ISSN : 2573-0320
Journal of Transmitted Diseases and Immunity
Factors Associated with Malaria Prevalence among Children under Five Years in the Hohoe Municipality of Ghana
Kweku M1, Takramah W1, Takase M2, Tarkang E1* and Adjuik M1
1School of Public Health, University of Health and Allied Sciences, Ho, Volta Region, Ghana
2School of Biological Sciences, University of Cape Cast, Cape Coast, Central Region, Ghana
- *Corresponding Author:
- Tarkang E
Department of Population and Behavioral Sciences
School of Public Health
University of Health and Allied Sciences
Ho, Ghana
Tel: +233 36 219 6122
E-mail: ebeyang1@yahoo.com
Received Date: April 26, 2017; Accepted Date: May 13, 2017; Published Date: May 20, 2017
Citation: Kweku M, Takramah W, Takase M, et al. Factors Associated with Malaria Prevalence among Children under Five Years in the Hohoe Municipality of Ghana. J Transm Dis Immun. 2017, 1:2.
Abstract
Background: Malaria continues to adversely impact the health of children in Ghana. Hohoe is an area of intense and prolonged, seasonal malaria transmission and malaria is still the leading cause of morbidity and mortality among children under five years. This study was set out to determine factors associated with malaria infection among children less than five years. Method: An unmatched case-control study was conducted in November 2015 involving children less than five years from 30 communities. Information on the background characteristics of the children and parents/guardians were collected using a pretested questionnaire. Children were screened to determine their malaria infection status using finger prick blood for RDT. Anthropometric indices and axillary temperature were measured, as well as blood film for malaria parasites and haemoglobin levels. T-test was used for means and Odds Ratios was used to determine the relationships and associations between the dependent and independent variables. Results: Out of 1697 children screened, 676 (39.8%) tested positive with RDT (cases) and 1,021 tested negative (controls). Older children aged 24-35 and 36-47, and 48 months and above were more likely to have malaria as compared to the younger age group 6-11 months (OR=1.66 (95% CI: 1.04-2.65); p=0.034), (OR=1.77 (95% CI: 1.10-2.87); p=0.019) and (OR=2.02 (95% CI: 1.24-3.29); p=0.005), respectively. Current fever, History of fever within one week and antimalarial drug use at home were 3.07, 1.75 and 4.03 times more likely to occur among cases than in the controls (OR=3.07 (95% CI: 1.21-7.81); p<0.018), (OR=1.75 (95% CI: 1.29-2.37); p<0.001) and (OR=4.03 (95% CI: 2.82-5.77); p<0.001), respectively. Anaemia (HB<11.0g/dL) was 1.87 times more likely among the cases than in the controls (OR=1.87 (95% CI: 1.40-2.48); p<0.001). Children of parents/guardians who were farmers and traders were more likely to have malaria (OR=1.73 (95% CI: 1.16-2.56); p=0.007) and (OR=1.83 (95% CI: 1.22-2.74); p=0.003), respectively. Conclusion: Age, current fever, history of fever within one week, anaemia, use of antimalarial drugs at home and parent/guardian’s occupation were factors associated with malaria infection. Targeted screening and treatment of older children and intensive education on malaria prevention in addition to the current control activities could serve as tools for controlling malaria to the level of elimination.
Keywords
Malaria infection; Cases; Controls; Risk factors; Hohoe Municipality; Ghana
Abbreviations
RDT: Rapid Diagnostic Test; LLIN: Long lasting Insecticide-Treated Net; GDHS: Ghana Demographic Health Survey; GHS-ERC: Ghana Health Service Ethical Review Committee; HRP2: Histidine Rich Protein 2; WBCs: White Blood Cells; ACT: Artemisinin-based Combination Therapy; SMC: Seasonal Malaria Chemo-prevention; WHO: World Health Organization; NMCP: National Malaria Control Programme; MICS: Multiple Indicator Cluster Survey; OPD: Out-Patient Department
Background
Malaria is considered to be one of the main global health problems, causing approximately 438,000 deaths in 2015 [1]. Since 2005, the Ghana National Malaria Control Programme (NMCP) has increased coverage and usage of available intervention tools such as using Long-Lasting Insecticide-treated mosquito Nets (LLINs), indoor residual spraying (IRS), intermittent preventive treatment during pregnancy (IPTp) and malaria case management; and indications are that the endemicity levels could be reduced [2]. In the world, there have been large reductions in the number of malaria cases and deaths between 2000 and 2015 [1]. The number of malaria cases and deaths decreased globally from 262 million and 839,000 respectively in 2000, to 214 million and 438,000 respectively in 2015 with most (88%) of the cases in 2015 occurring in the WHO African Region. Several factors have potentially contributed to recent health improvement in African countries, but there is substantial evidence that achieving high malaria control intervention coverage, especially with Insecticidetreated nets (ITNs) and targeted IRS, has been the leading contributor to reduced child mortality [3]. Malaria is endemic in Ghana with Plasmodium falciparum as the predominant species causing the disease. Malaria has been a major cause of mortality and morbidity in Ghana, especially among children under five years and pregnant women. It remains a public health concern and a leading cause of poverty and low productivity. According to the NMCP, malaria tops most out-patient department (OPD) cases and kills 3 children every day in Ghana [4]. As part of the millennium declaration, various countries have enjoined to have halted and reduced the incidence of malaria and other diseases by 2015. In order to achieve this target, Ghana has implemented a malaria control strategy that involves multi and inter-sectoral partnerships, working together to reduce illness and death caused by malaria by 50%, increase LLIN ownership to 80% and usage to 60% by 2010, and by 2015, 100% LLIN ownership and 85% usage [5]. These strategies include prevention through the use of ITNs, use of long-lasting insecticides nets (LLINs), early detection and appropriate prompt treatment with Artemisinin-based combination therapies (ACT’s). The other strategies include the use of intermittent preventive treatment (IPT) in pregnancy using sulphadoxine-pyrimethamine (SP), (SP-IPTp) and IRS. Seasonal Malaria Chemo-prevention (SMC) in some parts of the country. Although these control measures have successfully reduced the number of malaria cases in Ghanaian children over the past few years, there is still a high number of children under five suffering from malaria [5]. The Ghana Demographic Health Survey (GDHS) has shown that malaria is still hyper-endemic in Ghana with prevalence ranging from 11.2% to 40.0% [5]. The prevalence is said to be highest among children living in rural (37.7%) than in urban (15%) areas [5]. The 2011 Multiple Indicator Cluster Survey (MICS) in children under five years has shown endemicity ranging from hypo-endemicity in the Greater Accra Region, hyperendemicity in the Upper West Region and meso-endemicity in the rest of the country (14% in southern coastal areas, 28% in forest, and 44% in northern and central Savannah) [2].
According to the Hohoe Municipal Health Directorate (HMHD) annual report, malaria is still the leading cause of OPD attendance (28%) and the leading cause of deaths (19.4%) in the Hohoe municipality [6]. Out of 40,092 confirmed malaria cases at the Hohoe Municipal hospital (HMH), OPD in 2014, 3,452 (8.6%) were admitted and 1,452 (40.1%) of those admitted were children under five years [6]. In the light of this, a cross-sectional survey was carried out in the Hohoe Municipality of Ghana to examine the prevalence of malaria and anaemia in 2015. Reports from the GDHS showed malaria prevalence of 36.6% by rapid diagnostic test (RDT) and 25.2% by microscopy [5].
In order to apply successful implementations to substantially reduce the burden of malaria, there is a continuous need to understand the epidemiology and risk factors associated with the disease [7]. The aim of this study was to use the data collected in the 2015 cross-sectional survey in the Hohoe Municipality to investigate the relationship between malaria statuses of children under the age of 5 years and selected socioeconomic and demographic, as well as to identify significant risk factors associated with malaria.
Methods
Study area
The study area was Hohoe Municipality, which is one of the twenty-five administrative districts of the Volta Region, Ghana. The municipality has a total land surface area of 1,172 km square, which is 5.6% of the regional and 0.05% of the National land surface area. It is located at longitude 0 degrees 15 East and 0 degrees 45 East and latitude 6 degrees 45 North and 7 degrees 15 North and lies almost in the heart of the Volta Region. It shares boundary to the East with Togo, forming part of the International borders, on the southeast by the Afadzato District and the southwest with Kpando Municipal, on the northwest with Jasikan District and on the North West with Biakoye District. According to the 2010 population census, Hohoe has a total population of 167,016 representing 7.9 percent of the total population of the Volta Region. The Municipality has two main seasons, the wet and dry, with the major wet season from April to July and the minor one from September to November. The rest of the year is relatively dry. Malaria is hyper-endemic in the study area but with seasonal peaks in June-August and October-November coinciding with the period of the rains. Some economic activities engaged by the people of the municipality include agriculture, petty trading, construction and formal sector employment.
Study design
This study used an unmatched case-control design in which controls were enrolled without regards to the number or characteristics of the cases. This was done during a screening programme for malaria among children under five years in the communities. Both cases and controls were recruited from the community. The number of controls in this design was not equal to the number of cases. For instance, 676 cases and 1021 controls were recruited into the study. The study population included children aged 6 to 59 months, who were eligible, and with parental/guardian consent to participate in the study from 30 selected communities. Data were collected in the form of interviews and biological samples. The survey was carried out at the end of the high transmission season in November 2015. During the survey, axillary temperature and weight were measured and a finger prick blood sample was collected for the determination of malaria infection using RDTs and later confirmed with microscopy. The results obtained from RDT were used to select cases (positive for RDT) and controls (negative for RDT) and children were traced to various exposures of malaria infection.
Study population
The population of the study consisted of children less than five years in 30 selected communities in the Hohoe Municipality.
Inclusion and exclusion criteria
Children aged 6-59 months residing in the Hohoe municipality and whose parents/guardians consented to participate in the study was included. Children who did not reside in the Hohoe municipality aged less than 6 months or above 59 months and whose parents/guardians did not consent to participate, were excluded from the study.
Sample size determination
A sample size of 1697 children was obtained using OpenEpi, Version 3, open source calculator SSCC [8]. Briefly, the sample size was calculated assuming a power of 80%, an expected prevalence of 25%, 5% level of significance, Odds Ratio (OR) of 1.37 in the control and OR of 1.0 among cases and a ratio of proportionality of 2 cases: 3 controls. Cases and controls were automatically selected based on the RDT results. Children who tested positive for RDT became cases and those who tested negative for RDT served as unmatched controls (676 cases, 1021 controls).
Laboratory Methods
Rapid diagnostic testing of malaria in human blood
CareStart™ Malaria HRP2 test kit (Access Bio Inc, New Jersey, USA) was used for the rapid qualitative detection of Malaria Histidine-rich Protein 2 (HRP2) in human blood as an aid in the diagnosis of malaria infection. Using this kit, 5 μL of whole blood was introduced into the sample with the aid of a pipette after finger pricking. Three drops of assay buffer were added to the well. The result was read within 20 min.
Malaria blood films for microscopy
Thick blood films were prepared on a glass slide using 10 μL of blood, evenly spread to cover an area of 15 × 15 mm of the slide. The smear was stained with 10% Giemsa for 10 minutes and then examined under oil immersion with a light microscope (magnification × 100). The slides were double-read by trained Microscopists. Asexual parasite densities were estimated by counting the number of parasites per 200 white blood cells (WBCs) in the thick film. Parasite counts were converted to parasites per microliter (μL), using relative WBC of 8000 leukocytes per μL of blood. Similarly, gametocyte rate and density were determined by counting against 500 leukocytes and converted to parasites per microliter as for asexual parasites [9]. A sample was considered negative if no parasite was counted after 200 high power fields had been read. If there occurred discrepancies in the findings in a slide between the two initial technicians (positive or negative or a 50% or more difference in parasite density) a third, more senior Microscopist reading was deemed necessary and then adopted. Two senior Microscopists from the Noguchi Memorial Institute of Medical Research (NMIMR) and University of Health and Allied Science (UHAS), examined all the positive blood films including a 20% random sample of negative blood slides for quality control.
Haemoglobin and fever measurement
Haemoglobin was measured using URIT-12 Hemoglobin Meter (URIT Medical Electronic Co., Ltd. UK) whilst fever was measured using an electronic thermometer (MODE: ZC, SURGILAC Digital Thermometer, UK).
Anthropometric measurement
Children under 12 months were weighed naked while older children above 12 months were weighed with their pants on Seca weighing scales (Hamburg, Germany) to the nearest 10 g. The height of children aged less than 24 months were measured using non-stretchable tape to the nearest mm and a locally made measuring board precise to 1 mm. Children aged 24 months or more had their height measured while standing using a locally made measuring board precise to 1 mm. Mid-Upper Arm Circumference (MUAC) was measured on the left arm to the nearest 1 mm using a non-stretchable tape.
Dependent variable
For the purpose of this study, malaria infection in children under the age of 5 years (60 months) was according to the RDT test results. Thus, the dependent variable was binary, indicating whether a child tested positive or negative for RDT. Children who tested positive for RDT were classified as cases and those who tested negative as controls for malaria infection and were traced for various exposures to malaria infection.
Independent variables
The independent variables considered in this study comprised a number of socio-demographic and epidemiologic factors. Sociodemographic factors included the age of child, ownership and use LLIN, the age of parent/guardian of child, education level and occupation. Epidemiologic factors included variables such as fever (defined as axillary body temperature ≥ 37.5°C taken during the survey), history of fever within one week (defined as parents reporting children who experienced fever in the past one week), antimalarial drug use at home within one week (defined as parents reporting that they administered antimalarial drugs to their child due to fever/malaria within one week before the survey), anaemia (defined as an HB <11.0 g/dL), malaria parasitaemia (defined as any asexual parasite density), highdensity parasitaemia (defined as asexual parasite density>7000/ μmol/L) and gametocytaemia (defined as sexual parasitaemia).
Data Management and Statistical Analyses
Data from participants were recorded on specified forms and checked by field supervisors and the data manager for consistency and accuracy. All data were entered twice into a database using Epidata 3.1 software. The accuracy of data input was checked and validated using customized validation programmes. The cleaned data were converted to STATA version 12 file prior to analysis. All analyses were done with STATA version 12.0. Frequencies and percentages were used to summarize categorical variables whilst means and standard deviations were used for continuous variables. Data were analyzed for proportions of qualitative variables using chi-square and differences in means for quantitative variables were analyzed using t-test. Multivariable adjusted logistic regression analysis was used to determine associations between the dependent variable and the independent variables. A p-value less than 0.05 were considered statistically significant.
Ethical issues
The cross-sectional study was approved by the Ethical Review Committee (ERC) of the Ministry of Health/Ghana Health Service, (MOH /GHS), ID NO: GHS-ERC: 14/05/15. Before the commencement of the study, permission was sought from the Municipal Health Management Team (MHMT) and the municipal administration. Permission was also sought from the chiefs and elders in the selected communities. A written informed consent was obtained from the parents/guardians of the children. All the information collected was treated confidentially and used for research purposes only.
Results
Study population
The sample in this study was made up of 1697 children selected from 30 communities (Figure 1). Of those children, 676 tested positive for malaria RDT, resulting in an observed malaria prevalence of 39.8 %.
Background characteristics, anthropometric measurement of children and parents/guardians
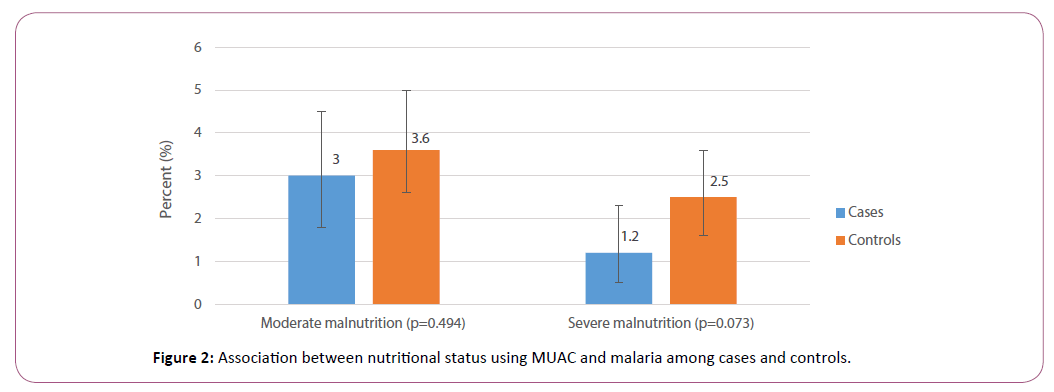
Six hundred and seventy-six (676) cases and 1,021 controls were involved in the study. The mean age of cases and controls were 33.4 ± 15.8 months and 29.0 ± 15.7 months, respectively. Children aged 6-11 months were 70 (10.4%) and 184 (18.0%) among the cases and controls, respectively, while 138 (20.4%) of cases and 242 (23.7%) of the controls were aged above 12-23 months. The majority of the children were between the ages of 24 months and above and were evenly distributed among the age groupings as shown in Table 1. There was a significant association between age and malaria (χ2=29.3, p<0.001). Among the cases and controls, the mean weight was 11.7 ± 2.6 and 11.07 ± 2.7 kg, respectively and the mean height, 85.5 ± 15.8 and 83.5 ± 13.9 cms respectively. Using Mid-upper arm circumference Normal among cases 648 (95.9%) and 959 (93.9%) among the controls. Moderate malnutrition was 20(3.0%) among cases and 37 (3.6%) among controls whilst severe malnutrition was 8 (1.2%) among the cases and 25 (2.5%) among controls. There was no significant association between malnutrition and malaria (χ2 =4.04, p=0.132) (Figure 2).
Table 1: Background characteristics and anthropometric indices of children and parents/guardians.
| Characteristics of children | Cases (N=676) n (%) | Controls (N=1,021) n (%) | Total | Chi square | P-value |
|---|---|---|---|---|---|
| (N=1,697) n (%) | |||||
| Mean age (in months) (SD) | 33.4 (15.8) | 29.0 (15.7) | - | ||
| Age-group (in months) | |||||
| 06-Nov | 70 (10.3) | 184 (18.0) | 254 (15.0) | 29.3 | <0.001 |
| Dec-23 | 138 (20.4) | 242 (23.7) | 380 (22.4) | ||
| 24-35 | 156 (23.1) | 230 (22.5) | 386 (22.7) | ||
| 36-47 | 156 (23.1) | 190 (18.6) | 346 (20.4) | ||
| 48+ | 156 (23.1) | 175 (17.2) | 331(19.5) | ||
| Mean weight (SD) (in kg) | 11.7 (2.6) | 11.07(2.7) | 11.3 (2.7) | - | - |
| Mean height (SD) (in cm) | 85.5 (15.8) | 83.5 (13.9) | 84.3 (14.7) | - | - |
| Mid-upper arm circumference | |||||
| Normal | 648 (95.9) | 959 (93.9) | 1,607 (94.7) | ||
| Moderate malnutrition | 20(3.0) | 37 (3.6) | 57 (3.4) | ||
| Severe malnutrition | 8 (1.2) | 25 (2.5) | 33 (1.9) | 4.04 | 0.132 |
| Axillary temperature | |||||
| No fever (temp<37.5°C) | 643 (95.1) | 1,010 (98.9) | 1,653 (97.4) | 23.3 | <0.001 |
| Fever (temp>=37.5°C) | 33 (4.9) | 11(1.1) | 44 (2.6) | ||
| Anaemia | |||||
| No Anaemia(Hb=11.0 g/dL) | 204 (30.2) | 461(45.2) | 665 (39.2) | 38.3 | <0.001 |
| Anaemia(Hb<11.0 g/dL) | 472 (69.8) | 560 (54.8) | 1,032 (60.8) | ||
| Malaria Parasites by microscopy | |||||
| Absent | 260 (38.5) | 986 (96.6) | 1,246 (73.4%) | 703.8 | <0.001 |
| Present | 416 (61.5) | 35 (3.4) | 451(26.6) | ||
| Parasite density | |||||
| High Parasite density (>=7000 µmol/L) | 71 (10.5) | 0 (0.0) | 71(4.2) | 111.9 | <0.001 |
| Low Parasite density (<7000 µmol/L) | 605 (89.5) | 1,021(100.0 ) | 1,626 (95.8) | ||
| Gametocytes | 4 (0.6) | 1(0.1) | 5 (0.3) | ||
| Mean weight (SD) | 11.7 (2.59) | 11.1 (2.75) | - | ||
| LLIN ownership and usage | |||||
| Own LLIN | 630 (93.2) | 971 (95.1) | 1,601 (94.3) | 7.7 | 0.005 |
| Use LLIN | 500 (74.0) | 735 (72.0) | 1235 (72.8) | ||
| Characteristics parents/guardians | |||||
| Mean age, (in years) (SD) | 30.6 (8.8) | 30.3 (8.2) | - | ||
| Age group (in years) | |||||
| 15-20 | 62 (9.2) | 96 (9.4) | 158 (9.3) | 4.6 | 0.325 |
| 21-30 | 331(49.0) | 477 (46.7) | 808 (47.6) | ||
| 31-40 | 216 (32.0) | 343 (33.6) | 559 (32.9) | ||
| 41-50 | 47 (6.9) | 87 (8.5) | 134 (7.9) | ||
| 50+ | 20 (2.9) | 18 (1.8) | 38 (2.2) | ||
| Educational level | |||||
| None | 67 (9.9) | 91(9.0) | 162 (9.5) | 7.7 | 0.052 |
| Basic | 530 (78.4) | 774 (75.8) | 1,304 (76.9) | ||
| Secondary | 69 (10.2) | 124 (12.2) | 193 (11.4) | ||
| Tertiary | 8 (1.2) | 30 (3.0) | 38 (2.2) | ||
| Occupation | |||||
| None | 114 (16.8) | 246 (24.1) | 360 (21.2) | 16.5 | |
| Artisan | 134 (19.8) | 206 (20.2) | 340 (20.0) | 0.006 | |
| Farming | 231 (34.2 | 291 (28.5) | 522 (30.8) | ||
| Trading | 181 (26.8 | 248 (24.3) | 429 (25.3) | ||
| Teaching/nursing/banking | 14 (2.1) | 23 (2.2) | 37 (2.2) | ||
| Others | 2 (0.3) | 7 (0.7) | 9 (0.5) | ||
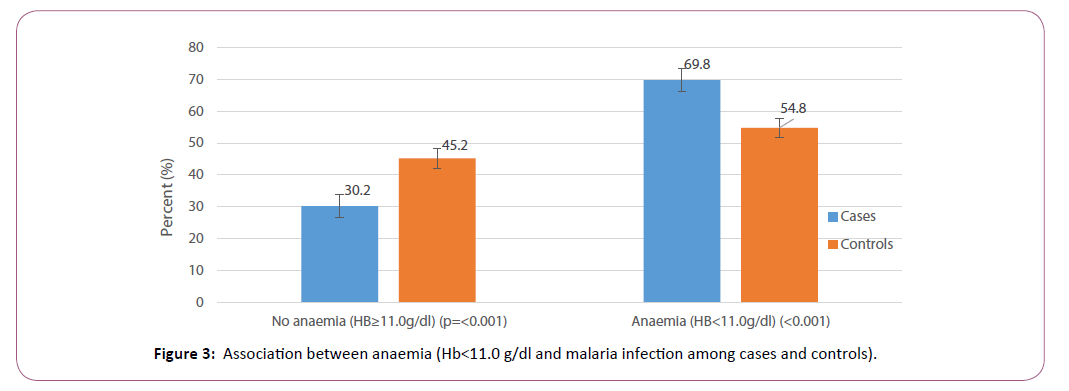
Table 1 also shows that, out of the 1,697 children, 44(2.6%) had fever (temperature ≥37.5ºC) at the time of the survey, 1,032 (60.8%) had anaemia (HB<11.0 g/dL), and 451(26.6%) had malaria parasites present in their blood by microscopy. Among the 676 cases, 33(4.9%) had a fever at the time of the survey, 472 (69.8%) had anaemia and 416 (61.5%) had malaria parasites present in their blood by microscopy. Among the 1021 controls, only 11(1.1%) had a fever at the time of the survey, 560 (54.8%) had anaemia and 35 (3.4%) had malaria parasites in their blood by microscopy, which were all P. falciparum; no other species was identified. There was a significant association between fever and malaria (χ2=23.3, p<0.001). There was also a significant association between anaemia and malaria (χ2=38.3, p<0.001) (Figure 3).
Figure 3: Association between anaemia (Hb<11.0 g/dl and malaria infection among cases and controls).
Overall, out of the 1,697 children, 71 (4.2%) had high parasite density (≥ 7000 μmol/L) and 5 (0.3) had gametocytes in their blood. Among the 676 case, 71 (10.5%) had high parasite density, and 4 (0.6) had gametocytes in their blood at the time of the survey. Among the 1021 controls, none (0.0%) had high parasite density and only 1 (0.1%) had gametocytes at the time of the survey. There was a significant association between parasite density and malaria (χ2 =111.9, p<0.001). LLIN ownership and use were high and similar among cases and controls (93.3% and 74.0% vs. 95.1% and 72.0%), respectively. There was a significant association between LLIN ownership and use and malaria (χ2=7.7, p=0.005). The mean age of parents/guardians in both cases and controls was 33.4 ± 15.8 years and 29.0 ± 15.7 years, respectively. The level of education and occupation of parents/guardians were similar among the cases and controls.
Socio-economic and demographic risk factors
Table 2 shows that the prevalence of malaria among children aged between 12 and 23 months was not significantly different from those aged 6-11 months (OR=1.17 (95% CI: 0.73, 1.88); p=0.507). Children aged between 24-35 months were 1.66 times more likely to be infected with malaria as compared with children aged 6-11 months (OR=1.66 (95% CI: 1.04, 2.65); p=0.034). Children aged 35-47 months and those aged 48 months and above were 1.77 times and 2.02 times more likely to have malaria infection as compared to children aged 6-11months (OR=1.77 (95% CI: 1.10, 2.87); p=0.019) and (OR=2.02 (95% CI: 1.24, 3.29); p=0.005), respectively. Moderate and severe malnourished were 0.70 and 0.39 less likely to have malaria, however, the differences were not statistically significant, (OR=0.70 (95% CI: 0.32, 1.55); p=0.383) and (OR=0.39 (95% CI: 0.12, 1.24); p=0.110), respectively. Fever was 3.07 times more likely to occur among cases than among controls (OR=3.07 (95% CI: 1.21, 7.81); p=0.018). Anaemia was 1.87 times more likely to occur in cases than controls (OR=1.87 (95% CI: 1.40, 2.48); p<0.001). Having malaria parasitaemia by microscopy was 50.11 times more likely among cases than among controls (OR=50.11 (95% CI: 33.95, 73.95; p<0.001). There was no significant difference in ownership and usage of ITN among cases and controls (OR=0.97 (95% CI: 0.51, 1.85); p=0.927) and (OR=0.94 (95% CI: 0.67, 1.31); p=0.695), respectively. Children with a history of fever within one week were 1.75 times more likely to be infected with malaria as compared to those without (OR=1.75 (95% CI: 1.29, 2.37); p<0.001). The odds of malaria infection among children who received antimalarial drugs within one week before the survey was 4.03 times more than in those who did not receive antimalarial drugs (OR=4.03 (95% CI: 2.82, 5.77); p<0.001). Children whose parents/guardians were farmers and traders were 1.73 and 1.83 times more likely to be infected with malaria compared to those without any occupation (OR=1.73 (95% CI: 1.16, 2.56); p=0.007) and (OR=1.83 (95% CI: 1.22, 2.74); p=0.003), respectively. Children whose parents/ guardians were artisans were also 1.53 times more likely to be infected with malaria as compared to those with no occupation but the difference was not statistically significant (OR=1.53 (95% CI: 0.99, 2.36); p=0.054).
Table 2: Socio-Demographic, anthropometric and Epidemiologic risk factors of children, parents/guardians and odds of malaria infection.
| Characteristics | Cases (N=676) | Controls (N=1,021) | AdjustedOR | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| n(%) | n(%) | |||||
| Age-group | ||||||
| 06-Nov | 70 (10.3) | 184 (18.0) | - | - | - | |
| Dec-23 | 138 (20.4) | 242 (23.7) | 1.17 | 0.73, 1.88 | 0.507 | |
| 24-35 | 156 (23.1) | 230 (22.5) | 1.66 | 1.04, 2.65 | 0.034 | |
| 36-47 | 156 (23.1) | 190 (18.6) | 1.77 | 1.10, 2.87 | 0.019 | |
| 48+ | 156 (23.1) | 175 (17.1) | 2.02 | 1.24, 3.29 | 0.005 | |
| Mid-upper arm circumference | ||||||
| Normal | 648 (95.9) | 959 (93.9) | ||||
| Moderate malnutrition | 20(3.0) | 37 (3.6) | 0.7 | (0.32, 1.55) | 0.383 | |
| Severe malnutrition | 8 (1.2) | 25 (2.5) | 0.39 | (0.12, 1.24) | 0.11 | |
| Fever (temperature=37.5ºC) | ||||||
| No | 643 (95.1) | 1,010 (98.9) | - | - | - | |
| Yes | 33 (4.9) | 11(1.1) | 3.07 | 1.21, 7.81 | 0.018 | |
| Anaemia (Hb<11.0 g/dL) | ||||||
| No | 204 (30.2) | 461(45.2) | - | - | - | |
| Yes | 472 (69.8) | 560 (54.8) | 1.87 | 1.40, 2.48 | <0.001 | |
| Malaria parasites | ||||||
| Absent | 260 (38.5) | 986 (96.6) | - | - | - | |
| Present | 416 (61.5) | 35 (3.4) | 50.11 | 33.95, 73.95 | <0.001 | |
| Own LLIN | ||||||
| No | 46 (6.8) | 50 (4.9) | - | - | - | |
| Yes | 630 (93.2) | 971(95.1) | 0.97 | 0.51, 1.85 | 0.927 | |
| Use LLIN | ||||||
| No | 176 (26.0) | 286 (28.0) | - | - | - | |
| Yes | 500 (74.0) | 735 (72.0) | 0.94 | 0.67, 1.31 | 0.695 | |
| History of fever within one week | ||||||
| No | 398 (58.9) | 787 (77.1) | - | - | - | |
| Yes | 278 (41.1) | 234 (22.9) | 1.75 | 1.29, 2.37 | <0.001 | |
| Antimalarial drug use within one week | ||||||
| No | 523 (77.4) | 924 (90.5) | - | - | - | |
| Yes | 153 (22.6) | 97 (9.5) | 4.03 | 2.82, 5.77 | <0.001 | |
| Age-group of parent/guardian | ||||||
| 15-20 | 62 (9.2) | 96 (9.4) | - | - | - | |
| 21-30 | 331(49.0) | 477 (46.7) | 1.17 | 0.71, 1.91 | 0.546 | |
| 31-40 | 216 (32.0) | 343 (33.6) | 1.1 | 0.66, 1.84 | 0.72 | |
| 41-50 | 47 (7.0) | 87 (8.5) | 0.97 | 0.50, 1.89 | 0.935 | |
| 50+ | 20 (3.0) | 18 (1.8) | 1.47 | 0.55, 3.93 | 0.445 | |
| Educational level of parent/guardian | ||||||
| None | 67 (9.9) | 91(8.9) | - | - | - | |
| Basic | 530 (78.4) | 774 (75.8) | 0.86 | 0.55, 1.35 | 0.505 | |
| Secondary | 69 (10.2) | 124 (12.1) | 8.8 | 0.45, 1.43 | 0.459 | |
| Tertiary | 8 (1.2) | 30 (2.9) | 0.52 | 0.18, 1.48 | 0.22 | |
| Occupation of parent/guardian | ||||||
| None | 114 (16.8) | 246 (24.1) | - | - | - | |
| Artisan | 134 (19.8) | 206 (20.2) | 1.53 | 0.99, 2.36 | 0.054 | |
| Farming | 231 (34.2 | 291 (28.5) | 1.73 | 1.16, 2.56 | 0.007 | |
| Trading | 181 (26.8 | 248 (24.3) | 1.83 | 1.22, 2.74 | 0.003 | |
| Teaching/nursing/banking | 14 (2.1) | 23 (2.2) | 0.92 | 0.33, 2.57 | 0.88 | |
| Others | 2 (0.3) | 7 (0.7) | 0.82 | 0.11, 6.10 | 0.847 | |
Discussion
The aim of this study was to investigate the relationship between the malaria status of children under the age of 5 years old and selected socioeconomic and demographic factors, as well as to identify significant risk factors associated with malaria.
Socio-economic and demographic risk factors
Based on the results of this study, the socio-demographic factors closely related to the risk of malaria were the age of children and occupation of parent/guardian of the child. It was found that older children 24 months and above were more likely to have malaria when compared to children aged 6-11 months. This is similar to other studies, which indicated that an older child was associated with a higher risk of malaria [7,10,11]. Age of child could also contribute to the individual’s way of life, where older children could move about freely and may go outside more often and therefore may be more susceptible to mosquito bites, and thus malaria.
Occupation of parent/guardian was significantly associated with malaria infection. This study found that children whose parents/ guardians were into farming and trading were 1.73 and 1.83 times more likely to have a malaria infection. This is in agreement with other reports where those with high socioeconomic status (SES) (OR=0.4, 95% CI: 0.3, 0.5, p<0.001) compared to low SES were associated with lower odds of malaria infection [11]. A parent/ guardian who is a farmer or trader may have lower education and be very busy with activities that bring lower income. Therefore, they may not be well informed and cannot afford to protect the child from getting malaria. One possible reason would be that household socio-economic status plays an important role in the management of illnesses because individuals with higher income have more resources to access health care on time. This study did not find any association between education of parents/guardians and malaria infection in children. This is however, contrary to results of other studies [12], where Children of educated parents (primary/secondary: OR=0.5, 95% CI: 0.4, 0.6, p<0.001; tertiary: OR=0.3, 95% CI: 0.2, 0.5, p<0.001) when compared to uneducated parents. Other studies have reported that educational level of an individual particularly that of a child’s caregiver, has been shown to be an important risk factor of malaria [12,13]. It is assumed that more educated individuals have a better understanding of health-related issues. In regards to malaria, compared to children who had caregivers with a secondary education, those with caregivers who had an unspecified level of education or no education were most at risk.
Long lasting insecticide net ownership and use
Although LLIN use plays a key etiologic role in malaria prevention in endemic countries, the ownership and use of LLIN were not significantly associated with a child’s malaria status. These results are in agreement with those of other authors, who also found no evidence of a lower odds of malaria infection among children sleeping under LLIN in relative to those not [9,11,14]. This observed lack of association in our study could possibly be attributed to an inconsistent or inappropriate use of the nets or perhaps a child was exposed to mosquito bites during other times of the day or evening when the net was not in use. However, our findings conflict the results of other authors, who found an association between LLIN ownership and use and malaria infection [15-18].
Fever, history of fever and antimalarial drug use
This study has shown that current fever, a history of fever within the past one week and antimalarial drug use at home was significantly associated with a child’s malaria status where the risk of malaria was substantially reduced in the event of antimalarial drug use. Fever was significantly higher among the cases than the controls. The likelihood of a child presenting with fever to have malaria infection was high, with 4.9% of those with fever, having malaria infection in cases compared to 1.1% in controls. Okebe et al. [14] also who found fever in 3% of those infected with malaria and there was increased odds of fever. This is in agreement with studies by Temu et al, in 2012 in Mozambique [11], who found that, children presenting with current fever were infected with malaria (56.7%; 95%CI: 43.7%, 68.9%) compared with those without fever (46.7%; 95%CI: 37.6, 55.9, p=0.037).
Malaria infection, anaemia and antimalarial drug intake
In this study, malaria infection was associated with anaemia (HB<11 g/dL). It was observed that 69.8% of children with malaria had anaemia when compared to 54.8% in children without malaria. This is in agreement with the findings by Okebe et al. [14] who also found that anaemia (HB<11 g/dL) was observed in 34% of those infected with malaria and there was increased odds of anaemia.
This study found an association between antimalarial drugs intake and malaria infection. Children without malaria infection were 0.27 times less likely to have used antimalarial drugs within one week before the survey. This is in agreement with results by Okebe et al. [14] who found that 38% of the patients were reportedly pre-treated including 6% with AL. The finding that recent ACTs treatment was positively associated with current malaria is remarkable.
Limitations
The following limitations were identified in this study: firstly the survey was conducted in November which coincided with the end of the rainy season with high malaria transmission and does not provide all information on factors determining the risk of malaria infection throughout the year. Secondly, malaria rapid diagnostic tests are subject to limitations in sensitivity and specificity, which could have led to an underestimation or overestimation of infection prevalence. Thirdly, some answers to questions such as enquiring about sleeping under LLINs were reported by the parents and not observed by interviewers. Fourthly, malaria infection is likely to be over-estimated in groups of children remaining at home (possibly sick from malaria) and underestimated in children who did not report for the survey (likely to be healthy children). Lastly, the validity of (malaria) treatment history frequently is questionable, and no data on the dose and duration of treatment were collected in the present study.
Conclusion
In conclusion, malaria burden among children 24 months and above is high in the Hohoe Municipality. The large upscale efforts of malaria control in Ghana can be seen since the last GDHS in 2014, however, Ghana still has a long way to go because we were not able to reach targets set for 2015 on morbidity and mortality due to malaria, LLIN ownership, and use. Although there has been a significant increase in the use of LLIN, these control measures alone may not be sufficient. Supplementing these control measures with the education of appropriate and consistent use of LLINs, as well as education of home management of malaria and targeted screening with RDTs and seasonal malaria chemoprevention (SMC) and treating of older children, can go a long way in aiding the reduction of the burden of malaria in Ghana.
Consent for Publication
Written informed consent was obtained from the study participants for publication of this research work. A copy of this form is available for review by the Editor of this journal.
Availability of Data and Material
Available upon request
Funding
None
Conflict of Interest
The authors declare that they have no competing interests.
Author’s Contributions
MK conceived the study. MK and MA and WT did the data analysis and wrote the methods section. MK, MA, WT, MT and ET were responsible for the initial draft of the manuscript. All authors reviewed and approved the final version of the manuscript.
Acknowledgements
We are grateful to Mr King Harrison Kpo for reading the slides, the Hohoe Municipal Health Management Team for their assistance during the survey. We are thankful to all the parents/guardian for giving us their consent to enroll their children, and we gratefully acknowledge the children who participated in the study.
References
- WHO (2015).World malaria report. Geneva: World Health Organization.
- NMCP: National Malaria Control Programme Review, Ghana, Final Report 2013, Ghana.
- Steketee RW, Campbell CC (2010)Impact of national malaria control scale-up programmes in Africa: magnitude and attribution of effects. Malaria Journal 9:299.
- NMCP (2016): National Malaria Control Programme, Ghana 2016, Ghana.
- Ghana Demographic and Health Survey (2014).
- Hohoe Municipal Health Directorate annual report (2014).
- Pullan R, Bukirwa H, Staedke S, Snow R, Baker S, et al. (2010) Plasmodium infection and its risk factors in Eastern Uganda. Malar J 9:2.
- OpenEpi, Version 3, open source calculator-SSCC, referenced from Kelsey et al., Methods in Observational Epidemiology 2nd Edition, Table 12-15, Fleiss, Statistical Methods for Rates and Proportions, formulas 3.18 &3.19.
- Drakeley CJ, Jawara M, Targett GAT, Walraven G, Obisike U, et al. (2004)Addition of artesunate to chloroquine for treatment of Plasmodium falciparum malaria in Gambain children casues a significant but short-lived reduction in infectiousness for mosquitoes. Trop Med Int Health.
- Gahutu JB, Steininger C, Shyirambere C, Zeile I, Cwinya-Ay N, et al. (2011) Prevalence and risk factors of malaria among children in southern highland Rwanda. Malar J10:134.
- Krefis AC, Schwarz NG, Nkrumah B, Acquah S, Loag W, et al. (2010) Principal component analysis of socioeconomic factors and their association with malaria in children from the Ashanti Region, Ghana. Malar J 9:201.
- Temu EA, Coleman M, Abilio AP, Kleinschmidt I (2012) High Prevalence of Malaria in Zambezia, Mozambique: The Protective Effect of IRS versus Increased Risks Due to Pig-Keeping and House Construction. PLoS ONE 7: e31409
- Snyman K, Mwangwa F, Bigira V, Kapisi J, Clark TD, et al. (2015) Poor housing construction associated with increased malaria incidence in a cohort of young Ugandan children. Am J Trop Med Hyg 92: 1207-1213.
- Nahum A, Erhart A, Maye A, Ahounou D, van Overmeir C, et al. (2010) Malaria incidence and prevalence among children living in a peri-urban area on the coast of Benin, West Africa: a longitudinal study. Am J Trop Med Hyg 83:3.
- Okebe J, Mwesigwa J, Kama EL, Ceesay SJ, Njie F, et al. (2014) A comparative case-control study of the determinants of clinical malaria in the Gambia. Malar J 13:306.
- Ayele D, Zewotir T, Mwambi H (2011) Prevalence and risk factors of malaria in Ethiopia. Malar J 11:195.
- Winskill P, Rowland M, Mtove G, Malima RC, Kirby MJ (2011) Malaria risk factors in north-east Tanzania. Malar J 10:98.
- Wotodjo A, Diagne N, Gaudart J, Richard V, Raoult D, et al. (2013) Malaria risk factors in Dielmo, a Senegalese malaria-endemic village, between October and November of 2013: a case-control study. Am J Trop Med Hyg 92: 565-568.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences