Focal and Segmental Glomerulosclerosis in a Patient with a Paternal Family History of Nephropathy of Unknown Cause
Rosales Montero Alejandro J1*, Alvarado Gutierrez Raul E1, Tura Rosales David1, Rodriguez Campon Alejandro1, Saus Sarrias Carlos2 and Carrillo Garcia Paula3
1Department of Nephrology, Hospital of Manacor, Balearic Islands, Spain
2Department of Pathological Anatomy and Nephropathology, Son Espases University Hospital (SEUH), Balearic Islands, Spain
3Department of Pathological Anatomy and Nephropathology, Son Llatzer Hospital, Balearic Island, Spain
- *Corresponding Author:
- Rosales Montero Alejandro J
Department of Nephrology,
Hospital of Manacor, Balearic Islands,
Spain,
Email: alejandrorodriguez25998@gmail.com
Received date: January 29, 2024, Manuscript No. IPJRM-24-18589; Editor assigned date: February 03, 2024, PreQC No. IPJRM-24-18589 (PQ); Reviewed date: February 24, 2024, QC No. IPJRM-24-18589; Revised date: March 05, 2024, Manuscript No. IPJRM-24-18589 (R); Published date: March 13, 2024, DOI: 10.36648/IPJRM.7.2.12
Citation: Alejandro RMJ, Raul AGE, David TR, Alejandro RC, Carlos SS, et al. (2024) Focal and Segmental Glomerulosclerosis in a Patient with a Paternal Family History of Nephropathy of Unknown Cause. Jour Ren Med Vol.7 No.2: 12.
Abstract
Focal Segmental Glomerulosclerosis (FSGS) is a disease of unknown cause that can sometimes have a familial presentation. In these cases, a hereditary mechanism has been suggested involved in the etiopathogenesis, caused by a series of genetic mutations in genes that encode proteins expressed mainly in the podocytes and their slit diaphragm and also in other components of the glomerular filtration barrier such as the membrane. One of the causes of this disease is Schimke Immune Osseous Dysplasia (SIOD) is an ultra-rare multisystemic, monogenic and autosomal recessive inherited disease caused by biallelic mutations in the SMARCAL1 gene. Approximately 100 cases have been reported worldwide. The disease is characterized by skeletal, renal and immunological abnormalities.
Keywords
Hereditary focal and segmental glomerulosclerosis; Schimke immuno-osseous dysplasia; SMARCAL1 gen
Introduction
Focal Segmental Glomerulosclerosis (FSGS) is a disease of unknown cause that can sometimes have a familial presentation. In these cases, a hereditary mechanism has been suggested involved in the etiopathogenesis, caused by a series of genetic mutations in genes that encode proteins expressed mainly in the podocytes and their slit diaphragm and also in other components of the glomerular filtration barrier such as the membrane. We present the clinical case of a patient from Romania, who came to our clinic for chronic kidney disease of unknown cause and with a paternal family history of nephropathy. Unknown cause (several members with chronic kidney disease on hemodialysis and others transplanted) in their country of origin and data of FSGS in the kidney biopsy. Given the suspicion of familial FSGS, a genetic study was requested, detecting a mutation (pathogenic variant) in heterozygosity in the SMARCAL1 gene, which encodes a protein member of the Switch/Sucrose Non-Fermenting (SWF/SWF) family necessary for the stability of the cytoskeleton of podocytes and the GBM, related to a rare cause of syndromic familial FSGS within a disease known as Schimke Immuno-Osseous Dysplasia (SIOD). Schimke Immune-Osseous Dysplasia (SIOD) is an ultra-rare multisystemic, monogenic and autosomal recessive inherited disease caused by biallelic mutations in the SMARCAL1 gene. The disease is characterized by skeletal, renal and immunological abnormalities, although there are mild forms of the disease associated with heterozygous pathogenic variants. Although there is evidence of temporary reductions in proteinuria using angiotensin-converting enzyme inhibitors and cyclosporine-A, in a large proportion of cases progression is observed and in cases with nephrotic syndrome, corticosteroid resistance occurs, with evolution to end-stage chronic kidney disease, with the need for renal replacement therapy and/or kidney transplant. Therefore, we conclude that our patient would be expressing a mild phenotype of this disease.
Case Presentation
A 33-year-old male originally from Romania, came to our clinic for a study of proteinuria in the nephrotic range:
• Paternal family history of nephropathy (father, grandfather, a paternal cousin, two paternal aunts and a brother) of unknown cause, some of them needing hemodialysis and others undergoing kidney transplant.
History of previous illnesses and/or habits
• Toxic habits: Former smoker up to 1 year of one pack of cigarettes/day since the age of 15. No alcoholism or consumption of psychoactive substances
• Obesity grade 2
• Rib fibrous dysplasia, in Department of Orthopedic Surgery and Traumatology
• Previous surgical interventions: No
Clinical history and follow-up
Patient under follow-up for 6 years in the Nephrology Department at the Hospital of Burgos (2012 to 2019) for proteinuria in the nephrotic range (8-12 gr/24 hours; Albumin: 6-10 gr/24 hours), without signs of nephrotic syndrome, with normal renal function and imaging studies, without alterations in renal morphology in the ultrasound study. Autoimmunity studies, viral serologies and proteino- gram were negative. A renal biopsy was proposed, which was rejected by the patient at that time. Since 2019, he has been lost to follow-up at his primary care medical center. Since then, he had not continued clinical follow-up until the end of 2020. Who goes to his primary care health center, in the town of Manacor, where he is evaluated by his usual doctor, who requests complete blood tests (hemogram, complete biochemistry and 24-hour urine study) and refers the patient to the Department of Internal Medicine.
It provides results of clinical analyzes in blood and urine, where deterioration of the Glomerular Filtration Rate (GFR) (Cr: 1.54 mg/dl) and proteinuria in the nephrotic range (proteinuria: 7.2 g/24 hours, albuminuria 5.76 g/24 hours) were observed. Alteration of the lipid profile is also observed with hypertriglyceridemia (328 mg/dl) and hyper- cholesterolemia with total cholesterol of 272 mg/dl and LDL (Low-Density Lipoprotein) Cholesterol levels of 157 mg/dl. HDL (High-Density Lipoprotein) cholesterol levels within normal. Red blood cell count, white blood cells, platelets, total proteins, albumin, thyroid function tests and liver function tests (alanine aminotransferase, aspartate amino- transferase, alkaline phosphatase, total bilirubin), screening for autoimmune diseases (ANAs, cANCA, pANCA, anti-dsDNA, anti-Ro/SSA and anti-La/SSB antibodies, rheumatoid factor) and serologies for bacterial, viral and parasitic diseases without alterations without alterations.
The Department of Internal Medicine decided to start treatment with the renin-angiotensin aldosterone system (enalapril), 10 mg every 24 hours and they decided to refer the patient to the Nephrology Department of our hospital. Asymptomatic patient at the time of consultation. During the physical examination, the following constants were recorded. Blood pressure was 165/110 mm Hg, Ambulatory Blood Pressure Monitoring (ABPM), with average morning BP 175/95 and average night BP 160/105. Heart rate was 91 bpm. On physical examination, no alteration was detected. In the initial analysis, renal function deteriorated, with creatinine of 1.44 mg/dl and proteinuria in 24 hour urine of 7.1 g/24 hours, albuminuria 5.76 g/24 hours. Autoimmunity studies, viral serologies and proteinogram were negative. A new renal ultrasonography was requested, with no changes observed in renal morphology.
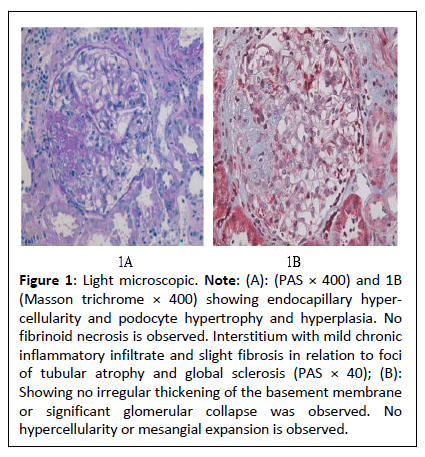
It was decided to perform a renal biopsy as seen in Figure 1 which showed cellular variant Focal Segmental Glomerulosclerosis (FSGS). Hygienic-dietary recommendations were started (salt-free, low-fat diet, with protein restriction of 0.8 gr/kg weight and physical exercise). Due to poor blood pressure control, a thiazide (hydrochlorothiazide), one 25 mg tablet daily and an sGLT2 inhibitor (dapaglifozin), one 10 mg tablet daily are added, dose of enalapril was increased to 10 mg every 12 hours and lipid-lowering treatment is started with a statin (atorvastatin), 1 daily tablet of 40 mg daily and fibrate (fenofibrate), 1 daily tablet of 145 mg, with improvement in blood pressure levels and stabilization of kidney function as shown in Table 1, improvement in proteinuria and lipid profile during follow-up in the first year and a half, except triglycerides, without reaching normality at any time.
Figure 1: Light microscopic. Note: (A): (PAS × 400) and 1B (Masson trichrome × 400) showing endocapillary hypercellularity and podocyte hypertrophy and hyperplasia. No fibrinoid necrosis is observed. Interstitium with mild chronic inflammatory infiltrate and slight fibrosis in relation to foci of tubular atrophy and global sclerosis (PAS × 40); (B): Showing no irregular thickening of the basement membrane or significant glomerular collapse was observed. No hypercellularity or mesangial expansion is observed.
| Laboratory tests | Normal values | Laboratory data of the patient on admission | 12 months after admission | 18 months after admission | 30 months after admission, irregular intake 6 months ago of enalapril, atorvastatin, fenofibrate and dapaglifozin |
|---|---|---|---|---|---|
| Urea (mg/dl) | 13-43 | 52 | 52 | 86 | 127 |
| Creatinine (mg/dl) | 0.7-13 | 1.54 | 1.54 | 2.87 | 4.6 |
| Albumin (g/dl) | 3.5-5.2 | 4.2 | 4.1 | 4.2 | 4.3 |
| Sodium (mEq/l) | 135-145 | 142 | 140 | 141 | 141 |
| Potassium (mEq/l) | 3.5-5 | 4.8 | 4.5 | 5 | 5.6 |
| Calcium (mg/dl) | 8.4-10.2 | 9.8 | 9.1 | 9.6 | 9.5 |
| Phosphorus (mg/dl) | 2.5-4.5 | 3.6 | 3.3 | 3.6 | 5.1 |
| Total proteins (g/dl) | 6.4-8.3 | 6.9 | 6.8 | 6.9 | 6.7 |
| Urine sediment analysis (blood, glucose, RBC WBC, Bacteria) | Glucose: Nill | Glucose: Nill | Glucose: Nill | Glucose: Nill | Glucose: Nill |
| RBC: <5* | RBC: <5 | RBC: <5 | RBC: 39 red blood cells per HPF | RBC: <5 | |
| WBC: <5* | WBC: <5 | WBC: < | WBC: <5 | WBC: <5 | |
| Bacteria: Nill | Bacteria: Nill | Bacteria: Nill | Bacteria:- Nil | Bacteria :- Nill | |
| 24-h urine protein (mg) | <150 | 3370 | 3140 | 5321 | 7429 |
| 24-h urine albumin (mg) | <150 | 2237 | 2673 | 4236 | 5896 |
| Lipid profile (TC, TGs, HDL and LDL cholesterol) mg/dl | TC | 256 | 209 | 169 | 240 |
| TGS | 253 | 282 | 285 | 316 | |
| LDL | 162 | 146 | 95 | 138 | |
| HDL | 43 | 49 | 45 | 48 | |
| Note: HPF: High Power Field; TC: Total Cholesterol; TGs: Triglycerides; HDL: High Density Lipoprotein; LDS: Low Density Lipoprotein. *Per high power field. | |||||
Table 1: Laboratory data of the patient on admission and later during follow-up.
Subsequently, from mid-2022, he lost follow up and came almost a year and a half later, with irregular intake of the pharmacological treatment, as well as not following the hygienic and dietary measures mentioned above, resulting in a progressive decrease in kidney function (Cr: 5.34 mg/dl), increased proteinuria (Proteinuria: 8.41 gr/24 hours; Albuminuria: 6.49 gr/24 hours), referring the patient to advanced chronic kidney disease consultation.
Results and Discussion
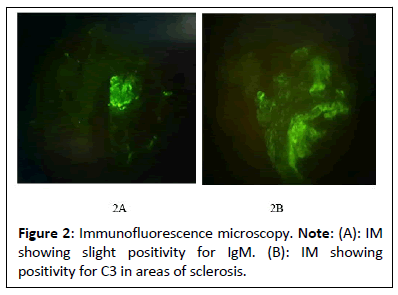
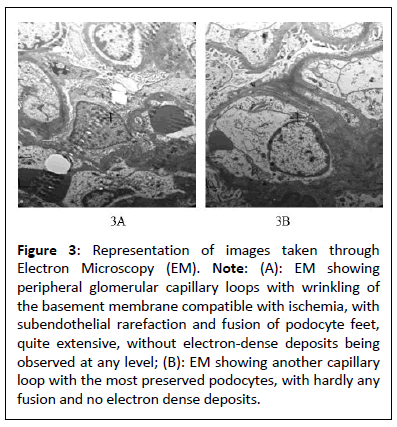
The medical history of this patient, characterized by progressive CKD associated with proteinuria in the nephrotic range without symptoms of nephrotic syndrome secondary to FSGS, which according to the findings found in the renal biopsy (light micrograph, direct immunofluorescence and electron microscopy), corresponds to FSGS variant cellular as shown in Figures 2 and 3. Despite the analytical abnormalities, the patient was asymptomatic at the time of diagnosis. With a family history of nephropathy in practically the entire paternal line with some relatives on hemodialysis and others with kidney transplants, it was decided to request a genetic study, performing an analysis of the genes associated with hereditary nephropathies (directed exome analysis), detecting a pathogenic variant in heterozygosis in the SMARCAL1 gene, which consists of a change in heterozygosity that consists of a transversion of a G for a T (c. 2542G>T) (HGVS: Human Genome Variation Society) that at the protein level produces, presumably, the change of glutamic acid at position 848 by a premature stop codon (p.E848*)(Protein change). There are at least 100 described mutations of the SMARCAL1 gene, of which the majority are described as nonsense/missense mutations, splice substitutions, small deletions, small insertions/duplications, 1. Small indel and 2. Macroscopic deletions. Generally, SMARCAL1 missense mutations are typically associated with later-onset disease, while missense, frameshift and splicing mutations are more frequently detected in severe SIOD [1]. The SMARCAL1 gene, which was assigned to 2q35 (Cytogenetic location), is composed of 18 exons, the first two exons being non-coding, encoding a protein member of the SWI/SNF family, also known as the SMARCAL1 protein [1,2]. The SMARCAL1 protein, which is also known as HepA-Related Protein (HARP) and has 954 amino acids, has helicase and ATPase activity and is believed to regulate the transcription of certain genes that alter the chromatin structure around these genes and plays an important role in addition to transcriptional regulation, in replication, repair, recombination and covalent modification [3,4]. Boerkoel et al. determined for the first time that mutations of the SMARCAL1 gene cause a deficiency in the SMARCAL1 protein, which in turn could cause a deterioration in cellular functions due to progressive DNA damage and are considered responsible for SIOD [5].
Figure 3: Representation of images taken through Electron Microscopy (EM). Note: (A): EM showing peripheral glomerular capillary loops with wrinkling of the basement membrane compatible with ischemia, with subendothelial rarefaction and fusion of podocyte feet, quite extensive, without electron-dense deposits being observed at any level; (B): EM showing another capillary loop with the most preserved podocytes, with hardly any fusion and no electron dense deposits.
Since it follows an autosomal recessive inheritance pattern, two pathogenic changes in the SMARCAL1 gene (homozygosity) are necessary for the patient to develop a complete clinical picture of the disease. SIOD is pan-ethnicity and has variable expressivity. Biallelic mutations in the SMARCAL1 gene are the only identified cause of SIOD. However, among patients from different families, these mutations have not been identified in all patients. This observation, variable expressivity and poor genotype-phenotype correlation has led to testing several hypotheses, including haplotype modification, oligogenic inheritance or locus heterogeneity in SIOD, indicating that pathogenic variants in homozygosity and compound heterozygosity in several affected individuals. Cosegregation with the disease in several families and that have been identified in patients with SIOD, this being less aggressive in patients with pathogenic mutations in compound heterozygosity in several affected individuals, indicates that the SMARCAL1 gene is a remodeling protein essential for genome integrity. In developing fetal kidneys, the SMARCAL1 gene is expressed in the ureteral epithelium, metanephric stromal mesenchyme and at all stages of nephron development. In postnatal kidneys. SMARCAL1 is expressed in the epithelial tubules of the nephron, collecting ducts and glomeruli (podocytes and endothelial cells). Studies suggest that alteration of genomic integrity during fetal kidney development contributes to the pathogenesis of FSGS in patients with SIOD. This disease is characterized by a combination of prominent spondyloepiphyseal dysplasia, short stature, pancytopenia with T cell immunodeficiency, cerebral ischemia phenomena, hypothyroidism and migraine-like headache. Hypertension and hyperlipidemia secondary to kidney disease combined with immune dysfunction could predispose the patient to atherosclerosis. Nephrotic proteinuria and/or corticosteroidresistant nephrotic syndrome and progressive chronic kidney disease associated with focal and segmental glomerulosclerosis. FSGS is the most common renal pathological finding associated with proteinuria in SIOD [6]. FSGS in people with SIOD is generally identified between 1 and 14 years of age and leads to end-stage renal disease in the short term, although it may appear later in adolescence or early adulthood in incomplete forms associated with heterozygous mutations in the gene SMARCAL1. FSGS is the main cause of Steroid Resistant Nephrotic Syndrome (SRNS) in SIOD. Minimal change disease, membranous nephropathy and mesangial proliferative glomerulonephritis have also been described as causes of SRNS [7]. In these individuals, there is evidence of temporary reductions in proteinuria with the use of angiotensin converting enzyme inhibitors or cyclosporine-A. The nephrotic syndrome of SIOD generally does not respond to steroid treatment, but there are reports of transient improvements in proteinuria using Angiotensin-Converting Enzyme Inhibitors (ACEIs), Renin-Angiotensin Channel Blockers (RABs) and cyclosporine [8]. Our patient only received supportive therapy. In more severe cases of nephrotic syndrome and/or stage of end-stage chronic renal failure, kidney transplant is indicated and there are no reports of post-transplant nephrotic syndrome, although infectious and cerebrovascular complications may still occur, since the SMARCAL1 mutation affects many systems, beyond the glomeruli [9].
Conclusion
We report a 31-year-old patient with a paternal family history of nephropathy of unknown origin, with a case of incomplete SIOD, of mild intensity, with costal bone dysplasia, arterial hypertension of unknown origin and from a nephrological point of view, with proteinuria and progressive deterioration of renal function. We emphasize the importance of early recognition and taking into account the wide range of clinical manifestations of this entity.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment and Funding Source
The authors would like to thank Dr. Paula Carrillo and Dr. Carles Saus for their help with the review of the images obtained by renal biopsy (optical microscopy, immunofluorescence and electron microscopy) and the genetics department of the Son Espases University Hospital and from the Institute of Genomic Medicine of Valencia for their help with DNA extraction and targeted exome analysis.
References
- Hunter KB, Lücke T, Spranger J, Smithson SF, Alpay H, et al. (2010) Schimke immunoosseous dysplasia: Defining skeletal features. Eur. J. Pediatr 169: 801-811.
[Crossref], [Google Scholar], [Indexed]
- Marie M (2016) Characterization of the disease pathogenesis of schimke immuno-osseus dysplasia.
- Wang L, Li J, Wu G, Kong X (2021) A novel compound heterozygous variant in SMARCAL1 leading to mild schimke immune osseous dysplasia identified using whole-exome sequencing. J. Int. Med. Res. 49
[Crossref], [Google Scholar], [Indexed]
- Jin J, Wu K, Liu Z, Chen X, Jiang S, et al. (2019) Whole exome sequencing identified a novel biallelic SMARCAL1 mutation in the extremely raredisease SIOD. Front Genet 18: 1-11.
[Crossref], [Google Scholar], [Indexed]
- Baradaran-Heravi A, Cho KS, Tolhuis B, Sanyal M, Morozova O, et al. (2012) Penetrance of biallelic SMARCAL1 mutations is associated with environmental and genetic disturbances of gene expression. Hum Mol Genet 21: 2572- 2587.
[Crossref], [Google Scholar], [Indexed]
- Pedrosa AK, Torres LF, Silva AC, Dantas AB, Zuntini KL, et al. (2016) Rare case of nephrotic syndrome: Schimke syndrome. J Bras Nefrol 38: 370-373.
[Crossref], [Google Scholar], [Indexed]
- Elizondo L I, Cho K S, Zhang W, Yan J, Huang C, et al. (2009) Schimke immuno-osseous dysplasia: SMARCAL1 loss-of-function and phenotypic correlation. J Med Genet 46: 49-59.
[Crossref], [Google Scholar], [Indexed]
- Sarin S, Javidan A, Boivin F, Alexopoulou I, Lukic D, et al. (2015) Insights into the renal pathogenesis in schimke immuno-osseous dysplasia: A renal histological characterization and expression analysis. J Histochem Cytochem 63: 32-44.
[Crossref], [Google Scholar], [Indexed]
- Woo HA, Lim S, Ahn YH, Heon KS, Il-soo H, et al. (2020) Clinical course of schimke immuno-osseous dysplasia after kidney transplantation. Transplantation 13-29.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences