Exfoliative Cytopathology of Human Lingual Neoplasm
Mohanta A* and Mohanty PK
Post-Graduate Department of Zoology, Utkal University, Bhubaneshwar, Odisha, India
- *Corresponding Author:
- Mohanta A
Research Scholar, Post-Graduate Department of Zoology
Utkal University, Bhubaneshwar, Odisha, India
Tel: +91 9937575859
E-mail: amohanta01@gmail.com
Received date: October 31, 2016; Accepted date: November 28, 2016; Published date: December 05, 2016
Citation: Mohanta A, Mohanty PK. Exfoliative Cytopathology of Human Lingual Neoplasm. J Clin Med Ther. 2016, 1:1.
Abstract
Objective: The present work is aimed to evaluate the prognostic and diagnostic importance of cytological atypias in exfoliative cytopathology of Human Lingual Neoplasm (HLN).
Methodology: In a hospital-based case-control study, 36 subjects (18 cases of lingual neoplasm and 18 control individuals) were included. Exfoliated scrape cytosmears were collected from the affected lingual site over the pre-coded cleaned microslides. Two slides were prepared from each case. Samples were immediately fixed in 1:3 aceto-alcohol fixatives. One set of the fixed samples was stained with Papanicolaou’s stain and the other set was counterstained with Giemsa’s stain. Stained slides were observed under Hunds-500 light microscope. 1000 cells were screened and suitable cytological atypias were scored. Photomicrographs were taken out as the supporting evidence. Software package PAST®, Version 2.17 was used for statistical analysis.
Result: Lateral borders (61.1%), of the tongue were found to be the most common sites of lingual carcinogenesis. Among the well observed cytological pleomorphism, occurrence of a number of moderately differentiated cytological atypias (KSC, KTC, KSC-A, KFC and KRC) in the cytosmears of premalignant cases indicates that the lesions were unexpectedly in an advanced stage. Furthermore, MNC, KSC and KTC are found to be the modal cytological atypias in the HLNs and so, these may be considered as the predictable potential biomarkers of lingual carcinoma.
Conclusion: Occurrence of multi-modal diagnostic cytological atypias such as MNC, KSC and KTC in the exfoliated cytosmears has a practical role in prognosis and diagnosis of HLNs. Therefore, exfoliative cytopathology will be helpful to defeat the dragon of diagnostic dilemma in HLNs in general and to detect the lingual carcinoma at an early hand in particular.
Keywords
Exfoliated cytosmears; Atypias; Pleomorphism; Human Lingual Neoplasm (HLN)
Introduction
Human Lingual Neoplasm (HLN) is one of the most commonly occurring neoplasms accounting for about 30% of all the human intra-oral malignancies and approximately 3% of all human malignant tumors [1]. Synonymously, HLN is popularly known as tongue cancer (TC), as it is pertaining to the tongue of the oral cavity and biomedically, it is known as squamous cell carcinoma of oral tongue (SCCOT), tongue carcinoma (TC), and lingual carcinoma (LC) as cancer of the tongue is originated from the epithelial squamous cells of the tongue. These are a homogeneous group of tumors characterized by aggressive behaviour, early spread to lymph nodes and a higher rate of regional failure compared to gingivo-buccal cases [2,3]. Specifically, the anterior movable tongue is the common affected site and accounts for 97% of all malignancies of the respective site [4]. Generally, the peak incidence is usually in the 5th and 6th decades but cases have been reported in young individuals too. Carcinoma of tongue is associated frequently with alcohol and tobacco chewing as a common etiology. However, the incidence of HLN among younger population (<50 years) is on the rise; many of whom lack the typical associated risk factors of alcohol and/or tobacco exposure [5].
In spite of technological advancement, early detection of HLN is still persisting as a great challenge. Although, Magnetic Resonance Imaging (MRI) is the imaging modality of choice for evaluation of tongue carcinomas [6], invasive method of biopsy remains as gold standard [7]. However, psycho-economically, the patients usually prefer to simple, non-invasive, time and cost-effective exfoliative cytopathology for diagnosis of oral tongue carcinoma. As more importance on molecular markers and histopathology than the exfoliative cytopathology of lingual carcinoma is given, more papers are published related to the former ones. On the other hand, the published papers on exfoliative cytopathology of lingual carcinoma are also found to be inadequate inconsistent and inappropriate so far as early detection of HLN is concerned. Therefore, the present work is aimed to analyse the importance of exfoliative cytopathology for early detection of human lingual carcinoma.
Materials and Methods
The subjects
In a hospital-based study, out of 136 oral cases, 18 (13.23%) lingual neoplasm cases (11 males and 7 females) registered at the Out-patient Department of Acharya Harihar Regional Cancer Centre (AHRCC), Cuttack, Odisha, India during May 2007 to May 2009 were included in this study. All the patients were referral cases, belonging to different regions of Odisha State. They did not have undergone either chemotherapy or radiotherapy. Detailed case-history including age, sex, oral site the nature and types of addiction of each individual was recorded prior to the collection of samples. Addicted individuals were habituated with different forms of tobacco and alcohol for more than 15 years. Age-group and sex matched non-addicted 18 healthy individuals were also included in this study as Control group. Thus, a total of 36 lingual cases were taken into account for this study.
Collection of samples and staining
Prior to the collection of sample, written consent of the respective subject was obtained. Two scalpel-scraped exfoliated cytosmears were collected from the affected site of the tongue on the pre cleaned-coded glass-slides. Collected cytosmears were fixed in 1:3 aceto-alcohols (1 part of glacial acetic acid and 3 parts of ethyl alcohol) immediately. A set of smears was stained with Papanicolaou’s stain and the other set was counterstained with Giemsa’s stain for cytopathological analysis. Stained slides were observed under Hunds-H500 light microscope fitted with a computer assisted Cat Cam 1.30 microscope camera. Photomicrographs were taken out as the supporting evidences.
Statistical analysis
Out of 1000 observed cells, the cytological atypias were scored. Software package Palaeontological Statistics (PAST)®, Version 2.17 was used for statistical analysis. Z-test was carried out at 1% (p<0.01) level of confidence.
Ethical considerations
This study was approved (Reference No EC/UU-38832/2007) by the Subject Research Committee (SRC) of Utkal University, Bhubaneshwar, Odisha, India and necessary permission from the Director, AHRCC, Cuttack, Odisha, India was also obtained for the same purpose.
Results
The cases: clinical aspects
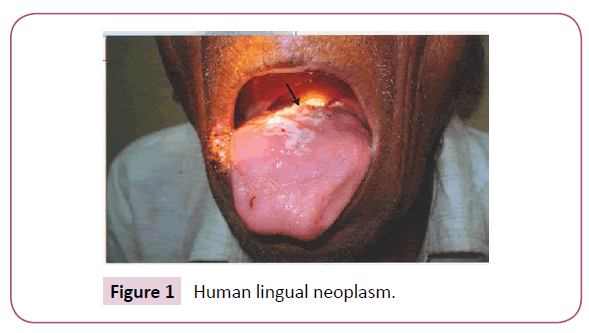
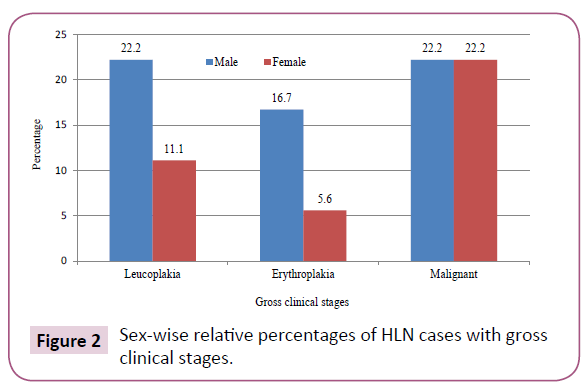
Out of 136 collected samples, a total of 18 (13.23%) cases of lingual neoplasm (Figure 1) were recorded in our study. Among them 11 (61.1%) were male and 7 (38.9%) were female. Ten (55.5%) cases were with premalignant lesion and 8 (44.5%) were malignant. Four (22.2%) male and 2(11.1%) female were suffering from leukoplakia. Three (16.7%) male and 1(5.5%) female were with erythroplakia. Out of 8 malignant cases, 4(22.2%) were male and 4(22.2%) were female (Figure 2). Lateral borders (61.1%), such as left lateral border (LLB-33.3%) and right lateral border (RLB-27.8%) of the tongue were found to be the most common sites of lingual carcinogenesis. Dorsal mid-central (DMC) of the tongue was affected in 5 (27.8%) cases and the ventral of the tongue (VOT) was found to be the least (11.1%) affected site of tongue cancer in this study.
Cervical Lymph Nodes (CLN) were observed in 12(66.7%) cases and absent in 6(33.3%) cases. Seventeen (94.4%) among 18 patients were addicted to different forms of tobacco and alcohol for more than 15 years and only one (5.6%) female (Case No 13) was non-addicted in 65 years of her life. The age of the patients was ranged from 34 year (Case no. 11) to 77 year (Case no. 9) with an average of 53.33 ± 12.17 year. A brief general attributes of the subjects is summarized in Table 1.
| Attributes | Male | Female | Total (%) |
|---|---|---|---|
| No of collected samples | |||
| Control | 11 | 7 | 18 (50) |
| Affected | 11 | 7 | 18 (50) |
| Age groupsin years | |||
| 30-49 | 5 | 2 | 7 (38.9) |
| 50-69 | 5 | 4 | 9 (50.0) |
| 70-89 | 1 | 1 | 2 (11.1) |
| Pathogenicity | |||
| Leukoplakia | 4 | 2 | 6 (33.3) |
| Erythroplakia | 3 | 1 | 4 (22.2) |
| Malignant | 4 | 4 | 8 (44.5) |
| Metastasis | |||
| CLN Positive | 6 | 4 | 10 (55.5) |
| CLN Negative | 5 | 3 | 8 (44.5) |
| Addiction pattern | |||
| Chewers/Snuff dippers | 3 | 4 | 7 (38.9) |
| Smokers | 3 | Nil | 3 (16.7) |
| Alcoholics | 2 | 1 | 3 (16.7) |
| Mixed | 3 | 1 | 4 (22.2) |
| Non-addicted | Nil | 1 | 1(5.5) |
| Occupation | |||
| Labourer | 5 | 4 | 9 (50.0) |
| Service | 6 | 3 | 9 (50.0) |
Table 1: General attributes of the patients.
Cytopathology
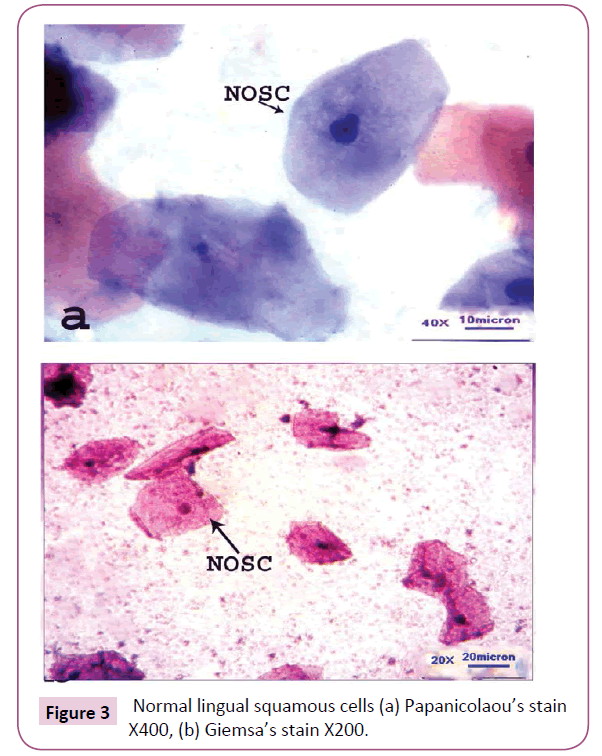
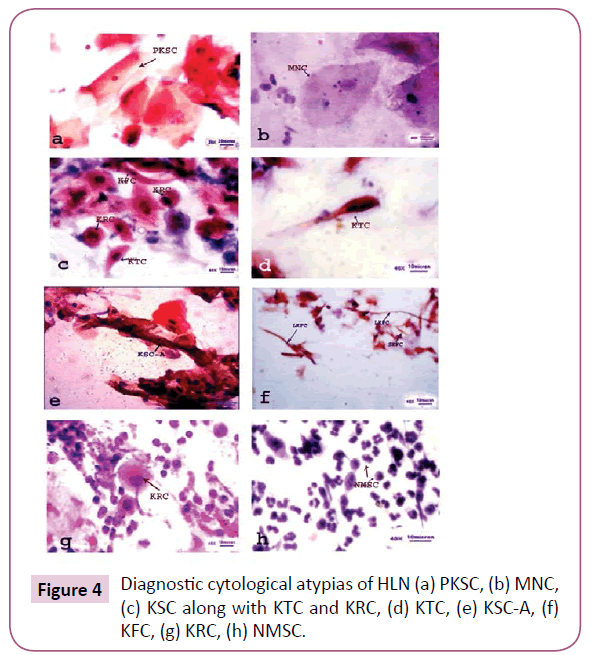
Cytological pleomorphism was well observed exhibiting a number atypias in the exfoliated cytosmears of HLN. Generally, the normal epidermal cells of the tongue were found to be more or less polyhedral with well-defined cell boundary, non-keratinized cytoplasm and centrally located rounded or oval nucleus (Figure 3). In due course of carcinogenesis, the normal cells of the tongue were metamorphosed into various pleomorphic cytological atypias, such as Micronucleated cell (MNC), Plump keratinized squamous cell (PKSC), Keratinized spindle cell (KSC), Keratinized strap (Anitschkow) cell (KSC-A), Keratinized fiber cell (KFC), Keratinized round cell (KRC) and Non-keratinized malignant squamous cell (NMSC) with drastic and drammatic modification. KFC and KRC were observed to be in two different forms-large and small. Thus, large keratinized fiber cell (LKFC), small keratinized fiber cell (SKFC), large keratinized round cell (LKRC) and small keratinized round cell (SKRC) have their own identity so far as cytological pleomorphism is concerned. MNC and PKSC were observed to be well differentiated squamous cell (WDSC); KSC, KTC, KSCA, KFC and KRC were moderately differentiated squamous cell (MDSC) and NMSC was absolutely poorly differentiated squamous cell (PDSC) (Figure 4). Universal occurrence of MNCs in all exfoliated cytosmear with gradual increase in its frequency from normal to malignant cases proves itself to be an onco-indicator as well as a potential biomarker of oral carcinogenesis [8].
Figure 3: Normal lingual squamous cells (a) Papanicolaou’s stain X400, (b) Giemsa’s stain X200.
PKSCs were chiefly found in premalignant lesions (leucoplakia and erythroplakia) and rarely found if present, in benign and malignant cases [9]. The moderately differentiated KSC, KTC, KSC-A, KFC and KRC are generally observed in pre-malignant and malignant tumors [10]. However, these were also frequently observed in the cytosmears of lingual premalignant lesions (as in case No 1, 6, 10, 15). Presence of these moderately differentiated in premalignant cases clearly indicates that the lesions were unexpectedly in an advanced stage and trigger aggressiveness. It is noteworthy that, in addition to MNC, both KSC and, KTC were found to be the modal cytological atypias in the HLNs. Therefore, to be frank, except PKSC and MNC, other detected pleomorphic cytological atypias may or may not be found in all cases, but presence of either one or more type of these atypias in a lingual cytosmear indicates the state of malignancy.
On the basis of Broder’s cytological differentiation [11], 11(61.1%) cases were well differentiated squamous cells (WDSC), 5(27.8%) cases were moderately differentiated squamous cells (MDSC) and the rest 2 (11.1%) were of poorly differentiated squamous cells (PDSC) type among lingual neoplasm cases. However, on the basis of cytopathological differentiation (pattern of keratinization, chromatisation and nuclear pleomorphism), our finding indicates that none of the cases was WDSC, 10 (55.6%) cases were MDSC and 8 (44.4%) were PDSC type (Table 2). Therefore, the detected cytological atypias have both prognostic and diagnostic importance during difficult diagnosis of HLN cases.
| Categories | Broder’scytological differentiation | Cytopathologicaldifferentiation* | ||||
|---|---|---|---|---|---|---|
| Male | Female | Total (%) | Male | Female | Total (%) | |
| WDSC | 6 | 5 | 11 (61.1) | Nil | Nil | Nil |
| MDSC | 3 | 2 | 5 (27.8) | 7 | 3 | 10 (55.6) |
| PDSC | 2 | Nil | 2 (11.1) | 4 | 4 | 8(44.4) |
| *On the basis of cytopathological analysis | ||||||
Table 2: A comparative account of cellular differentiation.
Statistical analysis
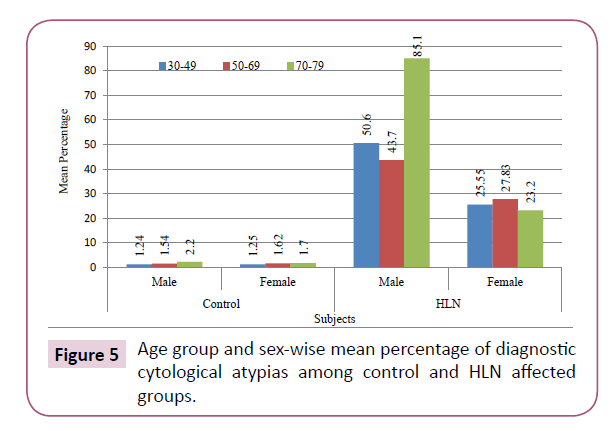
From the numerical point of view, highest number of NMSCs (3911 in male and 1116 in female) was recorded and KSCA was enumerated to be the lowest (only 5 in male and 8 in female) in the cytosmears of tongue neoplasm (Table 3). Lingual scraping of control group depicted that the number of cytological atypias recorded from 5, 5 and 1 males were 62, 77, 22 having their percentage to be 1.24, 1.54 and 2.2 and from 2, 4 and 1 females, 25, 65 and 17 atypical cells with 1.25, 1.62 and 1.70 per cent were scored in the age group 30-49, 50-69 and 70-89 years respectively. Thus, the mean percentage of atypias in males was 1.46 and in females, it was 1.52.
| Group | Age | No. of Samples | KSC | KTC | KSCA | LKFC | SKFC | LKRC | SKRC | MNC | PKSC | NMSC | Total | Mean % | Z-value | ||||||||||||||
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | ||
| 30-49 | 5 | 2 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | 25 | 12 | 37 | 13 | Nil | Nil | 62 | 25 | 1.46 | 1.52 | - | - | |
| 50-69 | 5 | 4 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | 36 | 28 | 41 | 37 | Nil | Nil | 77 | 65 | |||||
| 70-89 | 1 | 1 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | 8 | 7 | 14 | 10 | Nil | Nil | 22 | 17 | |||||
| 30-89 | 11 | 7 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | 69 | 47 | 92 | 60 | Nil | Nil | 161 | 107 | 1.46 | 1.52 | - | - | |
| Affected | 30-49 | 5 | 2 | 34 | 28 | 11 | 2 | 2 | 1 | 12 | 6 | 6 | 2 | 12 | 8 | 11 | 14 | 62 | Nil | Nil | 110 | 1935 | 308 | 2830 | 511 | 53.3 | 27.8 | 106.002* | 47.434* |
| 50-69 | 5 | 4 | 57 | 72 | 14 | 8 | 2 | 7 | 13 | 8 | 5 | 10 | 13 | 10 | 14 | 22 | 104 | Nil | Nil | 264 | 1481 | 648 | 2185 | 1133 | |||||
| 70-89 | 1 | 1 | 35 | 35 | 3 | 2 | 1 | Nil | 2 | 4 | 3 | 6 | 3 | 5 | 8 | 28 | 22 | 279 | 62 | 495 | 160 | 851 | 302 | ||||||
| 30-89 | 11 | 7 | 126 | 135 | 28 | 12 | 5 | 8 | 27 | 18 | 14 | 18 | 25 | 21 | 30 | 44 | 194 | 138 | 1506 | 436 | 3911 | 1116 | 5866 | 1946 | 53.3 | 27.8 | 106.002* | 47.434* | |
| M: Male; F: Female; *Significant at 1% (p≤0.01) level of confidence | |||||||||||||||||||||||||||||
Table 3: Age group and sex-wise comparative account of diagnostic cytological atypias in control and lingual neoplasm cases.
In lingual carcinoma group, the recorded number of cytological atypias from 05, 05and 01 males were 2830, 2185 and 851, having the percentage of 50.60, 43.70 and 85.10 and from 2, 4, 1 females, 511, 1133 and 302 atypias having percentage of 25.55, 27.83 and 23.20 in the age group of 30-49, 50-69 and 70-89 years respectively. The mean percentage of atypias was thus found to be 53.32 in males and 27.80 in females (Figure 5). The z-values, in lingual carcinoma were recorded to be 106.002 in males and 47.343 in females, which signified the test of significance (p≤0.01) itself.
Metastasis and TNM staging
Out of 18cases of lingual neoplasm, CLN were found in 12 (66.7%) cases (9 male and 3 female) and absent in 6 (33.3%) cases (2 male and 4 female) irrespective of their single or multiple (mixed) addiction habit. CLN were observed either in single, double or multiple in numbers with either ipsilatetal or contra-lateral in position. Pattern of metastasis differs from individual to individual is really unpredictable (Table 4). American Joint Committee for Cancer (AJCC) Staging and End Results Reporting-2010 was followed for Tumor-Node-Metastasis (TNM) staging of lingual neoplasms. Accordingly, only 1 (5.6%) case was recorded to be in Stage I, 5(27.8%) cases were in Stage II and 12 (66.6%) cases were in Stage IV. None of the cases were in Stage III (Table 5). It indicates that, due to robust aggressiveness, the HLN progresses from premalignant lesions towards malignancy recklessly.
| Case No. | Age | Sex | Specific site | Lesion size | Lesiontype | Cytological atypias | Addiction | Metastasis if any | TNM staging* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | Male | DMC | ≥2.5 cm | Erythroplakia, Ulcerative | KSC, KTC, PKSC, MNC, KRC | Chewers/Snuff dippers | CLN, SingleIpsilateral | T2N1M1 |
| 2 | 56 | Female | RLB | ≥3.2 cm | Exophyticmalignant tumor | KSC, KTC,KSCA, KRC, KFC, NMSC | Chewers/Snuff dippers | Absent | T2N0M0 |
| 3 | 36 | Male | LLB | ≥2.5 cm | Leukoplakia | PKSC, MNC, KTC,KSCA | Smokers | CLN, Single,Contra- lateral | T2N2cM1 |
| 4 | 42 | Female | RLB | ≥2.6 cm | Leukoplakia | PKSC, MNC, KSC, KTC,KSCA | Mixed | CLN, multiple,Ipsilateral | T2N2bM1 |
| 5 | 52 | Male | DMC | ≥3.6 cm | Malignant Ulcerative | KSC, KRC, KTC, KFC, NMSC | Chewers/Snuff dippers | CLN, Single,Ipsilateral | T2N1M1 |
| 6 | 58 | Female | RLB | ≥3.5 cm | Erythro-leukoplakia | MNC, KSC, KTC, KSCA, KFC, | Alcoholics | Absent | T2N0M0 |
| 7 | 72 | Male | RLB | ≥2.5 cm | Malignant Ulcerative | MNC, KSC, KTC, KSCA, KRC, KFC, NMSC | Mixed | CLN, multiple,Ipsilateral | T2N2bM1 |
| 8 | 48 | Male | LLB | ≥1.5 cm | Leukoplakia | PKSC, MNC, KTC, KSC | Alcoholics | Absent | T1N0M0 |
| 9 | 77 | Female | DMC | ≥2.8cm | Exophytic malignant tumor | MNC, KSC, KTC, KSCA, KRC, KFC, NMSC | Chewers/Snuff dippers | CLN, double,Ipsilateral | T2N2bM1 |
| 10 | 58 | Male | LLB | ≥2.5 cm | Erythroplakia | KSC,PKSC, MNC,KSCA, KTC, KRC, KFC | Smokers | CLN, SingleIpsilateral | T2N1M1 |
| 11 | 34 | Female | DMC | ≥2.3 cm | Leukoplakia | PKSC, MNC,KTC,KSCA, KRC | Mixed | Absent | T2N0M0 |
| 12 | 35 | Male | VOT | ≥1.7 cm | Exophytic malignant tumor | KSC, KRC, KFC, MNC, NMSC | Chewers/Snuff dippers | CLN, double,Contra- lateral | T1N2cM1 |
| 13 | 65 | Female | LLB | ≥2.6 cm | Malignant Ulcerative | MNC, KSC, KTC, KSCA, KRC, KFC, NMSC | Non-addicted | CLN,multiple, Contra- lateral | T2N2cM1 |
| 14 | 55 | Male | LLB | ≥3.2 cm | Leucoplakia | PKSC, MNC, KTC, KSC, KSCA | Mixed | CLN,multiple, Contra- lateral | T2N2cM1 |
| 15 | 54 | Male | VOT | ≥1.8 cm | Leucoplakia | PKSC, MNC, KSC,KTC, KRC | Alcoholics | CLN, double,Ipsilateral | T1N2bM1 |
| 16 | 62 | Female | RLB | ≥2.5 cm | Malignant Ulcerative | MNC, KSC, KTC, KSCA, KRC, KFC, NMSC | Chewers/Snuff dippers | Absent | T2N0M0 |
| 17 | 46 | Male | LLB | ≥3.3 cm | Exophytic malignant tumor | MNC, KSC, KTC, KSCA, KRC, KFC, NMSC | Mixed | CLN, double, Contra- lateral | T2N2cM1 |
| 18 | 66 | Male | DMC | ≥2.2 cm | Leucoplakia | PKSC, MNC, KSC, KTC, KSCA | Smokers | Absent | T2N0M0 |
| NB-LLB: Left Lateral Border; RLB: Right Lateral Border; DMC: Dorsal Mid-line Central; VOT: Ventral of the Tongue *American Joint Committee for Cancer(AJCC) Staging and End Results Reporting-2010 |
|||||||||
Table 4: A brief summary of case-history, clinical pathogenicity and diagnostic cytological atypias in 18 HLN cases.
| Stage | Tumor (T) | Node (N) | Metastasis (M) | No of cases (%) |
|---|---|---|---|---|
| Stage I | T1 | N0 | M0 | 1 (5.6) |
| Stage II | T2 | N0 | M0 | 5 (27.8) |
| Stage III | T | N | M | Nil (0) |
| Stage IV | T1 | N2b | M1 | 1 (5.6) |
| T1 | N2c | M1 | 1 (5.6) | |
| T2 | N1 | M1 | 3 (16.6) | |
| T2 | N2b | M1 | 3 (16.6) | |
| T2 | N2c | M1 | 4 (22.2) | |
| *American Joint Committee for Cancer(AJCC) Staging and End Results Reporting-2010 | ||||
Table 5: TNM Staging* of the HLN cases.
Discussion
The oral tongue located in the central part of the oral cavity is composed of numerous muscles wrapped in mucous membrane. It is formed by the anterior two-third of the tongue up to the circumvallate papillae while the posterior one‑third of the tongue, also called base tongue is a part of the oropharynx. The midline lingual septum divides the tongue into equal halves, consisting of the intrinsic and extrinsic muscles. Being a sense organ and tango-receptor, it enables the taste of the food we take and plays a critical role in mastication, formation of food bolus, deglutition, and swallowing of food. Not only that, it is also responsible for the unique production of sound, voice and speech.
Unfortunately, the multi-faceted mobile tongue becomes carcinomatous in a multi-step lingual carcinogenesis by accumulation of multiple genetic and epigenetic alterations [12]. Although, tongue is the commonest site of intra-oral carcinogenesis, it harbours a variety of carcinomas, such as, Verrucous Carcinoma (VC), Spindle Cell Carcinoma (SpCC), basal cell carcinoma, etc. Verrucous carcinoma (Ackerman’s tumour) is an uncommon but distinct variety of well differentiated squamous cell carcinoma first reported by Ackerman in 1948. He pointed out that VC of the tongue is clinically rare. Most patients are elderly males with smoking history [13]. The macroscopic appearance of Ackerman’s tumour depends on several factors like duration of lesion, degree of keratinization and the changes in adjacent mucosa. The fully developed carcinoma in an exophytic gray to red bulky lesion with a rough, shaggy, papillomatous surface. The term “verrucous” is used because of its fine, finger like projections over the surface of the tongue [14]. Microscopically, papillary proliferation and severe thickening of squamous epithelium are observed. Swelling of nuclei and mitoses are found occasionally [15].
SpCC is a relatively rare malignancy affecting the upper aerodigestive tract. It is a poorly differentiated variant of squamous cell carcinoma (SCC) [16] with a more aggressive behaviour [17-20]. Tumor cells are atypical with increased nuclear/cytoplasmic (N/C) ratio, having large, pleomorphic, hyperchromatic, vesiculated nuclei, and reasonable eosinophilic cytoplasm. Due to eosinophilic, the cytoplasm becomes keratinized and so it was named as Keratinized Spindle Cell (KSC). Mohanta et al. have reported that the KSCs are moderately differentiated, pleomorphic with increased N/C ratio which aggravates oral carcinogenesis [21]. In the present study, occurrence of a multiple number of moderately differentiated cells in the premalignant lesions exhibits the aggressiveness of lingual carcinoma. Also, in addition to MNC, both KSC and KTC are found to be the modal cytological atypias in the HLNs-for which, these may be considered as the potential predictive marker of lingual carcinogenesis.
HLN may arise in apparently normal epithelium, in areas of leukoplakia, or in an area of chronic glossitis. Typically, three types of gross morphologic growth patterns, such as exophytic, ulcerative, and infiltrative-are observed in tongue carcinoma with the lateral border being the most common sub-site of origin. In a retrospective study, Ranjan et al. have pointed out that the incidence of tongue cancer was more in males (male: female=6.1:1) particularly in their fifth decade (mean 52.7 years) and the most common location was anterior 2/3rd of the tongue mainly on right lateral side [22]. The average age of the patients was recorded to be 53.33 ± 12.17 year and the posterior lateral borders (61.1%), followed by DMC (27.8%) of the anterior tongue were found to be the most common sites of lingual carcinogenesis in this study which corroborates with the earlier finding.
HLN is an aggressive head and neck malignancy and is well-known for its high rate of proliferation and nodal metastasis. Although it is visibly located in oral cavity, 50% patients were already in advanced stage III and IV during presentation [23,24]. Generally, a correlation is recognized between tumor size, nodal presence, metastasis, and eventual prognosis. Currently, the site and size of the primary tumor are well recognized as factors that are more important. Asymptomatic early carcinomas smaller than 1 cm may be detected only during a routine clinical examination. However, these lesions are usually larger than 2 cm at presentation. Micheal et al. have pointed out that tumor thickness is an important predictive marker for lymph node metastases. As such, it can help in decision-making with regard to management of the primary tumour and neck, a ‘wait-and-watch’ policy is only warranted for superficial lesions with tumour thickness of less than 7 mm [25]. Shiga et al. also indicated that tumor thickness is a critical indicator of CLN metastasis in patients with T1 or T2 tongue cancers because a significant difference was observed even in their small number of patients [26]. In this study, it was observed that the dimensions of the lesions were measured to be ≥1.5 cm to ≥3.6 cm in diameter. Therefore, ignoring ‘wait and watch’ policy, the patients were advised for adopting ‘immediate and instant treatment’ policy.
It has also been reported that lymph nodes are occasionally located along some lymph vessels of the tongue and are known as Lingual Lymph Nodes (LLN) [27]. Rouviere first noted the presence of the LLNs and referred to them as the median and lateral LLNs. The existence of LLNs had received little attention and no studies of metastasis of oral cancer to these lymph nodes had been reported until Ozeki et al. [28]. The LLNs cannot be removed with usual neck dissection, so that metastasis to this node may cause neck recurrence of oral cancer [29].
Sano and Myers have emphatically stated that involvement of lymph node metastasis occurs independent of tumour size. Small primary tumours may have CLN metastasis whereas larger tumours do not which really corroborates the present findings. They have also emphasised that some of the predictive markers indicating a high risk for lymph node metastasis would have a significant role in determining the therapeutic strategy for these patients [30]. In the present study, we encountered with the similar situation where 2 (11.1%) female cases (Case no 2 and 16) with malignant neoplasm, but were devoid of CLN metastasis. Both are addicted to tobacco chewing and khaini/snuff-dipping. On the other hand, among 6 (33.3%) premalignant cases-of which 5(27.8%) male (Case no 1, 3, 10, 14 and 15) and only 1(5.6%) female case (Case no 4), multifarious CLN were noticed. All of them were also addicted to various forms of either tobacco and/alcohol. In order to defeat such difficult diagnostic dilemma, detected cytological atypias may be considered as the potential predictive markers of HLNs.
In a study conducted by Takagi et al., autopsy results of 83 cases with squamous cell tongue cancer were evaluated and it was shown that the frequencies of remote lymph node and hematogenous metastases were 35.4% and 58.5%, respectively [31]. Lymphatic metastasis in the neck is an important factor for influencing the prognosis of squamous cell carcinoma of the tongue. The survival rate in patients with negative lymphatic metastasis in neck was much higher than that of patients with positive results. In patients with positive lymphatic metastases, prognostic outcome was much worse in latter of stage N [32].
The prevalence of pulmonary metastases induced by squamous cell tongue carcinoma, among oral and maxillofacial tumors, is reported to be 2.5% [33]. It is also important to note that primary tumors metastasizing to the tongue are very unusual and only anecdotal cases have been reported. Primary tumors, originated at lungs, breast, skin, gastrointestinal tract, and liver rarely metastases to the tongue of oral cavity [34,35].
HLN is an aggressive tumor with rapid growth rate and high chance of regional and distant metastasis. Tumor dimension and the existence of extra-capsular spread (ECS) are predictors of survival [36]. In addition, regional spreading to the cervical lymph node and distant metastasis of tongue SCC are indicators of poor prognosis [37,38]. Involvement of a single node reduces survival by half [39]. However, clinical examination is unreliable for detection of nodes and the incidence of occult neck nodal metastases even in early oral cancers varies from 16% to 40% in tongue cancers [40,41].
Genomic instability and proliferative activity are important factors for tumour progression and metastatic growth in cancers. Wangsa et al. found that oral tongue carcinoma are genomically unstable cancers. They have reported that high expression of Ki- 67 is correlated with locoregional recurrence in stage I SCCOT patients, making it a potential marker for additional therapy in patients [42]. In a study, Wong et al. have evaluated the expression patterns of 156 mature miRNAs in tongue SCC using Taqman-based microRNA assays. Of these 156 miRNAs, miR- 133a and miR-133b were significantly reduced in tongue SCC cells in comparison with the paired normal epithelial cells. Their results suggested that aberrant reduction of miR-133a and miR- 133b was associated with the dysregulation pyruvate-kinase type M2 (PKM2), a potential oncogene in SCC of tongue. Furthermore, increased PKM2 expression was observed in all the tumors with reduced miR-133 levels. The inversely correlated expression of PKM2 and miR-133 in tongue SCC samples suggested that PKM2 and miR-133 might play a vital role in lingual carcinogenesis [43]. In another study, Wong et al. have observed that over-expression of miR-184 might play an oncogenic role in the antiapoptotic and proliferative processes of tongue SCC. In addition, plasma miR- 184 levels were associated with the presence of primary tumor. Further studies on the aberrantly expressed miRNAs in tongue SCC as well as using plasma miRNAs as novel tumor markers are warranted [44]. Gao et al. have also stated that the associations of long non-coding RNA (lncRNA) with the clinical features of TC patients substantiate the claim that lncRNA deregulation is linked to the biology activity of tongue SCC [45].
Conclusion
Human Lingual Neoplasm (HLN) is one of the most commonly occurring intra-oral neoplasms. Lateral borders of the tongue are observed to be the cancer prone site. Cytodiagnostically, appearance multiple number of moderately differentiated cytological atypias (KSC, KTC, KSC-A, KFC and KRC) in the exfoliated cytosmears of the premalignant cases not only indicates the aggressiveness of the lingual neoplasm but also shows the remarkable potentiality towards the state of malignancy. Furthermore, along with the MNC, both KSC and KTC are found to be the muti-modal cytological atypias in the HLNs-for which these may be considered as the potential predictive markers of lingual carcinogenesis. Irrespective of age, sex, and addiction of the subjects, metastasis of lingual neoplasm is unpredictable too. Therefore, the exfoliative cytopathology will be helpful to defeat the dragon of diagnostic dilemma in HLNs in general and to detect lingual carcinoma at an early hand in particular.
Acknowledgement
The authors are thankful to Prof. Gadadhar Parida, M.D, formerly Professor and Head, Department of Oncopathology, Acharya Harihar Regional Cancer Centre (AHRCC), Cuttack, Odisha, India for his guidance and supervision during cytopathological analysis. We are also indebted to the Head P.G. Department of Zoology, Utkal University, Vani Vihar, Bhubaneshwar, Odisha, India and to the Director, AHRCC, Cuttack, Odisha, India for permitting us to collect samples from oral cancer patients and also for providing library and laboratory facilities. One of us (AM) is grateful to the University Grants Commission (UGC), New Delhi, India for awarding UGC Meritorious Research Fellowship to carry out the research work.
References
- Amador VR, Pedraza LE, Carrillo FJO, Ortiz AC, Mendivil MF, et al. (1995) Cancer of the Mobile Tongue in Mexico: A Retrospective Study of 170 Patients. Eur J Cancer B Oral Oncol 31: 37-40.
- Llewellyn CD, Johnson NW, Warnakulasuriya KA (2001) Risk factors for squamous cell carcinoma of the oral cavity in young people--a comprehensive literature review. Oral Oncol 37: 401-418.
- Pathak KA, Das AK, Agarwal R, Talole S, Deshpande MS, et al. (2006) Selective neck dissection (I-III) for node negative and node positive necks. Oral Oncol 42: 837-841.
- Veeresh M, Kamala D, Triveni D,Ravikumar (2014) Early squamous cell carcinoma of the anterior tongue; Report of two cases. IOSR J Dent Med Sci 13: 1-4.
- Elizabeth MI, Manoj P, Aleyamma M, Gigi T, Paul S, et al. (2001) Squamous Cell Carcinoma of the Tongue Among Young Indian Adults. Neoplasia 3: 273-277.
- Ong CK, Chong VFH (2006) Imaging of tongue carcinoma. Cancer Imaging 6: 186-193.
- Cantaboni A, Pezzotta MG, Sironi M, Porcellati M (1992) Quality assurance in pathology. Cytologic and histologic correlation. ActaCytol 36: 717-721.
- MohantaA, Mohanty PK, Parida G (2015) An in vivo cytogenetic analysis of human oral squamous cell carcinoma. South Asian J Cancer 4:123-126.
- MohantaA, Mohanty PK (2016) Pattern of Keratin Expression and its Impact on Nuclear-Cytoplasmic Ratio in Plump Keratinized Squamous Cells during Oral Carcinogenesis. J Med Diagn Meth 5:200
- MohantaA, Mohanty PK (2016) Exfoliative cytopathology of lip neoplasm.J Med OncolTher 1: 41-46.
- Broders AC (1926) Carcinoma: grading and practical application. Arch Pathol Lab Med 2: 376.
- Hahn WC, Weinberg RA (2002) Rules for making human tumor cells. N Engl J Med 347: 1593-1603.
- Ackerman LV (1948) Verrucous carcinoma of the oral cavity. Surgery 23: 670-78.
- Mehta FS, Hammer JE (1983) Tobacco related, oral mucosal lesion and conditions in India. Publication: Basic Dental Research Unit. Tata Institute of Fundamental Research 3: 4.
- Kawakami M, YoshimuraK , Hayashi I, Ito K, Hyo S (2004) Verrucous Carcinoma of the Tongue: Report of two cases. Bulletin of the Osaka Medical College 50: 19-22.
- Stelowand EB, Mills SE (2005) Squamous cell carcinoma variants of the upper aerodigestive tract. Am J ClinPathol 124: S96-S109.
- Minami SB, Shinden S, Yamashita T (2008) Spindle cell carcinoma of the palatine tonsil:report of a diagnostic pitfall and literature review. Am J Otolaryngol 29: 123-125.
- Su HH, Chu ST, Hou YUY, Chang KP, Chen CJ (2006) Spindle cell carcinoma of the oral cavity and oropharynx: factor affecting outcome. J Chin Med Assoc 69: 478-483.
- Wenig BM (2002) Squamous cell carcinoma of the upper aerodigestive tract: precursors and problematic variants. Mod Pathol 15: 229-254.
- Oktay M, Unal TDK, Ocal B, Saylam G, Korkmaz MH, et al. (2011) Spindle Cell Carcinoma of the Tongue: A Rare Tumor in an Unusual Location. Patholog Res Int, pp: 1-6.
- Mohanta A, Mohanty PK, Parida G (2014) Keratinized Spindle cell: A diagnostic cytological atypia in oral neoplasm. IOSR J Dent Med Sci 13: 72-80.
- Ranjan V, Desai S, Joshi T, Kumar D, Pancholi M, et al. (2014) Current trends of carcinoma tongue at a Medical College in Central India: A retrospective study. Clin Cancer Investig J 3: 493-506.
- Yuen APW, Lam KY, Chan ACL, Wei WI, Lam LK (1998) Clinicopathological analysis of local spread of carcinoma of tongue. Am J Surg 175: 242-247.
- Yuen APW, Lam KY, Lam LK, Ho CM, Wong A, et al. (2002) Prognostic factors of clinically stage I and II oral tongue carcinoma – a comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez- Gemeno score and pathologic features. Head Neck 24: 513-520.
- Veness MJ, Morgan GJ, Sathiyaseclan Y, Gebskt V (2005) Anterior tongue cancer and the incidence of cervical lymph node metastases with increasing tumor thickness: should elective treatment to the neck be standard practice in all patients. ANZ J Surg 75: 101-105.
- Shiga K, Katagiri K, Nakanome A, Ogawa T, Kobayashi T ( 2007) Management of Early-Stage Tongue Cancer. Tohoku J Exp Med 212: 389-396.
- Rouviere H, Tobia MJ (1938) Anatomy of the human lymphatic system. Ann Arbor, Mich, Edwards Brothers, Inc.
- Ozeki S, Tashiro H, Okamoto M, Matsushima T (1985) Metastasis to the lingual lymph node in carcinoma of the tongue. J MaxillofacSurg 13: 277-281.
- Omura K, Yanai C, Yamashita T (1997) Diagnosis and management of lingual lymph node metastases. Int J Oral MaxillofacSurg 26: 45-47.
- Sano D, Myers J (2007) Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev 26: 645-662.
- Takagi M, Kayano T, Yamamoto H, Shibuya H, Hoshina M, et al. (1992) Causes of oral tongue cancer treatment failures. Analysis of autopsy cases. Cancer 69:1081-1087.
- Liu HY (1998) Types and prognosis of postoperative recurrent carcinoma of tongue. J Foreign Med: Stomatology 25: 183-184.
- Wu YT (1989) Pulmonary metastases of malignant tumors of the oral and maxillofacial region. Analysis of 70 cases. ZhonghuaKou Qiang Yi XueZaZhi 24: 130-133.
- Azam F, Abubakerr M, Gollins S (2008) Tongue metastasis as an initial presentation of renal cell carcinoma: a case report and literature review. J Med Case Reports 2: 249.
- Longo R, Baldini D, Gasparini G (2008) An atypical tongue metastasis of renal cell carcinoma in a patient with metachronous hepatocellular carcinoma. Cancer Ther 6: 707-710.
- Okuyemi OT, Piccirillo JF, Spitznagel E (2014) TNM staging compared with a new clinicopathological model in predicting oral tongue squamous cell carcinoma Survival. Head & Neck 36:1481-1489.
- Ferlito A, Rinaldo A, Devaney KO, MacLennan K, Myers JN, et al. (2002) Prognostic significance of microscopic andmacroscopicextracapsular spread from metastatic tumor in the cervical lymph nodes. Oral Oncol 38: 747-751.
- Santti HK, Atula T, Tikka J, Hollmen J, Makitie AA, et al. (2007) Predictive value of histopathologic parameters in early squamous cell carcinoma of oral tongue.Oral Oncol 43: 1007-1013.
- Som PM (1992) Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol 158:961‑969.
- Naaj IA, Leiser Y, Shveis M, Sabo E, Peled M (2011) Incidence of oral cancer occult metastasis and survival of T1‑T2N0 oral cancer patients. J Oral MaxillofacSurg 69: 2674-2679.
- Huang SH, Hwang D, Lockwood G, Goldstein DP, O’Sullivan B (2009) Predictive value of tumor thickness for cervical lymph‑node involvement in squamous cell carcinoma of the oral cavity: A Meta-analysis of Reported Studies. Cancer 115: 1489‑1497.
- Wangsa D, Ryott M, Lundqvist EAV, Petersson F, Elmberger G, et al. (2008)Ki-67 expression predicts locoregional recurrence in stage I oral tongue carcinoma. Br J Cancer 99: 1121-1128.
- Wong TS, Liu XB, Ho ACW, Yuen APW, Ng RWM, et al. (2008) Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int J Cancer 123: 251-257.
- Wong TS, Liu XB, Wong BYH, Ng RWM, Yuen APW, et al. (2008) Mature miR-184 as Potential Oncogenic microRNA of Squamous Cell Carcinoma of Tongue.Clin Cancer Res 14: 2588-2592.
- Gao W, Chan JYW, WongTS (2014) Long non-coding RNA deregulation in tongue squamous cell carcinoma. BioMed Res Int 2014: 1-10.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences