patients, 49 were heterozygous and 9 homozygous, respectively. The allele frequencies for FV Leiden (A1691G), was 4.8% and 4.43% in normal and thalassemia patients, respectively using Hardy–Weinberg equilibrium. The allele frequency of the mutant allele (T) for C667T in normal and thalassemia patients was 45.19% and 36.70%, respectively. Also the frequencies of the mutant allele (A) for G20210A in prothrombin gene were 0.96% and 0.0% in normal and patients, respectively. The current study has shown that 49.77% of normal individuals and 41.13% of thalassemia patients have at least one of these three mutations which are thrombotic risk factors. In fact, 6/77% of patients had thrombotic experiences, and 42.37% of them were splenectomized. We found one patient who was heterozygous for both Factor V Leiden and C667T mutations, but this case had no experience of thrombosis.
Table 2 The prevalence of genotypes and allele frequencies for tree thrombotic risk factors among thalassemia patients and normal individuals in north of Iran.
| Variants | Thalassemia patients |
Normal control |
|---|---|---|
| Factor V Leiden (G1691A) | ||
| Total cases | 158 | 104 |
| Homozygous Mutant (AA) | 0 | 2 |
| Heterozygous Mutant (GA) | 7 | 3 |
| Homozygous Normal (GG) | 151 | 98 |
| Frequency of mutant allele (A) | 4.43% | 4.80% |
| Prothrombin (G20210A) | ||
| Total cases | 158 | 104 |
| Homozygous Mutant (AA) | 0 | 0 |
| Heterozygous Mutant (GA) | 0 | 1 |
| Homozygous Normal (GG) | 158 | 103 |
| Frequency of mutant allele (A) | 0 | 0.96% |
| MTHFR ( C667T) | ||
| Total cases | 158 | 104 |
| Homozygous Mutant (TT) | 9 | 3 |
| Heterozygous Mutant (CT) | 49 | 44 |
| Homozygous Normal (CC) | 100 | 57 |
| Frequency of mutant allele (T) | 36.70% | 45.19% |
Discussion and Conclusion
Previous studies showed that thromboembolic has increased in patients with thalassemia. For example, an investigation in Italy revealed thrombosis in 29% of the thalassemia patients that did not transfuse blood regularly and 2 percent of beta-thalassemia major patient [21]. The disorder was more prevalent among splenectomized thalassemia patients. It is also observed that venous thromboembolism like pulmonary embolism, deep vein thrombosis (DVT), and portal vein thrombosis are more common among thalassemia patient especially in intermediate patients [22]. Zeinali et al. investigated the frequency of thrombolytic mutations, including Factor V Leiden and prothrombin (G202010A) in normal individuals in Tehran, Iran. They observed that 5.5% (allelic frequency 2.7%) of participants were heterozygous for Factor V Leiden and 3.1% (allelic frequency 1.5%) were heterozygous for prothrombin (G202010A) [23]. In our report, the number of individuals who were heterozygous for Factor V Leiden in normal cases is lower than Zeinali’s population, but the allelic frequency of this mutation is a little higher (4.8%). Moreover, the frequency of prothrombin (G202010A) in Mazandaran province (allelic frequency (0.96%) is lower compared to Tehran’s population. Rahimi et al. illustrated that in the west of Iran, the prevalence of prothrombin (G202010A) was 1.3% among thalassemia major, and 3.3% among normal individuals, while no one with thalassemia intermedia had this mutation [24]. This study confirmed another investigation by Karami et al. in 2007, that reported 3% prevalence of factor V Leiden, among 60 thalassemia intermedia patients from the same area in the north of Iran [25]. The prevalence of MTHFR (C667T) polymorphism among thalassemia patients was reported 93.2%, and 48.3% among normal individuals from western Iran [24]. Frequency achieved in this study was similar in normal individuals. Although our findings confirmed that the prevalence of this mutation is high among Iranians. In Italy, the prevalence of thrombosis among thalassemia patients was reported 13.55% [21]. In our study, 6.77% of patients had thrombosis experience. In Lebanon, heterozygous frequency of factor V Leiden was reported 14% in thalassemia patients and 12.4% in healthy individuals while we found 4.43% heterozygous in thalassemia patients and 4.8% in healthy individuals respectively, that are lower the prevalence compared with Lebanon [26]. A same study in Turkey declared that the prevalence of factor V Leiden in healthy individuals was 6%, which is close to report in this study [27]. Study of these three common genetic factors in thrombosis for both prevalence and causative role is still in progress in many areas which indicates the importance of them in coagulation disorders [10,14,28-30]. Even some other investigations which include Factor V, prothrombin (G202010A) and MTHFR (C667T) in another diseases have been conducted, represented the relevance of thrombosis genetic components and other maladies [31,32].
Since there is no difference in the frequency of thrombosis genetic risk factors between thalassemia patients and healthy individuals, the higher incidence of thrombotic event among thalassemia patients could be considered as a result of other components including: splenectomy, severe anemia, and deficiency in the action of coagulating factors [33]. Although, our study has showed that the higher incidence of thrombosis is not because of investigated mutations, yet it is suggested that thalassemia patients with these mutations should be under control.
Acknowledgements
We would like to appreciate the Research Institute of Mazandaran University of Medical Sciences for approving the proposals and devoting budget. We wish to thank the patients and the Boali Sina hospital staffs for their participation in this study.
Ethics Approval
The research was conducted within the guidelines under the terms of all relevant local legislation. All the patients have read and signed a consent form for participating in this research project. The study has been approved by Mazandaran University of Medical Sciences (MAZUMS) ethics committee.
Conflict of Interest
The authors declare that there are no conflicts of interest to disclose.
References
- Lane DA, Grant PJ (2000) Role of hemostatic gene polymorphisms in venous and arterial thrombotic disease. Blood95: 1517-1532.
- Bauduer F, Lacombe D (2005) Factor V Leiden, prothrombin 20210A, methylenetetrahydrofolate reductase 677T, and population genetics. Mol Genet Metab 86: 91-99.
- Bhakuni T, Sharma A, Rashid Q, Kapil C, Saxena R, et al. (2015) Antithrombin III deficiency in Indian patients with deep vein thrombosis: identification of first India based AT variants including a novel point mutation (T280A) that leads to aggregation. PloS one10: e0121889.
- Chen C, Yang L, Villoutreix B, Wang X, Ding Q, et al. (2017)Gly74Ser mutation in protein C causes thrombosis due to a defect in protein S-dependent anticoagulant function. Thromb Haemost.
- Suchon P, Germain M, Delluc A, Smadja D, Jouven X, et al. (2017) Protein S Heerlen mutation heterozygosity is associated with venous thrombosis risk. Scientific Reports7.
- Prajs I, Kuliczkowski K (2017) Predictive factors of thrombosis for patients with essential thrombocythaemia: A single center study. Advances in Clinical and Experimental Medicine26: 115-121.
- Kraaijenhagen RA, Haverkamp D, Koopman MM, Prandoni P, Piovella F, et al. (2000) Travel and risk of venous thrombosis. The Lancet356: 1492-1493.
- Poort SR, Rosendaal FR, Reitsma PH, Bertina RM(1996) A common genetic variation in the 3'-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 88: 3698-3703.
- Dahlbäck B, Carlsson M, Svensson PJ(1993) Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: prediction of a cofactor to activated protein C. Proceedings of the National Academy of Sciences90: 1004-1008.
- Sueta D, Ito M, Uchiba M, Sakamoto K, Yamamoto E, et al. (2017) A case of pulmonary thromboembolism due to coagulation factor V Leiden in Japan~ usefulness of next generation sequencing~. Thrombosis Journal 15: 8.
- Juul K, Tybjærg-Hansen A, Schnohr P, Nordestgaard BG (2004) Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Annals of Internal Medicine 140: 330-337.
- Rosendaal F, Koster T, Vandenbroucke J, Reitsma P (1995) High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 85: 1504-1508.
- Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, et al. (1994) Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 369: 64.
- Yoon U, Kwok L, Flessenkaemper I (2016) Bilateral Superficial Femoral Artery Thrombosis in a 15-Year-Old Caucasian Male with Homozygous Prothrombin G20210A Genotype and Associated Antiphospholipid Syndrome. International Journal of Angiology 25: e100-e105.
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. (1995) candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase.
- Sadewa AH, Sunarti, Sutomo R, Hayashi C, Lee MJ, et al. (2002) The C677T mutation in the methylenetetrahydrofolate reductase gene among the Indonesian Javanese population. Kobe J Med Sci 48: 137-144.
- Taher A, Isma'eel H, Mehio G, Bignamini D, Kattamis A, et al. (2006) Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost 96: 488-491.
- Belcaro G, Veller M, Nicolaides AN, Cesarone MR, Christopoulos D,et al. (1998) Noninvasive investigations in vascular disease. St Mary's Fellows. ISVI (Italian Society for Vascular Investigations). Angiology49: 673-706.
- Isma'eel H, Arnaout MS, Shamseddeen W, Mahfouz R, Zeineh N, et al. (2006) Screening for inherited thrombophilia might be warranted among Eastern Mediterranean sickle-beta-0 thalassemia patients. J Thromb Thrombolysis 22: 121-123.
- Cappellini MD, Grespi E, Cassinerio E, Bignamini D, Fiorelli G (2005) Coagulation and splenectomy: an overview. Ann N Y Acad Sci 1054: 317-324.
- Borgna Pignatti C, Carnelli V, Caruso V, Dore F, De Mattia D (1998) Thromboembolic events in beta thalassemia major: an Italian multicenter study. Acta haematologica 99: 76-79.
- Cappellini M, Grespi E, Cassinerio E, Bignamini D, Fiorelli G (2005) Coagulation and splenectomy: an overview. Annals of the New York Academy of Sciences 1054: 317-324.
- Zeinali S, Duca F, Zarbakhsh B, Tagliabue L, Mannucci P (2000) Thrombophilic mutations in Iran. Thrombosis and Haemostasis83: 351-352.
- Rahimi Z, Ghaderi M, Nagel RL, Muniz A (2008) Prevalence of thrombotic risk factors among ß-thalassemia patients from Western Iran. Journal of Thrombosis and Thrombolysis 26: 229-233.
- Karami H, Vahidshahi K, Kosarian M, Shahmohammadi S, Dabirian M, et al. (2010) Assessment of coagulation state and its related factors in thalassemia intermedia patients referred to thalassemia research center at Booali Sina Hospital Sari/IR Iran in 2007. Pakistan Journal of Biological Sciences 13: 448-451.
- Zalloua A, Shbaklo H, Mourad YA, Koussa S, Taher A (2003) Incidence of thromboembolic events in Lebanese thalassemia intermedia patients. Thrombosis and Haemostasis89: 767-768
- Eroglu A, Akar N (2011) Factor V Leiden, prothrombin G20210A and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphisms and the risk of tamoxifen-associated thromboembolism in breast cancer patients. Thrombosis Research 127: 384-385.
- Awad-Elkareem A, Elzaki SG, Khalid H, Abdallah MS, Adam I (2017) A low rate of factor V Leiden mutation among Sudanese women with deep venous thrombosis during pregnancy and puerperium. J Obstet Gynaecol: 1-2.
- Ekim M, Ekim H, Yilmaz Y (2015) The prevalence of Factor V Leiden, prothrombin G20210A, MTHFR C677T and MTHFR A1298C mutations in healthy Turkish population. Hippokratia 19: 309.
- Pasta L, Pasta F, D’Amico M (2016) PAI-1 4G-4G, MTHFR 677TT, V Leiden 506Q, and Prothrombin 20210A in Splanchnic Vein Thrombosis: Analysis of Individual Patient Data From Three Prospective Studies. Journal of Clinical and Experimental Hepatology 6: 10-14.
- Aydin H, Gunay M, Celik G, Gunay BO, Aydin UT, et al. (2016) Evaluation of Factor V Leiden, Prothrombin G20210A, MTHFR C677T and MTHFR A1298C gene polymorphisms in retinopathy of prematurity in a Turkish cohort. Ophthalmic Genet 37: 415-418.
- Gonçalves RO, Fraga LR, Santos WVB, Carvalho AFL, Cerqueira BAV, et al. (2016) Association between the thrombophilic polymorphisms MTHFR C677T, Factor V Leiden, and prothrombin G20210A and recurrent miscarriage in Brazilian women.
- Eldor A, Durst R, Hy-Am E, Goldfarb A, Gillis S, et al. (1999) A chronic hypercoagulable state in patients with ßthalassaemia major is already present in childhood. British Journal of Haematology 107: 739-746.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences