ISSN : 0976 - 8688
Der Pharmacia Sinica
Formulation and Evaluation of Nystatin Vaginal Tablet
Packiaraj JM*, Venkateswaran CS, Mohamed PS, Janakiraman K
Department of Pharmacy, Annamalai University, Annamalai Nagar, 608002, Chidambaram, Tamil Nadu, India
Abstract
The objective is to formulate vaginal tablet of Nystatin and evaluate the in vitro antimycotic effectiveness against the marketed product in Candida albicans. Nystatin is an antimycotic polyene antibiotic obtained from Streptomyces noursei. Vaginal tablet of Nystatin were formulated with 100,000 Units of Nystatin. The tablets were made with Ethyl Cellulose, 4 cps as polymer, Pregelatinized Starch (Starch 1500) as binder, PEG 8000 as wetting agent, Co-processed mixture of Lactose–Microcrystalline Cellulose (Cellactose) as diluent and Stearic Acid (Stellipress 1200 Poudre) as lubricant. The optimized formulation was subjected to stability studies at accelerated condition. Test and marketed formulations were evaluated for appearance, weight variation, thickness, hardness, friability, drug content and in vitro drug release. In vitro studies on anti mycotic evaluation were carried out for the optimized formulation in three sets of medium cultured with Candida albicans and the zone of inhibition were compared with the marketed one.
Keywords
Nystatin, USP, Vaginal tablet, Direct blending, Compression processd
Introduction
Nystatin was discovered in the New York State Health Laboratory is a antimycotic polyene antibiotic obtained from Streptomyces noursei. It is structurally similar to amphotericin B and has the same mechanism of action [1-3].
Nystatin is 20-(4-amino-3,5-dihydroxy-6-methyl-oxan-2-yl)oxy-4,22,24,28,29,32,34,36-octahydroxy-2,3,5-trimethyl- 26,38-dioxo-1-oxacyclooctatriaconta-6,8,12,14,16,18-hexaene-23-carboxylicacid. Its molecular formula is C47H75NO17. Nystatin is a yellow to light tan powder. It is freely soluble in dimethylformamide, slightly sparingly soluble in methyl alcohol, n-propyl alcohol and n-butyl alcohol, practically insoluble in alcohol, chloroform, and ether and insoluble in water. Nystatin vaginal tablet, 100,000 Units is available in the market manufactured by Duramed pharmaceutical, Inc., NY, US.
Microbiology
Nystatin is both fungi static and fungicidal in vitro against a wide variety of yeasts and yeast like fungi. Nystatin acts by binding to sterols in the cell membrane of sensitive fungi with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin exhibits no appreciable activity against bacteria, protozoa, trichomonas or viruses. Nystatin is not absorbed from intact skin or mucous membranes [1,2,4].
Chemistry and stability
Nystatin is an antifungal antibiotic produced by Streptomyces noursei. The drug is an amphoteric polyene macrolide that occurs as a hygroscopic, yellow to light tan powder with a cereal-like odour and is very slightly soluble in water and slightly to sparingly soluble in alcohol. Each mg of Nystatin contains not less than 4400 units of activity [5,6]. Nystatin deteriorates on exposure to heat, light, moisture, or air. Nystatin oral suspension and tablets should be stored in tight, light-resistant containers at room temperature (e.g. 15-30°C); exposure of the tablets to temperatures exceeding 40°C and freezing of the oral suspension should be avoided. Nystatin powder should be stored in tight, light-resistant containers and refrigerated at 2-8°C. Since extemporaneously prepared oral suspensions of Nystatin do not contain a preservative, such suspensions should be used immediately after preparation and should not be stored [7-9].
Indications and usage
The Nystatin vaginal tablet is effective for the local treatment of vulvovaginal candidiasis (moniliasis). The diagnosis should be confirmed, prior to therapy, by KOH smears and/or cultures. Other pathogens commonly associated with vulvovaginitis (Trichomonas and Haemophilus vaginalis) do not respond to Nystatin and should be ruled out by appropriate laboratory methods [4,8].
Dosage and administration
The usual dosage is one tablet (100,000 units Nystatin) daily for two weeks. The tablet should be deposited high in the vagina by means of the applicator [1,8].
Materials and Methods
Materials
Nystatin was obtained from Shreeji Pharma International. Ethyl Cellulose (Ethocel 4 cps) and Pregelatinized Starch (Starch 1500 LM) was obtained from Colorcon. Polyethylene Glycol 8000 (PEG 8000) was obtained from Dow. Coprocessed mixture of Lactose & Microcrystalline Cellulose (Cellactose 80) was obtained from Meggle. Stearic Acid (Stellipress 1200 Poudre) was obtained from Stearinerie Dubois.
Drug-excipient compatibility study
The active ingredients and excipients were mixed in appropriate ratio and mixed well in a polybag and it is passed through 40 ASTM Sieve and then taken in 2 ml glass vials [10]. Then these vials are kept at room temperature (control), 40°C ± 2°C/75 ± 5% RH and at 55°C. The samples were withdrawn at 2nd week for 55°C and at 4th week for 40°C ± 2°C/75 ± 5% RH and analysed for Appearance (Table 1), Fourier Transform Infrared Spectroscopy (Table 2), Assay (Table 3), and Moisture Content (Table 4).
| Physical Observation | |||||
|---|---|---|---|---|---|
| Pack: Glass Vials | |||||
| Ingredient | Appearance | ||||
| Title | D-E Ratio | Initial | 55°C 2nd Week |
40°C/75%RH 4th Week | |
| Nystatin | A | 1 | Yellow to light tan powder | Yellow to light tan powder | Yellow to light tan powder |
| Nystatin+Ethocel 4 cps | B | 1:25 | Yellow to light tan powder | Yellow to light tan powder | Yellow to light tan powder |
| Nystatin+Starch 1500 LM | C | 1:50 | Yellow to light tan powder | Yellow to light tan powder | Yellow to light tan powder |
| Nystatin+Polyethylene Glycol 8000 | D | 1:25 | Yellow to light tan powder | Yellow to light tan Semi solid mass | Yellow to light tan Semi solid mass |
| Nystatin+Cellactose 80 | E | 1:25 | Yellow to light tan powder | Yellow to light tan powder | Yellow to light tan powder |
| Nystatin+Stellipress 1200 Poudre | F | 1:1 | Yellow to light tan powder | Yellow to light tanSemi solid mass | Yellow to light tanSemi solid mass |
Table 1: Physical observation of drug-excipient compatibility study.
| S.No | Particulars | 55°C - 2nd Week |
|---|---|---|
| 1 | Nystatin | 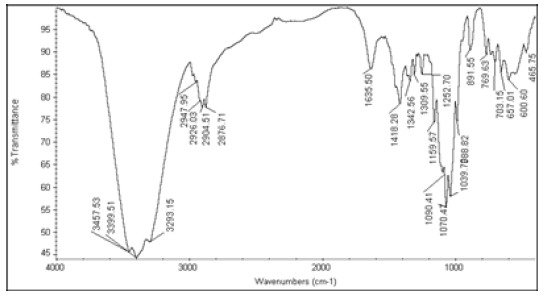 |
| 2 | Nystatin+ Ethocel 4 cps |
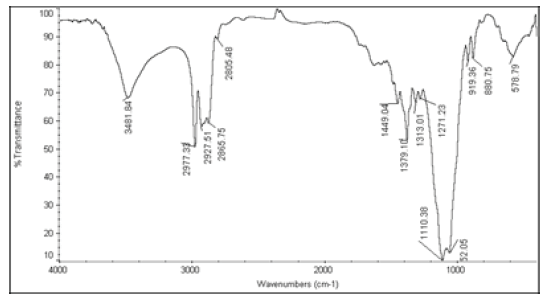 |
| 3 | Nystatin Starch 1500 LM |
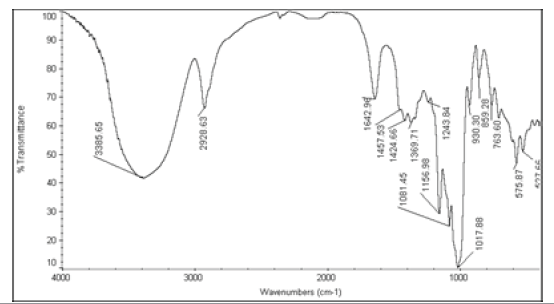 |
| 4 | Nystatin+Polyethylene glycol 8000 | 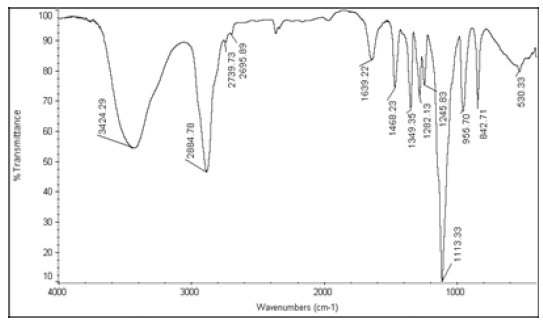 |
| 5 | Nystatin+Cellactose 80 | 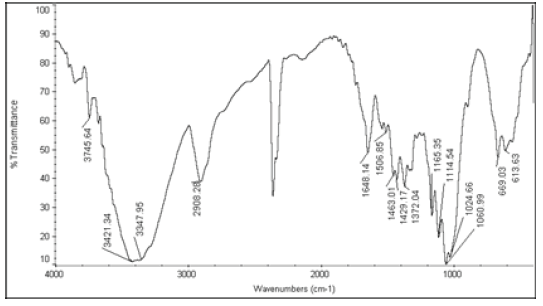 |
| 6 | Nystatin+Stellipress 1200 Poudre | 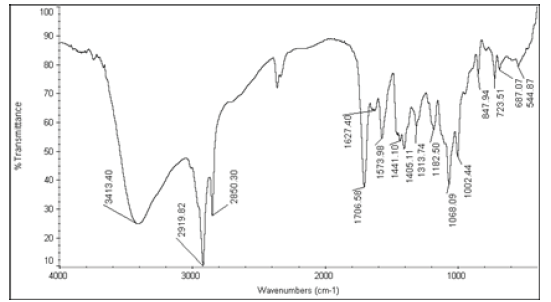 |
Table 2: FTIR interpretation graphs of 55°C-2nd week
| Assay (% Drug Content) | |||||
|---|---|---|---|---|---|
| Pack: Glass Vials | |||||
| Ingredient | Appearance | ||||
| Title | D-E Ratio | Initial | 55°C 2nd Week |
40°C/75%RH 4th Week |
|
| Nystatin | A | 1 | 98.5 | 96.8 | 97.2 |
| Nystatin+Ethocel 4 cps | B | 1:25 | 99.2 | 95.1 | 96.3 |
| Nystatin+Starch 1500 LM | C | 1:50 | 98.5 | 97.9 | 98.1 |
| Nystatin+Polyethylene Glycol 8000 | D | 1:25 | 99.5 | 93.1 | 94.3 |
| Nystatin+Cellactose 80 | E | 1:25 | 99.5 | 96.2 | 97.4 |
| Nystatin+Stellipress 1200 Poudre | F | 1:1 | 98.7 | 92.8 | 96.8 |
Table 3: Assay (% drug content) of drug-excipient compatibility study
| Moisture Content (% w/w) | |||||
|---|---|---|---|---|---|
| Pack: Glass Vials | |||||
| Ingredient | Appearance | ||||
| Title | D-E Ratio | Initial | 55°C 2nd Week |
40°C/75%RH 4th Week |
|
| Nystatin | A | 1 | 4.97 | 4.63 | 4.92 |
| Nystatin+Ethocel 4 cps | B | 1:25 | 3.95 | 2.97 | 3.24 |
| Nystatin+Starch 1500 LM | C | 1:50 | 8.57 | 6.98 | 8.27 |
| Nystatin+Polyethylene Glycol 8000 | D | 1:25 | 3.12 | 2.57 | 3.07 |
| Nystatin+Cellactose 80 | E | 1:25 | 4.26 | 3.56 | 4.09 |
| Nystatin+Stellipress 1200 Poudre | F | 1:1 | 3.12 | 2.25 | 2.98 |
Note: Moisture content by loss on drying at 105°C–15 min auto mode in IR moisture balance
Table 4: Moisture content (% w/w) of drug-excipient compatibility study
Prerequisite design attributes
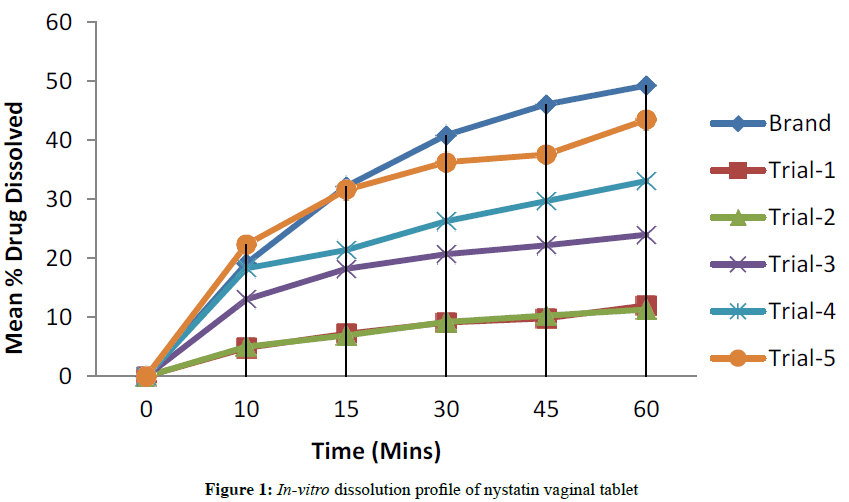
Nystatin Vaginal Tablet to be prepared will be designed to be similar to that of reference product; Excipients shall be similar to that of brand listed excipients. Tablet dimension is 25.53 mm × 12.75 mm oval shaped tablets. Tablet weight is 1100 mg. Disintegration Time about 5 min. Hardness is about 13 kP. % Friability NMT 1.0%. Dissolution profile should be comparable with the profile of reference product when tested in pH 4.5 acetate buffer (pH of Vaginal Fluid) Stability profile should have a comparable stability with that of reference product at accelerated stability condition (40°C/75%RH) for 12 weeks (Figure 1).
Formulations
Tablets were made by direct blending and compression process [4]. Formulation details are shown in (Table 5). All the lists of ingredients were weighed accurately (Except Lubricant) and passed through # 20 ASTM sieve. Sifted materials were blended for 10 min in a Double Cone Blender at 15 RPM. The blended materials were again sifted through #30 ASTM sieve. The sifted materials were again blended for 15 min in a Double Cone Blender at 15 RPM. The lubricant is sifted through # 60 ASTM sieve and added into the above blended materials, and blended for 5 min at 15 RPM. Compression was done in a Cadmach 16 station tablet press using ‘D’ type, 25.53 mm × 12.74 mm oval shaped flatfaced, bevel-edged punch tooling.
| Ingredients | Trial 1 | Trial 2 | Trial 3 | Trial 4 | Trial 5 | |
|---|---|---|---|---|---|---|
| mg/tab | ||||||
| Nystatin | 15.58 | 15.58 | 15.58 | 15.58 | 15.580 | |
| Ethocel 4 cps | 24.00 | 24.00 | 12.00 | 6.00 | 3.00 | |
| Starch 1500 LM | 82.50 | 82.50 | 82.50 | 123.75 | 165.000 | |
| Polyethylene Glycol 8000 | 15.50 | 15.50 | 15.50 | 15.50 | 15.500 | |
| Cellactose 80 | 956.92 | 951.42 | 963.43 | 928.17 | 889.92 | |
| Stellipress 1200 Poudre | 5.50 | 11.00 | 11.00 | 11.00 | 11.000 | |
| Total Tablet Weight | 1100.00 | 1100.00 | 1100.00 | 1100.00 | 1100.000 | |
| Final Blend Characteristics | ||||||
| Bulk Density (g/ml) | 0.69 | 0.66 | 0.74 | 0.75 | 0.71 | |
| Tapped Density (g/ml) | 1.01 | 0.99 | 0.98 | 1.02 | 1.03 | |
| Compressibility Index (%) | 31.7 | 33.3 | 24.5 | 26.5 | 31.1 | |
| Hausner Ratio | 1.46 | 1.50 | 1.32 | 1.36 | 1.45 | |
| Angle of Repose (°) | 30 | 30 | 31 | 33 | 29 | |
| Loss On Drying (% w/w) | 1.66 | 1.72 | 2.12 | 2.57 | 2.55 | |
| Blend Uniformity (% Assay) in Final blend (10 no’s) (2.0 cc die used twice) |
Avg: 96.20 Min: 91.20 Max: 101.20 %RSD: 5.20 |
Avg: 94.30 Min: 90.90 Max: 97.60 %RSD: 3.55 |
Avg: 99.20 Min: 97.20 Max: 101.20 %RSD: 2.02 |
Avg: 97.95 Min: 95.20 Max: 100.70 %RSD: 2.81 |
Avg: 98.05 Min: 95.80 Max: 100.30 %RSD: 2.29 |
|
| Tablet Characteristics | ||||||
| Weight (mg) | 1089-1103 | 1102-1118 | 1095-1111 | 1093-1115 | 1098-1108 | |
| Thickness (mm) | 3.72-3.83 | 3.71-3.80 | 3.68-3.76 | 3.77-3.83 | 3.74-3.80 | |
| Hardness (kP) | 13.2-17.1 | 12.8-15.7 | 12.5-17.4 | 11.7-14.8 | 13.2-16.8 | |
| Friability (%) | 0.23 | 0.35 | 0.29 | 0.42 | 0.19 | |
| Disintegration Time (mins) | 15.10-18.40 | 16.20-17.45 | 11.32-13.11 | 8.12-9.41 | 3.45-5.12 | |
| pH of 1% Tablet Slurry | 6.25 | 6.13 | 6.47 | 6.32 | 6.38 | |
| Loss On Drying (% w/w) | 1.88 | 1.83 | 2.30 | 2.71 | 2.69 | |
| Remarks | Picking, Sticking and Striation Observed. DT to be reduced. | No Tablet defects observed. DT to be improved. | No Tablet defects observed. DT to be improved. | No Tablet defects observed. DT to be improved. | No Tablet defects observed. DT is satisfactory. | |
| Uniformity of Dosage Units (% Assay) Composite Sample (10 no’s) |
Avg: 96.00 Min: 91.60 Max: 100.40 %RSD: 4.58 |
Avg: 95.35 Min: 93.80 Max: 96.90 %RSD: 1.63 |
Avg: 97.90 Min: 96.10 Max: 99.70 %RSD: 1.84 |
Avg: 98.10 Min: 95.90 Max: 100.30 %RSD: 2.24 |
Avg: 98.20 Min: 96.50 Max: 99.90 %RSD: 1.73 |
|
| Dissolution Apparatus-II (Paddle), 50 RPM, 900 ml, pH 4.5 Acetate Buffer |
Time(min) | % Drug Dissolved (Mean of n=6 Units) | ||||
| 10 | 4.8 | 5.0 | 13.0 | 18.3 | 22.3 | |
| 15 | 7.2 | 6.9 | 18.2 | 21.4 | 31.6 | |
| 30 | 9.1 | 9.2 | 20.7 | 26.3 | 36.3 | |
| 45 | 9.8 | 10.3 | 22.2 | 29.7 | 37.6 | |
| 60 | 12.0 | 11.3 | 24.0 | 33.1 | 43.5 | |
Table 5: Formulation development and evaluation of nystatin vaginal tablet
Evaluation
The tablets were evaluated for different physicochemical parameters such as appearance, weight variation, thickness, hardness, friability, drug content and in vitro release [10,11]. In vitro release was studied using Apparatus-II (Paddle) dissolution test apparatus in pH 4.5 Acetate buffer for a period of 60 min. HPLC method was used as the method of analysis [11,12].
Stability studies
The finalized formulation (Trial 5) was loaded for stability in Alu-Alu blister packs at accelerated condition (40°C/75%RH for 12 weeks). The results are shown in Table 6; the stability results show the stability of Nystatin in Nystatin Vaginal Tablet. The results obtained for each tests is well within the prescribed and predefined in-house limits [7,9].
| S.No. | Particulars | 40°C/75% RH 4th Week |
|---|---|---|
| 1 | Nystatin | 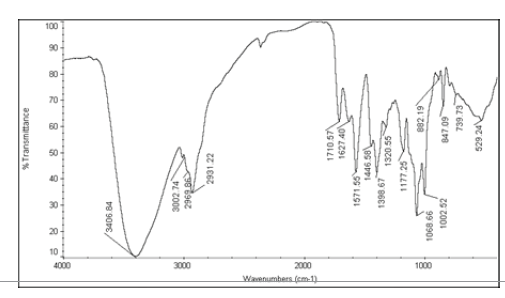 |
| 2 | Nystatin+Ethocel 4 cps | 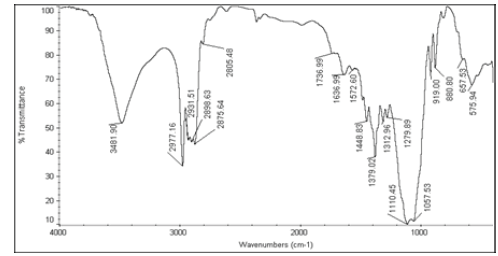 |
| 3 | Nystatin+Starch 1500 LM | 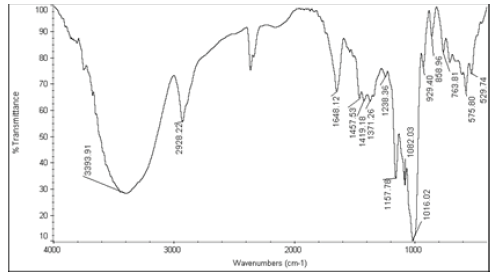 |
| 4 | Nystatin+Polyethylene glycol 8000 | 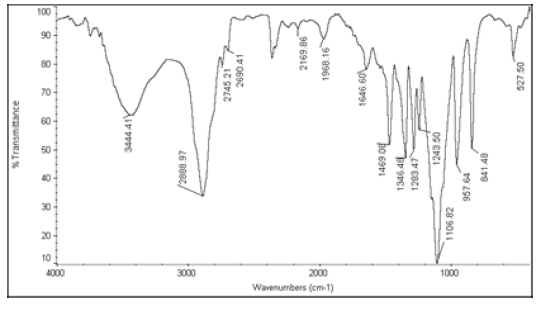 |
| 5 | Nystatin+Cellactose 80 | 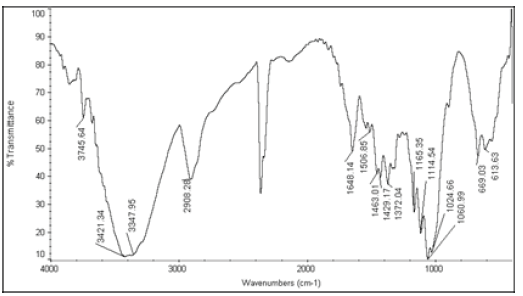 |
| 6 | Nystatin+Stellipress 1200 Poudre | 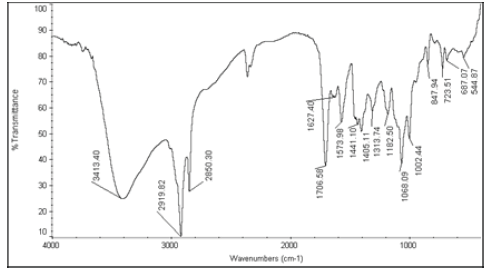 |
Table 6: FTIR interpretation graphs of 40°C/75% RH 4th week
Anti-mycotic effectiveness study Procedure
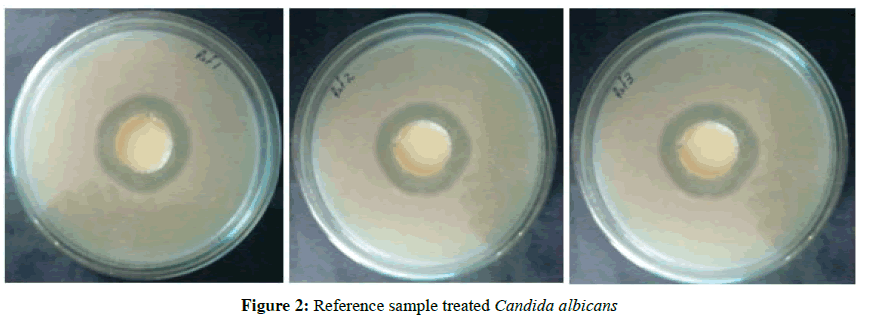
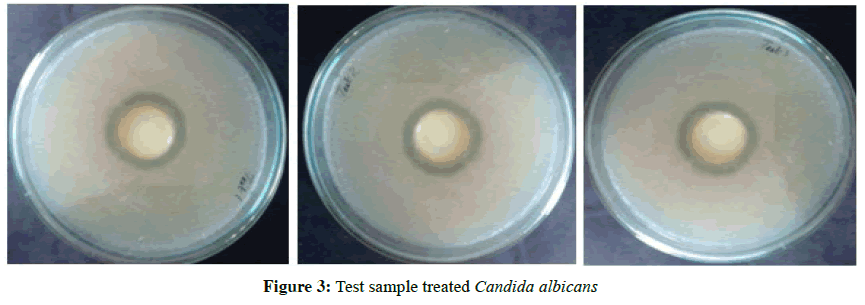
Test organism was spread on the test plates- Muller Hinton Agar (MHA). Sterile wells were made with the help of a borer with 30 mm diameter-with the test and reference samples. Samples were prepared in Stock with DMSO. The test plates were incubated for 48 h. The zone of inhibition (in mm diameter) were read and taken as the activity of the sample against the test organisms (Table 7). Strain-Candida albicans (MTCC 227) (Figures 2 and 3).
| S.No. | Culture | Well (diameter–mm) |
Sample Content (ml) |
Test (zone–mm) |
Reference (zone–mm) |
|---|---|---|---|---|---|
| 1 | Candida albicans | 30 | 10 | 49 | 52 |
| 2 | Candida albicans | 30 | 10 | 49 | 52 |
| 3 | Candida albicans | 30 | 10 | 48 | 53 |
| Average | 49 | 52 | |||
| % Activity of Test with respect to Reference (Avg T/ Avg R) × 100 | 94% | ||||
Table 7: Zone of inhibition for reference and test sample treated with Candida albicans
Results and Discussion
Drug Excipient compatibility data shown in Tables 3 and 4 suggests that both the temperature and moisture doesn’t affect the stability of mixture indicating compatibility of drug with Excipients studied. All the five Trial blend flow is good. Drug distribution in the blend and in tablets is satisfactory [13,14]. Able to achieve hardness up to 16 kP to 17 kP. Tablet defects of trial-1 (Picking, Sticking and Striation) observed during compression and trial 2, 3, 4, 5 (Picking, Sticking and Striation) was not observed during compression. Disintegration time needs to be reduced and dissolution profile needs to be improved in Trial 1, 2, 3, 4. The Trial-5 Disintegration Time is similar to that of marketed product. Dissolution profile is comparable with that of marketed product (Table 8).
| S.No. | Tests | Specification | Initial | 40°C/75%RH | |
|---|---|---|---|---|---|
| 4th Week | 12th Week | ||||
| 1 | Description | Pale yellow mottled oval shaped, flat face bevel edged tablet | Complies | Complies | Complies |
| 2 | Assay (%) | NLT 90.0% to NMT 110.0% of labeled amount of Nystatin | 98.2 | 96.4 | 94.9 |
| 3 | Related Substances (%) | NLT 85.0% of Nystatin A1 is found. NMT 4.0% of any other individual compound. |
Nys A1–92.8 UI–0.78 |
Nys A1–91.5 UI–1.12 |
Nys A1–90.9 UI–1.54 |
| 4 | Loss On Drying (% w/w) |
NMT 5.0% | 2.69 | 2.51 | 2.57 |
| 5 | Disintegration Time (min) | NMT 15 min | 4 min 30 s | 5 min 41 s | 6 min 18 s |
| 6 | *Dissolution (% Drug Dissolved) | NLT 35% (Q) at the end of 60 min. |
43.5 (S1) |
44.1 (S1) |
43.8 (S1) |
| *Apparatus-II (Paddle) 50 RPM 900 ml pH 4.5 Acetate Buffer Nys A1–Nystatin A1,UI–Unknown Impurity |
|||||
Table 8: Stability studies of finalized formulation (Trial-5)
Conclusion
The marketed product of Nystatin Vaginal tablet (Manufactured by Duramed Inc., USA) was characterized for Dimension, Weight, Thickness, Hardness, Disintegration Time, Dissolution, pH of Tablet Slurry and Loss on Drying. A systematic Drug-Excipient compatibility study was done by packing the samples in Glass vials and exposed at 55°C–2 weeks and 40°C/75% RH–4 weeks and the exposed samples were characterized for Morphological Change (Physical Observation), Assay, Related Substances, Moisture Content and FTIR Finger Printing with respect to Initial samples (Table 9). This study was done foremost before taking up the prototype formulation activity [15,16]. Intra-vaginal tablet of Nystatin, 100,000 units was prepared by a simple direct blending manufacturing process which provided the necessary uniform distribution of drug in the blend for tableting and comparable disintegration time and dissolution profile to that of marketed product was achieved. Physical characteristics of the prepared vaginal tablet like Weight, Thickness, Hardness, Friability, Disintegration Time, Loss On Drying and pH of 1% Tablet Slurry were determined and were comparable to that of marketed product. Dissolution test was conducted for both test and reference products in Apparatus-II (Paddle), 50 RPM, 900 ml, pH 4.5 Acetate Buffer, Time Points: 10, 15, 30, 45 and 60 min; and the results were comparable, based on which an In-house single point dissolution limit was fixed as NLT 35% (Q) at the end of 60 min. The finalized Nystatin Vaginal Tablet were packed in Alu-Alu blister packs and charged for stability at accelerated condition (40°C/75%RH for 12 Weeks) and was characterized for Description, Assay, Related Substances, Loss On Drying, Disintegration Time and Dissolution. The results were comparable with the initial values showing the stability of Nystatin in prepared vaginal tablet. Anti-mycotic effectiveness of the prepared Nystatin Vaginal tablet against the marketed product (in triplicate) was done in the cultures of Candida albicans (Outsourced and monitored the activity in Biozone Research Technologies based at Chennai) The prepared Nystatin Vaginal Tablet showed 94% anti-mycotic activity with respect to the marked product.
| FTIR Interpretation | |||||
|---|---|---|---|---|---|
| Pack: Glass Vials | |||||
| Ingredient | Finger Print Comparisons | ||||
| Title | D-E Ratio | Initial | 55°C 2nd Week |
40°C/75%RH 4th Week |
|
| Nystatin | A | 1 | Typical Wave Numbers (cm-1) of Nystatin includes, 3400, 2930, 1710, 1627, 1570, 1446, 1398, 1320, 1177, 1068, 1002, 847, 739 and 529 | ||
| Nystatin+Ethocel 4 cps | B | 1:25 | Complies | Complies | Complies |
| Nystatin+Starch 1500 LM | C | 1:50 | Complies | Complies | Complies |
| Nystatin+Polyethylene Glycol 8000 | D | 1:25 | Complies | Complies | Complies |
| Nystatin+Cellactose 80 | E | 1:25 | Complies | Complies | Complies |
| Nystatin+Stellipress 1200 Poudre | F | 1:1 | Complies | Complies | Complies |
Table 9: FT-IR interpretation drug-excipient compatibility study
References
- Aulton, M., Pharmaceutics-The Science of Dosage form Design, 2002.p. 113-138.
- Martin, A., Swarbrick, J.,Cammarata, A.,Physical Pharmacy: Physical Chemical in the Pharmaceutical Science, 2011.p. 352.
- Katzung, B.,Basic and Clinical Pharmacology,2012. 10(48): p. 849-860.
- Craig, C.,Stitzel, R.,Modern Pharmacology with Clinical Applications, 2004.p. 596-605.
- Remington, G., The Science and Practice of Pharmacy, 2000. p. 889.
- https://www.expresspharmaonline.com
- https://www.durmed.com
- https://www.accessdata.fda.gov/scripts/cder/dis solution/index.cfm
- https://www.fda.com
- Herbert, L.,Leon, L.,The Theory and Practice of Industrial Pharmacy,1986.p. 293-344.
- Lawrence, L.,Lazo, B., Parker, K.,The Pharmacological Basis of Therapeutics, 2006.p.1125-1240
- Montvale, N., Physicians Desk References, 2007. 61th Edn.:p. 2478-2505.
- Richard, A.,Lippincott’s lllustrated Reviews Pharmacology, 2000. p. 337-344.
- Rowe, R., Sheskey, P.,Hand Book of Pharmaceutical Excipient, 2006.p. 1-832.
- Beggs, S.,Salinas, E.,Introductions of Clinical Pharmacology, 2002. 7th Edn.: p. 129-137.
- Foye, W.,Lemke, T.,Williams, D.,Principles of Medicinal Chemistry,2008.p. 807-955.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences