ISSN : 2348-9502
American Journal of Ethnomedicine
Extraction and Evaluation of Hypoglycemic and Wound Healing Potential of Hydro-Ethanolic Extract of Alhagi pseudalhagi Wild
Anupam K Sachan1*, Rao CV2 and Nikhil K Sachan3
1Dayanand Dinanath College, Institute of Pharmacy, Ramaipur, Kanpur-209214, India
2National Botanical Research Institute, Lucknow-226001, India
3Department of Education, University Grants Commission, New Delhi-110002, India
- *Corresponding Author:
- Sachan AK
Dayanand Dinanath College
Institute of Pharmacy, Ramaipur
Kanpur-209214, India
Tel: +91-9936273358
E-mail: anupamkrsachan@gmail.com
Received Date: August 07, 2017; Accepted Date: August 22, 2017; Published Date: August 29, 2017
Abstract
Objective: The present investigation envisaged to evaluate antidiabetic and wound healing potential of hydro-ethanolic extract of Alhagi pseudalhagi (family: Leguminosae) plant from Chambal region.
Experimental section: The hydro-ethanolic extract of A. pseudalhagi obtained by hot Continuous extraction was subjected to phytochemical examination and pharmacological screening for antidiabetic activity in male wistar rats after intraperitoneal administration using 18 h rat fasted model, oral glucose tolerance test and STZ induced diabetic rat model. The wound healing activity was evaluated through excision wound model and the progressive change in wound area was monitored planimetrically by tracing the wound margin on graph paper.
Results: The hydro-ethanolic extract of A. pseudalhagi observed containing the presence of different phyto-constituents of alkaloids, carbohydrates, glycosides, protein and amino acids, phenolic compounds, tannins which has potential antidiabetic and wound healing activity on standard pharmacological models Conclusion: The observations from present study suggest A. pseudalhagi as a promising candidate with potential antidiabetic activity having wound healing property as well. It may prove to be effective in the treatment of diabetic wound healing.
Keywords
Jawasa; Antidiabetic; Wound healing; Folk medicine
Introduction
Diabetic mellitus is manifested by hyperglycemia which is also characterized by excessive urine excretion [1]. It is a chronic metabolic syndrome causing high glucose level with disturbance of carbohydrate, lipid and protein metabolism resulting from defects in insulin secretion, action or both [2]. Craving for new antidiabetic drugs from natural plants is still attractive because the plants contain substances which provide alternative and safe cure of diabetic mellitus. In India, there are a number of medicinal plants having been used traditionally for treatment of various ailments but only a few of them are tapped for biological and biochemical profiling to scientifically rationalize their medicinal usage [3]. However, in recent past the search for hypoglycemic agents has focused on plants used in traditional medicine, partially because of leads provided by traditional medicine to natural products with potentials of being better treatments compared to currently used drugs [4].
The diabetic mellitus may cause other secondary ailments like diabetic retinopathy, gestational difficulties, hypertension and delayed wound healing etc. Therefore, future therapeutic strategies may require combination of multiple natural remedies for ailment which are likely precipitate as resultant of primary disease. At this juncture, botanicals with free radical scavenging activity are emerging as the primary components of holistic approaches to diabetic management through poly-herbal formulations. Considering the diabetic interference on, inter alia, pathophysiology of wound for concurrent management and mitigation of malaise. Wound is a disruption of cellular and anatomical or functional continuity of living tissue which, in fact, it is a process to repair that follows injury to the skin and other soft tissues itself [5,6].
Alhagi pseudalhagi is a medium-sized, intricately branched perennial small thorny shrub which belongs to the family of leguminosae is known as Yavasaka in Ayurveda and Camel thorn in English also well known as Jawasa in Hindi. The plant is bitter and acrid with a district flavor, widely distributed in Semiarid zone of India and finds uses in the indigenous system of medicine for a variety of purposes [7,8]. A. pseudalhagi is used as traditional herbal medicine and also an important ingredient of many Ayurvedic formulations [9,10]. A. pseudalhagi is possibly one of the most traditionally used medicinal herbal plants for the treatment of various diseases in rural and remote areas of district Eta in Uttar Pradesh, India [11]. The various parts of plant are reported being used as antibacterial, anti-urolithic, anti-ulcer, wounds healers, cholegogue, astringent, antianginal, antipyretic, antioxidants, diuretic, hypoazotemic and for treatment of cholitis, gastritis, dysenetry, nasopharynx diseases, hemorrhoids, eczema. pyrexia and sexual disorders [12-18]. Gulzar et al. reported the A. pseudalhagi being rich in biologically active phytochemicals such as phenolics, flavanoids, glycosides, alkaloids and polysaccharides alonge with different essential minerals, protiens and lipids [19]. The present study envisaged to investigate antidiabetic and wound healing potential of hydro-ethanolic extract of Alhagi pseudalhagi using standard pharmacological models.
Materials and Methods
Collection and authentication plant material
The plant material (whole plant) was collected from wild sources around the Udi area of Chambal Valley, District Etawah, Uttar Pradesh, in month of June and July during 2016 taking into account the good collection practices for medicinal plants from wild sources [20]. The plant was identified and authenticated at source by Pharmacognosy and Ethnopharmacology Division CSIR-National Botanical Research Institute, Lucknow. A voucher specification (No.: NBRI-SOP-216) has been deposited in Institute repository.
Preparation of crude drug for extraction
Plant material was cut into pieces and shade dried at room temperature; the dried material was subject to size reduction to a coarse by using a dry grinder (Philips, India), passed through a sieve and stored in air tight glass container for further use [21].
Extraction of plant material
The air dried powder of A. pseudalhagi was extracted by hot continuous hydro-ethanolic extraction with 500 mL of 50% v/v ethyl alcohol as menstruum using soxhlet extractor [22,23]. The hydro-ethanolic extract so obtained was filtered through muslin cloth and filtrate was evaporated under reduced pressure by using rotary evaporator and vacuum dried. Brownish black colour residue was obtained. The residue was then stored in desiccators.
Preliminary phytochemical screening methods
The extract was subjected to preliminary phytochemical investigation for the presence of various phytoconstituents using standard qualitative phytochemical tests for detection of compounds viz alkaloids, carbohydrates, glycosides, protein and amino acids, phenolic compounds, tannins, saponins, phytosterols, fixed oil and fats [24-26].
Experimental animals
Wistar male albino rats (180-200 g) were used in this investigation. The animals were maintained under standard environmental conditions, fed with balanced commercial pallet diet and had free access to and water ad libitum. Experimental protocol was duly approved by institutional animal ethics committee (IAEC) and animals care was carried out as per the guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA) India in approved laboratory (Reg.No.222/2000/CPCSEA). Determination of antidiabetic activity relied on blood glucose estimation and mean at the end of the study.
Safety profile study
An acute toxicity study of the hydro-ethanolic extract of A. pseudalhagi was carried out for the determination of LD50 by adopting the fixed dose method (Annexure 2d) of CPCSEA, OECD guidelines [27]. Wistar albino rats (180-200 g) were used for the study. The different doses of 150, 500, 1000 and at the maximum dose of 2000 mg/kg was administered orally and number of dead or surviving rats after 24 h was recorded.
Anti-diabetic activity
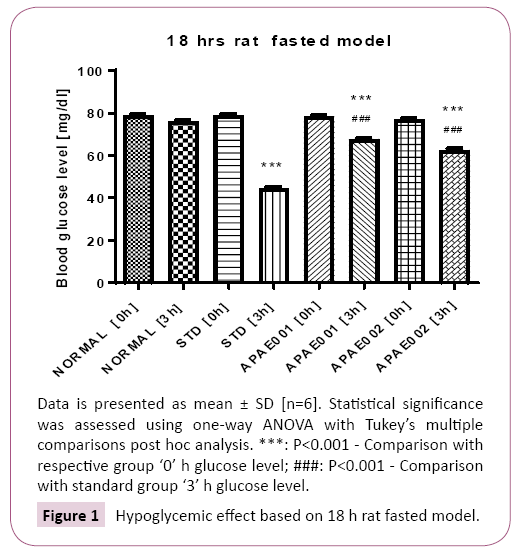
18 h rat fasted model: The animals were divided into 4 groups - Normal vehicle control, test extract treated and reference (glibenclamide) treated group with 06 animals in each group and were fasted overnight. Normal animals receives tween 80 solution (1 mL/kg), reference group receives glibenclamide (0.5 mg/kg), Test groups receives test extracts APAE001 (250 mg/ kg) and APAE002 (500 mg/kg). Blood glucose determination was done at 0 h (prior to any treatment) and 3 h (Post-drug administration) on same day [4].
Oral glucose tolerance test (OGTT): The animals were divided into normal untreated control, negative control (glucose primed), test (glucose primed-test extract) treated and reference (glucose primed+Glibenclamide) treated group with 06 animals in each group and were fasted overnight. Normal and negative control groups received tween 80 solution (1 mL/kg), Test group receives test extracts APAE001 (250 mg/kg) and APAE002 (500 mg/kg) and reference group receives glibenclamide (0.5 mg/kg). The test and reference drug substances were administered at 0 h to respective group and glucose (1.5 g kg-1; 10% sol.) administered to all groups except normal untreated group. Blood glucose determination was done at 0 h (prior to any treatment), 0.5 and 1.5 h (Post-glucose administration) same day [28].
STZ induced diabetic rat model
Induction of diabetes: Overnight fasted animals were treated with streptozotocin (STZ), 60 mg/kg body weight, dissolved in ice cold citrate buffer (0.1 M, pH 4.5) administered intraperitoneally. Experimental diabetic condition was established in STZ treated experimental rats over a period of after 7 days followed by blood collection using retro orbital puncture method and the plasma glucose level of each rat was determined. Animals with fasting blood glucose (FSG) of range 250-300 mg/dL were considered diabetic and included in the study. Blood samples were collected on 0, 7, 14, 21 and 28 days. All the animals were allowed to free access to normal saline and pellet diet and maintained at room temperature (25 ± 30°C) [29].
Treatment protocol for STZ induced diabetic rat model: Animals were divide in to five groups (n=6); normal group received 1% v/v tween 80 (1 mL/kg); diabetic control group (STZ, 45 mg/ kg), reference group (glibenclamide, 0.5 mg/kg) and test groups received extracts of two different doses (250 and 500 mg/ kg) in 1% v/v Tween 80 (1 mL/kg). The treatment protocol was continuing for period of 28 days.
Wound healing study
Excision wound model: The animals were divided into 3 groups (n=6); Normal control, test extract treated and reference treated group. Skin hairs of dorsal surface were shaved off and surface sterilised with 70% alcohol prior to creation of wound. Transdermal wounds of 8 mm diameter were made on dorsal surface with the help of biopsy punch by excising skin and panniculus carnosus on both side of thoraco-lumber region of rat under ether anesthesia. Wounds were left undressed to the open environment. Subsequently, normal, reference group and test group animals were treated topically with water soluble ointment base, 0.2% w/w nitrofurazone ointment and herbal ointment respectively twice daily until the wounds completely healed [30].
Results and Discussion
The phytoconstituents extracted using 50% v/v hydro-ethanolic menstrum by soxhlet extractor obtained as black colour residue after drying. The extractive value was found to be 3.11% for hydro-ethanolic extract. The phytochemical analysis conducted on A. pseudalhagi detected the presence of different phytoconstituents of alkaloids, carbohydrates, glycosides, protein and amino acids, phenolic compounds, tannins presented in Table 1.
Table 1: Qualitative phytochemical profile of extract.
| Phytochemicals | Tests | Inference |
|---|---|---|
| Alkaloids | Dragendorff’s, Wagner’s, Mayer’s, Hanger’s Test | (+) |
| Flavonoids | Shinoda’s test | (+) |
| Saponins | Froth’s test | (-) |
| Carbohydrates | Molisch’s test, Fehling’s test,Benedict’s Test | (+) |
| Protien and amino acid | Million’s test, Biuret andNinhydrin test reagent | (+) |
| Phenolic compoundsand Tannins | Ferric chloride test and Gelain test | (+) |
| Glycosides | Legal’s test, Borntrager’s test, Libermann-Burchard’s test | (+) |
| Phytosterols | Salkowski’s test and Libermann-Burchard’s test | (-) |
| Fats and oils | Saponification and Filter paper test | (-) |
| Terpenoids | Chloroform test | (+) |
The preliminary observations of hypoglycemic effect of Alhagi pseudalhagi extract in 18 h Rat fasted model showed significant reduction in blood glucose level of fasting rat at different time intervals of 0 h and 3 h in test groups. Blood glucose level decreased in studied animals in dose dependent manner. The plant extract may be beneficial for prophylaxis of diabetes. Blood glucose level with hypoglycemic effect of Alhagi pseudalhagi extract is presented in Figure 1. Results of 18 h fasted model showed that blood glucose level of animals in standard group at 0 h was higher whereas significant lower level was observed at 3 h. Similarly, treatment with APAE001 and 2 also resulted significant lower blood glucose level at 3 h interval while comparing with control as well as standard groups. On the other hand, OGTT level in APAE001 and 2 treatments (at 30 and 90 min interval) and STZ induced animal study was observed with significant levels as compared with control and standard.
Oral glucose test was performed in overnight fasted rats to observe the potency of extract in glucose primed condition. Both the test group exhibited fall in blood glucose level in dose dependent manner. The reduction in blood glucose level in standard treated group was more in compare to test group (Table 2).
Table 2: Oral glucose tolerance test.
| S.No. | Treatment group | Dose(mg kg-1p.o.) | Blood Glucose Level (mg dl-1) Mean ± S.D. |
||
|---|---|---|---|---|---|
| 0 h | 30 min | 90 min | |||
| 1. | Normal Control | Untreated | 77.16 ± 2.44 | 75.69 ± 1.71 | 73.23±2.33* |
| 2. | Glucose Primed Control | Vehicle | 78.45 ± 2.51 | 183.41 ± 3.66*** | 148.39 ± 6.65*** |
| 3. | Glibenclamide | 0.5 mg/kg | 80.45 ± 3.49 | 147.69 ± 4.43*** | 88.50 ± 3.30** |
| 4. | APAE001 | 250 | 79.30 ± 3.05 | 147.56 ±4.44*** | 128.57 ± 5.13***,### |
| 5. | APAE002 | 500 | 78.39 ± 3.19 | 141.29 ± 3.41*** | 121.30 ± 5.77***,### |
Data is presented as mean±SD (n=6). Statistical significance was assessed using one-way ANOVA with Tukey’s multiple comparisons post hoc analysis.*P<0.05: Comparison with ‘0’ h in respective group;**P<0.001: Comparison with ‘0’ h in respective group;***P<0.0001: Comparison with ‘0’ h in respective group.###: P<0.0001: Comparison with '90 mins’ of standard group.
In diabetes mellitus, there are many underlying factors that contribute to high blood glucose levels out of which insulin resistance in peripheral tissues and reduced production of insulin in β-cells are primary contributing factors. STZ exposure to animal models induces diabetes through selective destruction of β-cells of pancreas by necrosis. During diabetic condition increased blood glucose and excess production of antioxidants are primarily responsible for progression and complication of diabetes in mammals.
The curative response of medicinal plants largely depends on synergy of phytoconstituents, cumulatively accounting for the overall bioactivities. The hypoglycemic effect of A. pseudalhagi was observed comparative to standard antidiabetic drug (Table 2). The exact mechanism is not yet clear for anti-hyperglycemic effect of medicinal plants; however, it may be cumulatively due to restoration of pancreatic tissue function causing improved insulin output and to some extent decrease in intestinal absorption saccharides because of active ingredients present in extract (Tables 3 and 4). The presence of phenolic and flavonoid contents is largely correlated with antidiabetic potential of plant extract [31].
Table 3: Blood glucose levels in STZ induced diabetic rat model.
| S. No. | Groups | Blood Glucose Level (mg dl-1) (Mean ± S.D.) |
|||||
|---|---|---|---|---|---|---|---|
| Dose (mg /kg) |
Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| 1 | Normal Control | Untreated | 77.63±3.45 | 92.13±5.24 | 107.43±5.33*** | 87.61±6.05 | 82.74±5.74 |
| 2 | STZ Control | Vahicle1ml/kg | 80.13±3.83 | 353.94±12.98*** | 326.37±8.16*** | 286.37±8.16*** | 269.43±10.74*** |
| 3 | Glibenclamide | 0.5 | 78.01±3.27 | 348.09±13.05*** | 289.66±13.05*** | 177.44±5.83*** | 115.51±9.67*** |
| 4 | APAE001 | 250 | 76.87±3.39 | 358.29±10.33*** | 323.82±10.98***, ### | 273.17±10.31***, ### | 214.19±13.61***, ### |
| 5 | APAE002 | 500 | 79.14±3.55 | 350.59±12.15*** | 303.83±19.30*** | 213.82±12.27***, ### | 144.66±8.58***, ### |
Data is presented as mean±SD (n=6). Statistical significance was assessed using one-way ANOVA with Tukey’s multiple comparisons post hoc analysis.*P<0.05: Comparison with day ‘0’ in respective group;**P<0.001: Comparison with day ‘0’ in respective group;***P<0.0001: Comparison with day ‘0’ in respective group. ###: P<0.0001: Comparison with respective day of standard group.
Table 4: Wound contraction rate in experimental animals.
| Topical Treatment | Wound Contraction Rate (%) | |||
|---|---|---|---|---|
| 4 day | 7 day | 15 day | 21 day | |
| Control | 26.06 ± 5.04 | 38.95 ± 3.77 | 61.37 ± 5.08 | 76.71 ± 4.14 |
| Standard*** | 39.92 ± 3.11 | 63.98 ± 5.26 | 86.74 ± 5.66 | 95.52 ± 2.59 |
| APAE001 | 33.81 ± 5.42* | 56.60 ± 4.93*** | 80.65 ± 3.91*** | 86.23 ± 3.55** |
Data is presented as mean ± SD (n=6). Statistical significance was assessed using one-way ANOVA with Tukey’s multiple comparisons post hoc analysis. Comparison with control: *P<0.05;**P<0.001;***P<0.0001.
Tannins present in A. pseudalhagi may be held responsible to promote the wound healing through several cellular mechanisms, chelating of the free radicals and reactive species of oxygen, promoting contraction of the wound and increasing the formation of capillary vessels and fibroblasts. Tannins also serves as free radical scavengers. The Phenolic compounds like phenolic acids, flavonoids and tannins are considered important plant metabolites that play a significant role in the diabetic wound healing [32]. The plant contains tri-terpenoids and flavonoids which promote wound healing due to their astringent and antimicrobial property which appear to be responsible for wound contraction and elevated rate of re-epithelialization. Flavonoids also posses potent antioxidant and free radical-scavenging effect, enhancing the level of antioxidant enzymes in granuloma tissue.
Conclusion
The observations from present study suggest A. pseudalhagi as a promising candidate with potential antidiabetic activity having wound healing property as well. It may prove to be effective in the treatment of diabetic wound healing.
References
- Sachan NK, Kumar Y, Pushkar S, Thakur RN, Gangwar SS, et al. (2009) Antidiabetic potential of alcoholic and aqueous extracts of Ficusracemosa Linn bark in alloxan induced diabetic rats. International Journal of Pharmaceutical Sciences and Drug Research1: 24-27.
- Ahmed Z, Chisthi MZ, Johri RK, Bhagat A, Gupta KK, et al. (2009) Antihyperglycemic and Antidyslipidemic Activity of Aqueous Extract of Dioscoreabulbifera Tubers, DiabetologiaCroatica 38:63-72.
- Sachan NK (2010) Contribution of Indian Traditional and Holistic Medicine to New Drug Development Book Chapter. In: Kumar A, Das G(eds.) Biodiversity, Biotechnology and Traditional Knowledge,Narosa Publishing House, New Delhi, India.
- Sheweita SA, Mashaly S, Newairy AA, Abdou HM, Eweda SM (2016) Changes in Oxidative Stress and Antioxidant Enzyme Activities in Streptozotocin-Induced Diabetes Mellitus in Rats: Role of Alhagimaurorum Extracts. Oxidative Medicine and Cellular Longevity.Hindawi Publishing Corporation, pp: 1-8.
- Earley MJ (2008) Wound, Tissue Repair and Scares. In: Williams NS, Christopher JKB (eds.) Baieley and Loves: Short Practice of Surgery. London: Edward Arnold Publishers Limited 25: 24-50.
- Lakshmi A, Rao YM, Bhargavi CH, Seelam U (2011) Antidiabetic and Wound Healing Activity of Various Bark Extracts of Polyalthialongifolia. Asian Journal of Pharmaceutical and Clinical Research4: 109-113.
- KirtikarKP, BasuBD (1932) Indian Medicinal Plants. Allahabad, India.
- Chopra RN,NayarSL, ChopraC (1956)Glossary of Indian Medicinal Plants. Council of Scientific and Industrial Research, New Delhi, India.
- Sachan NK, Sachan AK, Rao V (2015) Ethnobotanical Survey of Indigenous Medicines Practiced in Chambal Valley of Uttar Pradesh. BharatiyaVaigyanikEvamAudyogikAnusandhanPatrika (CSIR-NISCAIR) 23: 132-135.
- Srivastava B, Sharma H, Dey YN, Wanjari MM,Jadhav AD (2014) Alhagipseudalhagi: A Review of its Phyto-chemistry, Pharmacology, Folklore Claims and Ayurvedic Studies. International Journal of Herbal Medicine 2: 47-51.
- Varshney K, Singh AK (2008) Inventory of Some Ethnomedicinal Plant Species used by Rural People of Etah District, UP, India, Plant Achieves 8: 757-759.
- Muhammad G, Hussain MA, Anwar F, Ashraf M, Gilani AH (2015) Alhagi: A Plant Genus Rich in Bioactives for Pharmaceuticals. Phytotherapy Research; 29: 1-13.
- Khushbaktova ZA, Syrov VN, Kuliev Z (1992) The Effect of Proanthocyanidins from Alhagipseudoalhagi. Desv on Course of Experimental Myocardial Infarction. EKSP KlinFarmakol 55:19-21.
- Khaiitbaev KK, Sultan A, Ganiev SS, Aslanov KA (1993) Quantitative Determination of the Total Flavonols in Alhagipseudoalhagi, Chemistry of Natural Compounds 29: 586.
- Altmysheva AA (1976) Medicinal Wealth of Kirgizia [in Russian].(2nd Edn), Frunze, Kyrgyzstan.
- Cirous A, Goudarzi D, Jahangiri V (2010) The Effect of Alhagipseudalhagi Distillate on Ureteral Stone Expulsion. AMUJ 13: 56-62.
- Rahmatullah M, Khatun MA, Morshed N (2010a) A Randamized Survey of Medicinal Plants used by Folk Medicinal Healers of Sylhet Division Bangladesh.Adv Nat ApplSci 4: 52-62.
- Rahmatullah M, Ferdausi D, Mollik MOH, Jahan R, Chowdhary MH, et al. (2010b) A Survey on Medicinal Plants used by Kaverajes of Chalna Area, Khulna District, Bangladesh. Afr J Tradit Complement Altern Med 7: 91-97.
- Gulzar M (2015) Alhagi: A plant genus rich in bioactives for pharmaceuticals, Phytotherapy Research 29:1-13.
- Sachan NK, Srivastava U, Sachan AK (2015) Livelihood Empowerment Opportunities through Planned Tapping of Medicinal Plants as Minor Forest Produce. CSIR-BharatiyaVaigyanikevamAudyogikAnusandhanPatrika 23: 24-30.
- Evans WC (2005)Trease and Evans: Pharmacognosy. Edinburgh: Saunders/Elsevier.
- Harbone JB (1998) Phytochemical methods: A guide to modern techniques of plant analysis. London: Champman and Hall.
- Doughari JH (2012)Phytochemicals: Extraction Methods, Basic Structures and Mode of Action as Potential Chemotherapeutic Agents, Phytochemicals - A Global Perspective of Their Role in Nutrition and Health. Rao DV(ed.) Croatia: InTech Open.
- Khandelwal KR (2004) Practical Pharmacognosy, Techniques & Experiments, XIII edn. NiraliPrakashan, Pune.
- Chandira M, Vankateswarlu BS, Gangwar RK, Sampathkumar KP, Bhowmik D, et al. (2010) Studies on Anti-stress and Free Radical Scavenging Activity of Whole Plant of Cocciniaindica Linn.Internat Res J Pharmaceutical Sci 1: 0050-0055.
- Sachan NK, Kumar Y (2010) Phytochemical Investitagion of Ficus racemosa Bark-an Ethnomedicinal Plant. Annals of Pharmacy and Pharmaceutical Sciences 1: 80-84.
- Veeraraghavan P (2000) Expert consultant CPCSEA, OECD guidelines # 420.
- Rathore K, Singh V, Jain P, Rao SP, Ahmed Z, et al. (2014)In-vitro and In-vivo Antiadipogenic, Antidiabetic and Hypolipidemic Activity ofDiospyrosmelanoxylon (Roxb.). Journal of Ethnopharmacology 155: 1171–1176.
- Singh MP, Pathak K (2015) Animals Models for Biological Screening of Antidiabetic Drugs: An Overview.European Journal of Experimental Biology5: 37-48.
- Rathnakumar K, Verma R, Jaikumar S, Sengottuvelu S (2013) Wound healing Activity of Ethanolic Extract of Euphorbia hirta Leaves on Excision Wound model in Rats. Global J Res Med plants &Indigen Med 2: 871-575.
- Khan TY, Raina R, Verna PK, Sultana M, Jyoti MK (2014) Phytochemical Constituents and Antidiabetic Potential of Ipomoea carneaJacq Leaves Extracts. Journal of Experimental and Integrative Medicine4:137-142.
- Singh A, Singh PK, Singh RK (2014) Antidiabetic and Wound Healing Activity of Catharanthusroseus L. in StreptozotocinInduced Diabetic Mice. American Journal of Phytomedicine and Clinical Therapeutics 2: 686-692.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences