ISSN : 0976 - 8688
Der Pharmacia Sinica
Formulation and Evaluation of Taste Masked Doxycycline HCl Medicated Jelly
Syed Nisar Hussain Shah, Shahnila Aslam, Hina Javed*, Asma Nawaz, Kashif Kamboh and Muhammad Mohsin Javaid
Faculty of Pharmacy, Bahauddin Zakariya University, Multan
Abstract
Aim: The aim of present study was to formulate and evaluate the taste mask Doxycycline HCl medicated oral jelly by making complex with β cyclodextrin.
Methods: The slurry was prepared by using glucose 53%, sugar 34%, gelatin 25% and sorbitol 2.52%. All physicochemical characteristics like viscosity, homogeneity and viscosity, pH, synersis, palatability and taste were performed on this medicated jelly.
Results: In vitro dissolution studies were performed on this formulation in artificial saliva which showed maximum 99.3% drug release within 20 min. The release pattern of this medicated jelly followed the first order release kinetics. Stability study at 40 ± 5ºC was performed for three months which has shown the satisfactory result.
Conclusion: Doxycycline HCl is absorbed from buccal mucosa as well as from GIT. The chewing has shown drug release of greater than 90%. Thus it can be said that oral medicated jelly of Doxycycline HCl was made successfully.
Keywords
Doxycycline, β-cyclodextrin, Medicated oral jelly, Artificial saliva, Ex-vivo release study
Introduction
Jellies are transparent or translucent non-greasy semisolid solutions or suspensions made up of small inorganic particles or large organic molecules interpenetrated by a liquid. These are meant for oral or external application to mucous membranes and can be ingested without water [1]. By systemic administration of drug through the oral mucosa, it has the ability to overcome the difficulties of short lived action and differences in release of drug and retaining times [2-4]. Oral medicated jelly avoids first pass metabolism. The drugs which are released from jelly and swallowed, will be introduced in the GIT either by dissolving or suspending in saliva and so will be present in a readily bioavailability form. These novel formulations are more suitable for children & old age people offering quick dissolution and absorption of drugs thus early onset of action [5]. The dysphagic patients can be choked by water while consuming liquid formulation and this problem can be eliminated by administering liquid formulations with high viscosity in the form of jellies. Medicated jellies are used for lubrication and some other purposes [4,6].
In the current study, Doxycycline hydrochloride is used as model drug. It is bacteriostatic with broad spectrum antimicrobial activity, belongs to tetracyclines. It is also reported to be more active against protozoa particularly plasmodium species [7,8]. It is pale yellow crystalline powder, having molecular weight 480.3Da and has solubility in water and alcohol. The objective of this study was to formulate the taste mask Doxycycline medicated jelly complexed with β-cyclodextrin [9-11]
Materials and Methods
Doxycycline HCl was provided as a gift sample from Hamaz Pharmaceutical (Pvt.) Ltd. Glucose was used as sweetening and preserving agent and was available in pharmaceutical laboratory of BZU, Gelatin was used as gelling agent and citric acids was used as preservative and were also used from laboratory. 2-hydroxypropyl β-cyclodextrin (Sigma Aldrich) was purchased from GM scientific store. Starch was obtained from Rafhan industry, Faisalabad by Volka foods international.
Preparation of taste masked medicated jelly of Doxycycline HCl β-cyclodextrin complex
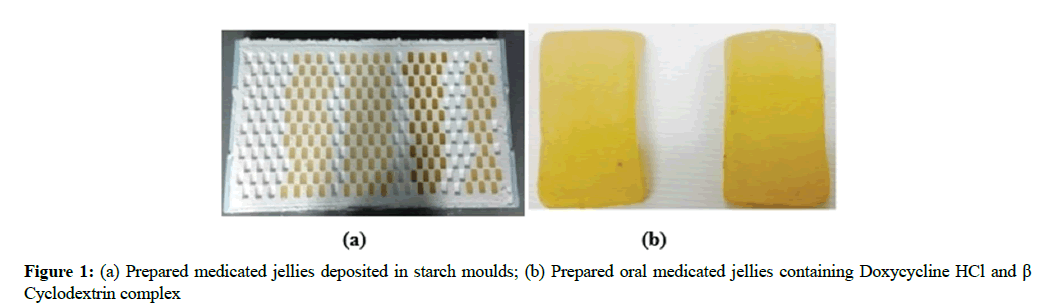
Weighed quantity of β-cyclodextrin was added in a suitable quantity of distilled water in a mortar so as to gain a consistent paste. Then weighed amount of Doxycycline was added slowly to make β-cyclodextrin and Doxycycline complex [11,12]. The whole mixture was then grounded for an hr. During this procedure, suitable amount of water was added to the mixture to get a suitable consistency. After this, the ingredients of first portion were mixed and cooked till a 115°C temperature was achieved. Then ingredients of second portion were mixed separately and added in the first cooked portion and cooked it again tills 118ºC. Citric acid and flavors were added in this mixture. Then Doxycycline HCl and β-Cyclodextrin complex was dissolved in small amount of water and added in the mixture. Then the whole mixture was transferred in starch moulds at humidity range of 40-45% at 38-43ºC temperature. When jelly was prepared, it was kept for 16 hrs at 20-22ºC and 40-45% humidity. Then it was degusted and polished with cooking oil as shown in Figure 1. The formulation of medicated jelly was given in Table 1.
| Ingredient | Quantity(g) |
|---|---|
| Doxycycline | 4 |
| β-cyclodextrin | 1 |
| First Portion | |
| Water | 9 |
| Glucose | 103 |
| Sugar | 67 |
| Second Portion | |
| Gelatin | 10 |
| Sorbitol | 6.56 |
| Water | 64 |
Table 1: Formulation of medicated jelly
Assay of Doxycycline HCl
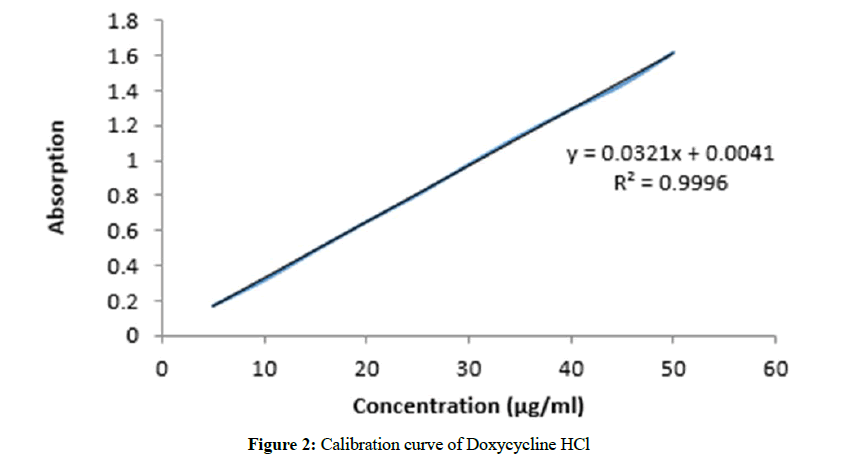
Volumetric flask of 100 mL was taken, washed it thoroughly and rinsed with distilled water. 0.1 g of DOX-HCl was dissolved in small amount of distilled water and then final volume was made up to 100 ml by adding sufficient amount of distilled water. 10 ml of this solution was added in another flask and its volume was made up to 100 ml by adding sufficient distilled water to make stock solution. Further dilutions were made in the following manner by using this stock solution. The absorbance of these dilutions was observed at 272 nm using UV Spectrophotometer. The calibration curve was made for Doxycycline in construction range from 0.25-5 μg/mL as shown in Figure 2. The regression equation for calibration curve was Y=0.0321X+0.0041 with regression coefficient (R2)=0.9996, where Y=absorbance at 272 nm, m=slope (0.0321), b=intercept (0.0041), and X=concentration (μg/mL) of Doxycycline HCl.
Solubility studies
Seven glass sample vials having volume of 5 ml were taken, dried and cleaned. Then 2 ml of methanol (pure), methanol solution in water (20, 40, 60 and 80%), PBS (pH 7.4) and distilled water were taken, and each was individually added in each vial. Then sufficient amount of Doxycycline HCl was added in all vials step by step until it started to settle down in the bottom of vials. This was then allowed to stir with magnetic stirrer at constant temperature (37ºC) and left for 24 hrs. The upper layer was pipette out and suitable dilutions were made with particular solvents and were analyzed by using the Ultraviolet visible spectrophotometer at 272 nm to conclude the concentration (μg/ml) with the aid of calibration curve. The solubility of Doxycycline HCl in each of these solvents was determined in triplicate [13].
Partition coefficient studies
Partition co-efficient determines distinctional aptitude of a solute to be dissolved in two immiscible phases [14]. Separating funnel of 100 ml volume was taken, cleaned & dried. Then 5 ml of distilled water and the same quantity of octanol was added in it. 0.05 mg of Doxycycline HCl was put in the separating funnel and wobbled strongly for 10-15 min and left for 24 hrs in vertical position. Then water and octanol layers were separated in separate test tubes. The absorbance of each layer was determined at 272 nm by UV spectrophotometer after suitable dilutions with particular solvents. All this process was carried out at room temperature in triplicate. Then conc. of Doxycycline HCl in each layer was determined with the aid of calibration curve.
Preparation of artificial saliva
Artificial saliva is prepared for the dissolution study of medicated jellies [15,16]. The compositions of artificial saliva are given in Table 2. The volumetric flask of 1 liter was used and adequate volume of water was added in it. Then all ingredients were weighed and added in it one after another and agitated constantly until fully dissolved. The adequate amount of distilled water was added to make volume up to 1 liter and was filtered using Whatmann filter paper.
| Ingredients | Quantity |
|---|---|
| Sodium bicarbonate | 5.208g |
| Dipotassium hydrogen phosphate trihydrate | 1.369 g |
| Sodium chloride | 0.877 g |
| Potassium chloride | 0.477 g |
| Calcium chloride dehydrate | 0.441 g |
| Distilled water | Quantity sufficient up to 1000 ml |
Table 2: Composition of artificial saliva
In vitro characterization
The medicated jellies were subjected to tests in order to determine its physical appearance, taste and palatability, syneresis, texture, clarity, precipitation, viscosity, homogeneity and pH [17-19].
The physical appearance of prepared jellies was evaluated visually for the clarity, color and presence of any type of particulate particles. Appearance must be appealing for the patient compliance and acceptance.
Taste and palatability test was performed on five healthy volunteers. Each of them was given a jelly containing almost 100 mg of Doxycycline HCl dose. They were asked to place the jelly in mouth for 30 seconds and then spit out. The bitterness was noted at interval of 1 min up to 5 min.
Syneresis is the contraction of jelly upon storage and release of water from the jelly. It occurs if the gelling agent is used in lower concentration [20]. All the jellies were noted for the signs of syneresis at room temperature (25 ± 5ºC) as well as 8 ± 1ºC. The jellies having syneresis were rejected and not used in further studies.
Texture of medicated jelly was evaluated by visual inspection to check the stickiness and grittiness of it. The jellies were mildly rubbed between two fingers.
The pH of medicated jelly had been measured using Digital pH meter at 25 ± 1°C. 1% solution of prepared jelly was made in water and its pH was determined [18].
For weight variation test ten prepared jellies were taken randomly and their individual weight was determined on analytical balance [21]. Then variation in their weight was noted.
Stability studies
Stability studies of prepared jellies were performed by keeping these jellies wrapped in aluminum foil at room temperature (25 ± 5ºC) for 90 days. Then these jellies were kept after wrapping in an oven at 35, 40, 45 and 50ºC temperature for 7 days at each temperature [22].
Drug release kinetics
Drug release kinetics reproduces different release mechanisms of drug release. Five different kinetic models may help in ex-vivo data analysis as well as finding suitable flitting Equation including Zero order (Ft=K0 t), where Ft is drug release fraction with time and K0 is drug release constant; First order (F=100 × [1-Exp(-K1 × t)]), where K1 is the first order release constant and F is the fraction of drug release at time t; Higuchi (F=kH × t^0.5), where KH is Higuchi’s constant and F is the fraction of drug release at time t; Korsmeyer–Peppas model (F=kKP × t^n), where kKP is Peppas constant, F is the fraction of drug release at time t and n is the diffusion constant and Hixson Crowell (F=100 × [1-(1- kHC × t)^3]), where kHC is Crowell’s constant and F is the fraction of drug release at time t. DD solver (Microsoft excel 2013) was used by entering data in it.
In vitro dissolution profile studies
In vitro drug release is measured by using an apparatus (masticator). Dissolution study was done in water as well as in artificial saliva at 37ºC [23]. Jellies were added in it and masticated artificially for 3 min. Then sample of 5 ml was drawn with help of pipette and added in a test tube. Then 5 ml of aliquot was added in it and repeated the same procedure. The samples were taken each 3 min till 18 min. Suitable dilutions were made of each sample and then were analyzed by the UV-spectrophotometer at 272 nm to determine the drug concentration and amount of drug release within specific time period.
Results and Discussion
Solubility studies
The Doxycycline HCl is freely soluble in distilled water in comparison of methanol and sparingly soluble in Phosphate buffer saline. The solubility of Doxycycline HCl reduces as the concentration of methanol increases. The solubility studies of Doxycycline HCl showed that this is more soluble in water (1021.4 ± 0.01 mg/ml) and methanol (261.3 ± 0.01 mg/ml).
Partition coefficient studies
The value of partition co-efficient (Ko/w) was 0.638. The result showed that the given drug consist of nearly sufficient hydrophilicity value which is considered important to formulate the drug for oral delivery.
Physical appearance
The physical appearance including clarity, precipitation, homogeneity as well as other features of the prepared Doxycycline HCl jellies were observed that showed all jellies were clear, yellow in color, having semisolid consistency, homogenous and have pleasant fruity aroma.
pH determination
The pH of the formulation influences the taste and stability of oral jellies. The pH of the prepared formulations was found in the range of 3.0 ± 1 to 3.5 ± 0.1 at 25 ± 5ºC which was acidic.
Syneresis
Due to the low concentration of gelling agent, there will be more chances of syneresis in jellies. There was observed no syneresis in all jellies after 24 hrs.
Determination of taste and palatability
The jellies were very sweet and in start and sweetness decreased gradually but were palatable till end.
Determination of stickiness and grittiness
The stickiness and grittiness of the prepared Doxycycline HCl jelly were observed that showed the all jellies have no stickiness and grittiness.
Weight Variation Test
Weight variation result of medicated jellies of was found between 3.72 ± 0.01 g and 4.01 ± 0.01 g.
Stability studies
A physically stable medicated oral jelly should retain its viscosity, color, clarity, taste, and odor throughout its shelflife. The stability studies were carried out on formulations at 0ºC, 25ºC and 40ºC and the observations revealed no significant changes in physical characteristics of optimized jelly formulations of Doxycycline HCl during stability studies.
In vitro drug release study
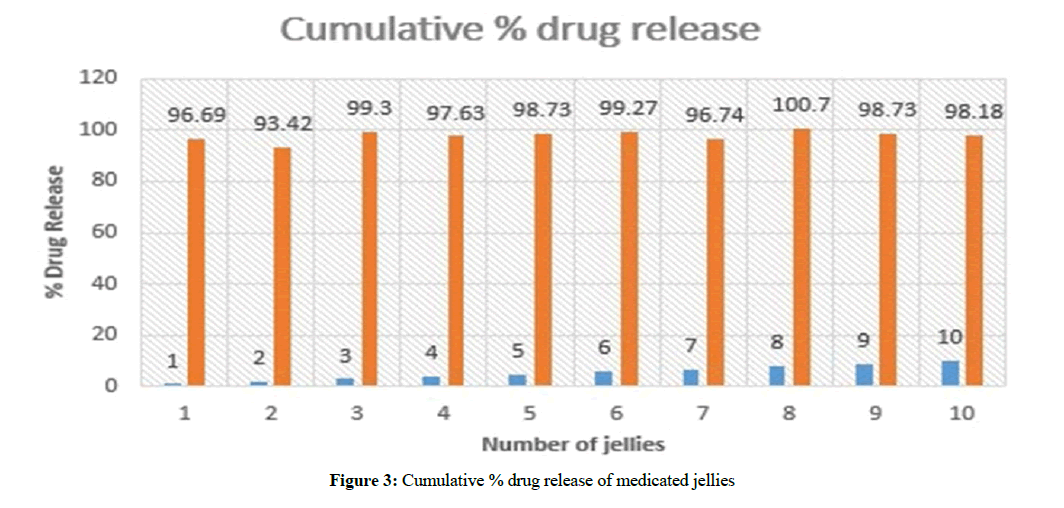
All samples of medicated jellies from JI-J10 were analyzed by the UV-spectrophotometer at 272 nm to determine the drug concentration and amount of drug released. It was observed that J3 showed 99.3% drug released within 18 min as shown in Table 3 and cumulative % drug release graph was drawn as shown in Figure 3. J3 batch was considered best formulation and further in vivo studies can be carried out.
| S. No. | %Drug released from 0-3 min (mg) |
%Drug released from 3-6 min (mg) |
%Drug release from 6-9 min (mg) |
%Drug release from 9-12 min (mg) |
%Drug release from 12-15 min (mg) |
%Drug release from 15-18 min (mg) |
Total %drug release over time (mg) | Average Percentage drug release (%)=Sum of all percentages/No. of percentages |
|---|---|---|---|---|---|---|---|---|
| J1 | 74.1 | 15.8 | 2.30 | 1.64 | 1.49 | 1.36 | 96.69 | 97.93 % |
| J2 | 72.2 | 12.4 | 2.52 | 2.30 | 2.11 | 1.99 | 93.42 | |
| J3 | 78.7 | 13.1 | 2.31 | 2.30 | 1.50 | 1.39 | 99.3 | |
| J4 | 75.04 | 15.8 | 2.30 | 1.64 | 1.49 | 1.36 | 97.63 | |
| J5 | 70.68 | 21.7 | 1.92 | 1.77 | 1.52 | 1.14 | 98.73 | |
| J6 | 72.55 | 20.21 | 1.92 | 1.80 | 1.61 | 1.18 | 99.27 | |
| J7 | 71.93 | 19.2 | 1.52 | 1.46 | 1.36 | 1.27 | 96.74 | |
| J8 | 73.8 | 19.9 | 1.95 | 1.87 | 1.67 | 1.52 | 100.7 | |
| J9 | 70.68 | 21.7 | 1.92 | 1.77 | 1.52 | 1.14 | 98.73 | |
| J10 | 72.54 | 19.5 | 1.77 | 1.67 | 1.52 | 1.18 | 98.18 |
Table 3: Drug release of medicated jellies
Kinetic drug release from medicated oral jelly
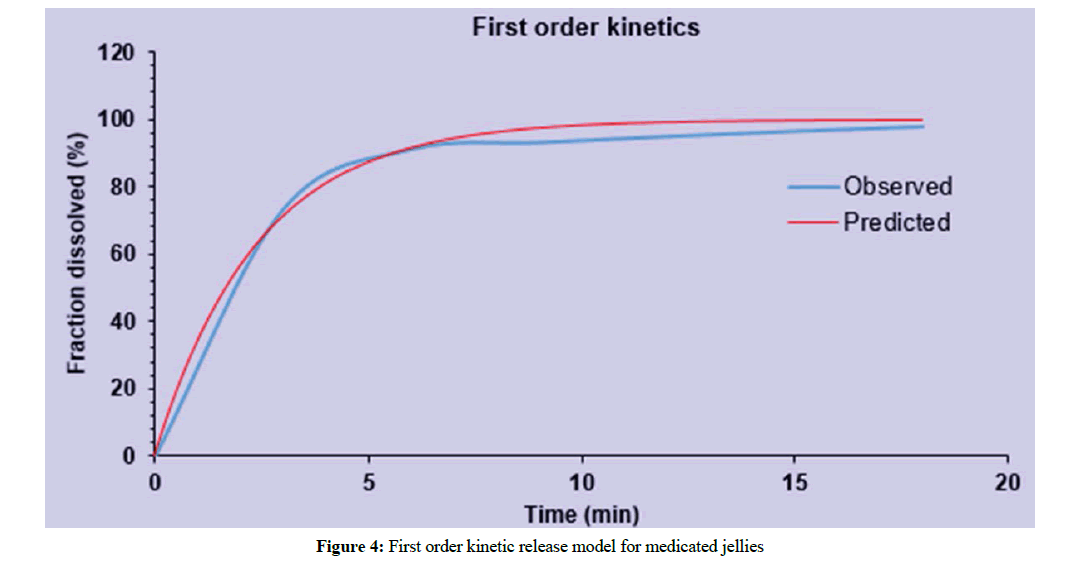
Data from drug release kinetics showed that the drug release from medicated jelly J3 has followed the first order release kinetic model i.e., the drug released was increased by incorporating in the form of complex as shown in Figure 4. The most suitable fit model that best fits the release data was evaluated by value of regression co-efficient (R2).
Figure 4: First order kinetic release model for medicated jellies
Conclusion
Medicated oral jelly containing Doxycycline HCl was successfully prepared by making its complex with β-cyclodextrin. Doxycycline HCl is absorbed from buccal mucosa as well as from GIT. The chewing has shown drug release of greater than 90%. Doxycycline HCl is bitter in taste and this bitter taste was masked in our formulation. Thus it can be said that oral medicated jelly of Doxycycline HCl was made successfully. These formulations if tested for in vivo studies will gain importance especially for children and elderly people who have bacterial infections.
Acknowledgement
The authors hereby acknowledge the lab facilities provided by the Chairman, Department of Pharmacy, Bahauddin Zakariya University, Multan.
Conflict of Interst
The authors declare that there are no potential conflicts of interest associated with this study.
References
- Malhotra, M., et al., Drug Delivery System, 2014. 1141(2): p. 233-247.
- Natarajan, R., Prabhu, C., Rajendran, N., International Journal of Research in Pharmacy and Chemistry, 2014. 4(2): p. 479-483.
- Surana, A.S., International Journal of Pharmaceutical Sciences Review and Research, 2010. 4(2): p. 68-71.
- https://patentimages.storage.googleapis.com/pdfs/US5932235.pdf
- Dosani, M.A., et al., International Journal of PharmaTech Research, 2011. 3(3): p. 1705-1713.
- Archer, J.S, Archer, D.F., Journal of the American Academy of Dermatology, 2002. 46(6): p. 917-923.
- Tan, K.R., et al., The American Journal of Tropical Medicine and Hygiene, 2011. 84(4): p. 517-531.
- Santos, M., et al., Journal of Veterinary Pharmacology and Therapeutics, 1996. 19(4): p. 274-280.
- Ali, N., Harikumar, S., Kaur, A., International Research Journal of Pharmacy, 2012. 3(4450): p. 11.
- Del Valle, E.M., Process Biochemistry, 2004. 39(9): p. 1033-1046.
- Salunke, T., Mayee, R., International Journal of Pharmaceutical Innovations , 2013. 3(5): p. 1-14.
- Mohapatra, A., Parikh, R.K., Gohel, MC., Asian Journal of Pharmaceutics, 2014. 2(3): p. 1-9.
- Philip, A.K. Pathak, K., AAPS, 2006. 7(3): p. E1-E11.
- Squier, C.A., Cox, P., Wertz, PW., Journal of Investigative Dermatology, 1991. 96(1): p. 123-126.
- van Ruth, S.M., et al., Journal of Agricultural and Food Chemistry, 2001. 49(5): p. 2409-2413.
- Shojaei, A.H., Journal Pharmacy & Pharmaceutical Sciences, 1998. 1(1): p. 15-30.
- Inoue, Y., et al., Indian Journal of Pharmaceutical Sciences, 2013. 75(4): p. 435.
- Covington, A., Bates, R., Durst, R., Pure and Applied Chemistry, 1985. 57(3): p. 531-542.
- Beeram, V.V.R., Indian Journal of Pharmaceutical Sciences, 2010. 78(2): p. 68-73.
- Suda, N., et al., Journal of Pharmaceutical Health Care and Sciences, 2005. 31(3): p. 355-359.
- Prakash, K., et al., Asian Journal of Pharmaceutics, 2014.7(3): p. 241-247.
- Mohapatra, A., Parikh, R., Gohel, M., Asian Journal of Pharmaceutics, 2008. 2(3): p. 172-177.
- Galey, W.R., Lonsdale, H., Nacht, S., Journal of Investigative Dermatology, 1976. 67(6): p. 713-717.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences