Folic acid in the Prevention of Neural Tube Defects
RD Department Laboratori Baldacci SpA Pisa, Italy.
- *Corresponding Author:
- Cecilia Viegi
RD Department Laboratori Baldacci SpA Pisa, Italy.
E-mail: infoeu@baldaccilab.com
Received date: May 07, 2018; Accepted date: May 22, 2018; Published date: May 28, 2018
Citation: Viegi C, Bertini M (2018) Folic acid in the Prevention of Neural Tube Defects. J Birth Defects. 1:5
Abstract
The global analysis of the published studies and reviews confirms the necessity of folic acid supplementation in women at the early stage of conception and until organogenesis during pregnancy, as the folate concentration is a critical factor that plays a fundamental role in the prevention of NTDs. Different strategies have been adopted, from promoting a diet rich in folate or a supplementation with folic acid tablets, which ensure better bioavailability compared with the natural one. Despite worldwide public health campaigns recommending periconceptional daily supplementation of folic acid, many women that are planning a pregnancy don’t follow these recommendations. In addition to that, about 40% of pregnancies are unplanned. Every efforts must be done to enhance the awareness of prevention among health institutions, medical communities and women of childbearing age.
Keywords
Folic acid supplementation; Neural tube defects; Dihydrofolate reductase; Organogenesis
Introduction
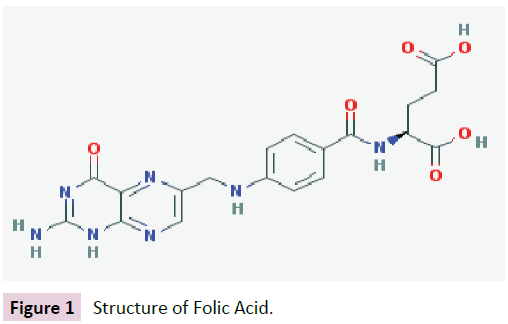
Folic acid or vitamin B9 is a water-soluble vitamin with a chemical structure including an aromatic pteridine ring linked to p-aminobenzoic acid and a glutamate residue, as shown in Figure 1.
The folates, that are polyglutamate derivatives, can be found in nature in leafy green vegetables, from which their name derives (from latinum word “folium”). Folate-rich foods include animal liver, legumes and oranges. Mammals are incapable of de novo folate synthesis, which is why it is essential that folate is obtained through the diet. Because of the high chemical stability of the folic acid instead of its reduced form (folate), folic acid is the suitable product used in supplementation.
Folate plays a critical role in cell division and therefore in embryonic development. Rapidly dividing cells are particularly vulnerable to a lack of folates or B12 vitamin, caused by an insufficient intake, an increased need, altered absorption and metabolism or drugs treatments (e.g, anticonvulsivants). This deficit causes a defective DNA synthesis in dividing cells, particularly evident in bone marrow, with the risk of megaloblastic anemia, and in embryo, with higher risk of congenital defects.
ADME
Folic acid is rapidly absorbed from the gastrointestinal tract, mainly from the duodenum and jejunum. The naturally occurring folate polyglutamates are largely deconjugates, and then reduced by dihydrofolate reductase (DHFR) in the intestines to form 5-methylentetrahydrofolate [1]. The bio-availability of natural folates is stated to be reduced by as much as 25-50 percent with respect to the synthetic folic acid [2,3]. After folate absorption into the portal circulation, it can either be taken up by the liver, which is the principal storage site of folate, or it can be released into blood or bile.
Folate undergoes enterohepatic circulation. Folate metabolites are eliminated mainly in the urine, and in small quantities via biliary excretion [4].
Causes of Folate Deficiency
Folate deficiency commonly results from inadequate dietary supply or lack of intestinal absorption. Several conditions such as hepatic diseases and alcoholism may impair the enterohepatic cycle of folate. Additionally, drugs that inhibit dihydrofolate reductase (e.g., methotrexate) or interfere with the absorption and storage of folate (e.g., anticonvulsivants and oral contraceptives) can reduce the concentration of folate in plasma. Mutations of methionine synthetase and in particular of 5.10-methyltetrahydrofolate reductase (MTHFR) are nowadays considered the most frequent causes of an altered folate metabolism and subsequent hyperhomocysteinemia [1].
Clinical studies have shown that in pregnant women there was an increased demand of folate that led to an average reduction of serum folate [5], due to the growth of the foetus and uteroplacental organs, and probably to an increased catabolism and urinary folate excretion. Whatever the reasons of the decline, probably due to a combination of different mechanisms, it essential that plasma folate be kept above a critical level (>7.0 nmol/L) for the transplacental folate delivery to the fetus.
NTDs and Causes
Neural Tube Defects (NTDs) are severe birth defects of the central nervous system that originate during embryogenesis, between 21 and 28 days following conception, and result from failure of neural tube closure [6]. NDTs cause infant disability or even death, with a mean incidence of 300,000 new cases a year [7]. In the US, 95% of children with NTDs are born to couples with no family history of these defects. By contrast, among couples with a previous history of newborn with a NTD, the recurrence risk is 2%-3% in subsequent pregnancy. This increased risk is explained by genetic defects, mainly the 677CT-MTHFR mutation [5].
From EFSA data [8] the prevalence of NTDs across the EU ranges from about 0.4 to 2 per 1,000 births and an estimated 4,500 pregnancies are affected by NTDs each year. Data by EUROCAT (the European network of population-based registries for the epidemiological surveillance of congenital anomalies) [9] during the period 2004-2008 reported that the total prevalence of NTD was about 1.0 per 1000 births with more than two-thirds of the cases accounted by Termination of Pregnancy for Fetal Anomaly (TOPFA) [10].
In Italy the incidence among survivors is about 0.68 per 1,000 and a fetal incidence is assessed around 1 per 1,000. Anencephaly contributes with approximately 50% of cases, spina bifida with 40% and encephalocele with 10% [11].
The causes of NTDs are multifactorial, the most economically disadvantaged population groups having the highest incidence: they include genetic predisposition (which may be related to the high prevalence in certain populations, e.g., in Ireland), environmental risk factors, life styles, and dietary patterns leading to inadequate and/or unbalanced intake of essential nutrients [12,13].
Folate deficiency or genetically rooted error in folate metabolism can cause developmental defects through disturbances of DNA biosynthesis and/or the methylation cycle. The nervous system becomes evident on post-ovulation day 18, and neural tube closure occurs during days 22-28. Thus, NTDs occur so early that most women are unaware of their pregnancy [14]. Efforts were also made to relate NTD risk to variants of folate-related genes [5,15], or altered folate transfer [16].
Folic Acid Efficacy
Since the 1960s there has been a clear association in the reduction of recurrence of NTD-affected pregnancies through multivitamin supplementation, but a limit was in the non-randomized nature of these investigations. The first large multicenter study by the Medical Research Council of UK [17] terminated early because of overwhelming evidence that folic acid 4 mg daily, taken from before conception till the twelfth week of gestation, by women with a history of past pregnancies affected by NTDs, reduced the incidence of such defects by about two-thirds. Multivitamins alone (A, D, B1, B2, nicotinamide, B6, and C) or non-supplemented groups did not demonstrate a similar benefit. This was followed by other studies supporting the importance of periconceptional folic acid supplementation [18-20].
In particular, the randomised, population-based community intervention study carried out in China [19] suggested that multivitamin supplement containing folic acid (0.4 mg) significantly reduced the risks of NTDs with the beneficial effect dependent on the frequency and timing of the supplementation. Ten intervention trial counties and 8 control counties were selected from the provinces of Henan, Guizhou, Hunan, and Jilin. Participants were current resident women planning a pregnancy who volunteered to participate in the follow up were included in the trial. Women from intervention counties received a supplement containing 400 μg (0.4 mg) folic acid, with other vitamins and elements), while women in the control counties did not receive supplementation. Participants were followed up according to periconceptional supplementation for 2 years. During 2000 and 2002, all of the women having pregnancies with birth defects and women whose pregnancies were without any birth defects were interviewed. Nine NTDs were recorded from 25,444 pregnancies (NTD birth prevalence = 0.35/1,000 pregnancies) in the intervention group and 48 NTDs among 26,599 pregnancies (NTD birth prevalence = 1.80/1,000 pregnancies) in the control group. The protective rate was 80.4%.
Similar results were found in a Chinese-US study [21] exploring the efficacy of this dosage of folic acid and reporting a 79% reduction in the risk of NTDs in areas with high rates of NTDs (6.5 per 1000), while this reduction was 41% in areas with low rates of NTDs (0.8 per 1000).
A recent publication [22] highlighted the results of some observational studies in agreement with the previous randomized studies on the evidence that folic acid-containing supplements could reduce the risk for NTD-affected pregnancies; these results are also supported by a Cochrane review of 5 trials [23] on periconceptional supplementation involving 6708 births: conclusions are that periconceptional folate supplementation substantially reduces the incidence of NTDs compared with no interventions/placebo or vitamins and minerals without folic acid (RR 0.31, 95% confidence interval 0.17 to 0.58). Folic acid also had a significant protective effect for recurrence of these defects.
Folic Acid Safety
Folic acid is well tolerated. No evidence of harm has ever been obtained in trials involving periconceptional supplementation, but only mild side effects like nausea, vomiting, constipation or diarrhoea in all groups of treatment [24]. Folic acid rarely has allergic responses but these may include erythema, rash, itching.
Even though folate is a water-soluble vitamin that shows low toxicity because of the elimination of excess amounts through urine, recently two international expert panels, the U.S. National Toxicology Program and the UK Scientific Advisory Committee on Nutrition, assessed the risks associated with folate overexposure [25].
Overall, the totality of the evidence examined by these panels, as well as studies published since the release of their reports, suggested that there are no established risks related to adverse health effects.
Nevertheless, the two major safety concerns related to folic acid have been hypothesized to be the potential of masking the evert clinical signs of vitamin B12 deficiency at high doses and the increasing risk of cancer associated with long-term use. However large evidence in literature shows that the amount of folic acid used in the prevention of NTDs (0, 4 mg/day) is not associated with the potential of masking the signs of vitamin B12 deficiency [26] and the same conclusions have been reported by a review of Scientific Advisory Committee on Nutrition [27]. As far as pro-carcinogenic potential, a literature review shows no new evidence for increased risk of cancer related to folic acid use before and during pregnancy [28].
Folic Acid Campaign
The global analysis of the published studies confirms the efficacy of folic acid, at the dosage of 0.4 mg/day, in the prevention of NTDs (e.g., spina bifida, anencephaly), in women in the periconceptional period of pregnancy, as the folate concentration during organogenesis is a critical factor: as regards NTDs the first 28 days after conception are very important. As the most common and serious BDs occur between conception and 8th- 12th week of gestation, an effective vitamin supplementation has to assure an adequate maternal folate status at least from 1 month prior conception through the second-third month of pregnancy.
These serious concerns lead many countries to recommend women who might become pregnant to supplement their diet with folic acid. Public health campaigns by countries promote the awareness of this message and supplement intake prior to and for the first weeks after conception.
Campaings differ between countries, although the broad trend is to recommend a healthy diet plus a folic acid supplement of 400 μg/day from preconception until the end of the first trimester of pregnancy (8-12 weeks). The same recommendation is suggested by the WHO [29].
Finally another purpose of these campaigns, in addition to the well-being and prevention of fearsome and rare diseases, is to limit the economic impact of NTDs in terms of health costs [30].
Conclusion
Many countries have set up fortification and supplementation campaigns, but never the less, this is not enough to achieve blood levels sufficient for protection, requiring additional supplementation by consumption of folic acid [31]. Strategies have been diverse, from promoting a diet of foods high in folate (the form of the vitamin in foods) to FA supplement tablets which ensures better bioavailability of the compound compared with that of natural origin.
Although experience has been clearly showed that folic acid may prevent neural tube defects (anencephaly, spina bifida) most women in Europe are still not taking supplements prior to conception.
In addition, about 40% of pregnancies are unplanned, and strategies to increase consumption of the vitamin B complex have failed in most countries. In this context institutions and health professionals recommend the use of folic acid to women prior and during pregnancy, but it is necessary to enhance an awareness of prevention, not only of adverse events during pregnancy, but many aspects of our health. In the case of congenital defects, folic acid makes the difference between a healthy baby and one with disabilities. For this reason, folic acid intake should be a policy similar to the application of vaccines due to the prevention level obtained from these equally disabling and mortal diseases [31].
References
- Greenberg JA, Bell SJ, Guan Y, Yu Y (2011) Folic Acid Supplementation and Pregnancy: More Than Just Neural Tube Defect Prevention. Rev Obstet Gynecol 4: 52-59.
- Gregory J F (1997) Bio-availability of folate. Eur J Clin Nutr 51: 554-559.
- Cuskelly CJ, McNulty H, Scott JM (1996) Effect of increasing dietary folate on red-cell folate: implications for prevention of neural tube defects. Lancet 347: 657-659.
- Toxnet- Toxicology Data Network - Folic Acid- US National Library of Medicine.
- Tamura T, Picciano M (2006) Folate and human reproduction. Am J Clin Nutr 83: 993-1016.
- Mitchell LE (2005) Epidemiology of neural tube defects. Am J Med Genet C Semin Med Genet 15: 88-94.
- Blencowe H, Cousens S, Modell B, Lawn J (2010) Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol 39: 110-121.
- EFSA (European Food Safety Authority) ESCO Report on Analysis of Risks and Benefits of Fortification of Food with Folic Acid. 6 October 2009.
- EUROCAT (European Surveillance of Congenital Anomalies) Special Report: Prevention of Neural Tube Defects by Periconpceptional Folic Acid Supplementation in Europe - December 2009- Part I- Overview.
- Khoshnood B, Greenlees R, Loane M, Dolk H (2011) EUROCAT Project Management Committee; EUROCAT Working Group. Paper 2: EUROCAT public health indicators for congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol 91: 16-22.
- Taruscio D, Carbone P, Granata O, Baldi F, Mantovani A (2011) Folic acid and primary prevention of birth defects. Biofactors 37: 280-284.
- ISS- Rapporto ISTISAN 04/26 (2004) Folic acid: from research to public health practice. Istituto Superiore di Sanita` Press, Rome.
- De Marco P, Merello E, Calevo MG, Mascelli S, Pastorino D, et al. (2011) Maternal periconceptional factors affect the risk of spina bifida-affected pregnancies: an Italian case-control study. Childs Nerv Syst 27: 1073-1081.
- Digra NC (2004) Primary Prevention of Neural Tube Defects. JK Science.
- Van der Put NMJ, van Straaten HWM, Trijbels FJM, Blom HJ (2001) Folate, Homocysteine and Neural Tube Defects: An Overview. Exp Biol Med 226: 243-270.
- Rothenberg SP, da Costa MP, Sequeira JM, Cracco J, Roberts JL, et al. (2004) Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. N Engl J Med 350: 134-142.
- MRC Vitamin Study Research Group (1991) Prevention of neural tube defects: results of the Medical Research Council vitamin study. Lancet 338: 131-137 .
- Werler MM, Shapiro S, Mitchell AA (1993) Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 269: 1257-1261.
- Chen G, Song X, Ji Y, Zhang L, Pei L, et al. (2008) Prevention of NTDs with periconceptional multivitamin supplementation containing folic acid in China. Birth Defects Res A Clin Mol Teratol 82: 592-596.
- Milunsky A, Jick H, Jick SS, Bruell CL, MacLaughlin DS (1989) Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA 262: 2847-2852.
- Berry RJ, Li Z, Erickson JD, Li S, Moore CA, et al. (1999) Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med 341: 1485-1490.
- Wolff T, Witkop CT, Miller T, Syed SB (2009) Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Update of the Evidence for the U.S. Preventive Services Task Force. Ann Intern Med 150: 632-639.
- De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, Rayco-Solon P (2015) Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev 14: CD007950.
- Czeizel AE, Dudás I, Métneki J (1994) Pregnancy outcomes in a randomised controlled trial of periconceptional multivitamin supplementation. Final report. Arch Gynecol Obstet 255: 131-139.
- Field MS, Stover PJ (2017) Safety of folic acid. Ann N Y Acad Sci.
- Mills JL, Dimopoulos A, Bailey RL (2016) What is standing in the way of complete prevention of folate preventable neural tube defects? Birth defects research Part A, Clinical and molecular teratology 106: 517-519.
- SACN- Scientific Advisory Committee on Nutrition- Update on Folic Acid. July 2017.
- Mortensen JH, Oyen N, Fomina T, Melbye M, Tretli S (2015)Supplemental folic acid in pregnancy and maternal cancer risk. Cancer Epidemiol 39: 805-811.
- Gomes S, Lopes C, Pinto E (2016) Folate and folic acid in the periconceptional period: recommendations from official health organizations in thirty-six countries worldwide and WHO. Public Health Nutr 19: 176-189.
- Yi Y, Lindemann M, Colligs A, Snowball C (2011) Economic burden of neural tube defects and impact of prevention with folic acid: a literature review. Eur J Pediatr 170: 1391-1400.
- Martínez-Garza L (2016) Twenty-five years of knowledge of the prevention of neural tube defects with folic acid. Medicina Universitaria 18: 187-188.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences