ISSN : 2348-9502
American Journal of Ethnomedicine
Evaluation of the Toxicity of Secondary Metabolites in Aqueous Extracts of Ficus thonningii Blume in Wistar rats
1Department of Pharmaco-toxicology and Pharmacokinetics, Faculty of Medicine and Biomedical Sciences, University of Yaoundé 1, Cameroon
2Department of Pharmacognosy and therapeutic Chemistry, Faculty of Medicine and Biomedical Sciences, University of Yaoundé 1, Cameroon
3Department of Plant Biology, Faculty of Science, University of Dschang, Cameroon
4Revance Therapeutic Incorporated, Newark California, USA
- *Corresponding Author:
- Charles Fokunang
Department of Pharmaco-toxicology and Pharmacokinetics
Faculty of Medicine and Biomedical Sciences
University of Yaoundé 1, Cameroon
Tel: +237670902446
E-mail: ccharlesfokunang@yahoo.co.uk
Received Date: October 08, 2018; Accepted Date: November 12, 2018; Published Date: November 16, 2018
Citation: Estella TF, Jessica PK, Joseph N, Nono NB, Evrard N, et al. (2018) Evaluation of the Toxicity of Secondary Metabolites in Aqueous Extracts of Ficus thonningii Blume in Wistar rats. Am J Ethnomed Vol.5 No.2:13
DOI: 10.21767/2348-9502.100013
Abstract
Peptic ulcer has become one of the major public health problems that affect about 10% of world’s population. It is a disease resulting from an imbalance between gastro protective and aggressive factors of the gastric mucosa. The treatment of this disease is usually long, expensive and also the difficulty in accessibility to modern medications to the local population in rural zones. This makes poor patients in the community to rely on traditional herbal medicine to address their health problems. F. thonningii Blume is a tree used by the population of the West Region of Cameroon to treat gastric ulcers. The objective of this study was to assess the toxicity of the major classes of secondary metabolites present in extracts of F. thonningii Blume in Wistar rats.
The experimental model used to induce the gastric ulcers was absolute ethanol 100%. Thirty rats represented in six groups: one group without treatment, three pretreated groups with the extract (125, 250 and 500 mg/kg), a group receiving a pretreatment with the omeprazole (20 mg/kg) and another receiving a pretreatment with distilled water. We evaluated acute toxicity by applying the 420 OECD guidelines on 20 rats. Various biochemical parameters for toxicity such as the: Aspartate Amino Transferase (ASAT), Aspartate Amino Transferase (ALAT), Creatinine, XO, total proteins, was quantified. The administration of 2000 mg/kg extract to rats did not cause the death of animals and no modification of animal behaviour, increase dose dependence in body weight, water intake was observed.
The administration of 2000 mg/kg extract to rats was well tolerated and there was no death. Targeted organ toxicity indicated positive effect in the liver (ASAT and ALAT) but negative effects in the kidneys (creatinine). The results of the liver functions tests showed that the conjugating ability of the liver was intact and there was no hepatocellular damage induced by the administration of the F. thonningii stem bark extract, as revealed by the low levels of ASAT and ALAT, hence showing that this plant had a hepatic-protective effect on the liver.
Keywords
Ficus thonningii stem bark hydro-ethanolic extract; Gastric ulcer; Antacid; Acute toxicity
Introduction
Ulcers, gastric or duodenal, cause a loss of a small or extended portion of the bowel tissue wall. It is either erosions or abrasions or superficial abrasions that do not reach the muscle layer and which heal without scarring [1-3]. Gastric ulcer which is chronic and recurrent in the majority of cases results from an imbalance between chlorhydro-peptic stressors (HCl, pepsin, and gastrin) and gastric mucosal defences (mucus, bicarbonate, blood flow mucosal cyto-protection). Gastric ulcer occurs in case of rupture in the mucosa that allows the pepsin and hydrochloric acid attack the stomach wall. Gastric ulcer is a disease that affects both men and women [2,4].
It represents 31.65% of cases of consultations in gastroenterology services in Cameroon [5-7]. The three most deadly digestive diseases in Cameroon during 2013 were paralytic ileus and intestinal obstruction, peptic ulcer disease, and other digestive diseases. The annual mortality rate per 100,000 people from peptic ulcer disease in Cameroon has decreased by 21.6% since 1990, an average of 0.9% a year [8].
Treatment of this disease requires in most cases a combination of several molecules with specific mechanisms of action. This treatment has 4 goals: relieve pain, accelerate healing, prevent complications and reduce the frequency of relapses. But while effective, treatment using conventional medicines is not usually well attended by patients [9]. The reasons included their high cost and low availability to a large majority of the population especially those living in rural areas. In many developing countries, the health infrastructure is poor and a large majority of the population, mainly rural, has no access to primary health care and modern medicines. These patients use the resources of traditional herbal medicine as an alternative treatment.
However, traditional herbal medicine is facing a number problems for its vulgarisation including lack of sufficient studies on therapeutic properties as well toxicity tests to provide sufficient guarantees for their rational use.
The common wild plant, F. thonningii, is extensively used in African ethno medicine for treating a number of disease conditions which include diarrhoea, urinary tract infections, diabetes mellitus, gonorrhoea, respiratory infections, and mental illnesses. Traditional medicine has a long history. It is the sum total of the knowledge, skill, and practices based on the theories, beliefs, and experiences indigenous to different cultures, whether explicable or not, used in the maintenance of health as well as in the prevention, diagnosis, improvement or treatment of physical and mental illness [10,11].
The leaves of F. thonningii contains various bioactive compounds which include alkaloids, terpenoids, flavonoids, tannins and active proteins, all of which contribute to its curative properties. In vitro and in vivo pharmacological studies revealed that F. thonningii possesses antimicrobial, antidiarrheal, antihelmintic, antioxidant, antiinflammatory and analgesic properties [10,12]. Scientific research has validated the ethno medicinal claims that F. thonningii is useful in disease management. However, there is need to continue identifying, isolating and quantifying the active principles and possibly determine the mechanisms underlying the curative properties of its bark [10]. They are medicines from the local traditional pharmacopoeia, with toxicity limits determined, with pharmacological activity confirmed by scientific research, at dosage quantified and quality controlled. Among the molecules with pharmacologically proven activity of plants. Toxicity is the totality of adverse events following the administration of a substance. It is influenced by: dose, duration of exposure, chemical structure, exposure, physiological factors (sex, age, health status, pregnancy, and nutritional condition), hereditary factors and many others [13-15]. The different types of toxicity tests are the acute toxicity studied, the sub-acute toxicity (14-28 days), and the chronic toxicity (90 days). It is in this context that the current study was conducted to investigate phytochemically screen, evaluate the anti-ulcer, and toxicity of the stem bark extract on wistar rats.
Materials and Methods
This was an experimental in vitro and in vivo preclinical study on wistar rats conducted from the 11 November 2016 to the 25th May 2017. The study was done in the Preclinical Animal toxicology and Pharmacology Laboratory of the Faculty of Medicine and Biological Sciences, of the University of Yaoundé 1, Cameroon, while the quantification of biochemical parameters was done in the biochemistry laboratory of the same university.
Ethical consideration
Ethical approval was given by the institutional review board (IRB) of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé 1 and administrative authorization was obtained to conduct study in the animal house of this faculty.
Collection, identification preparation of plant material
Fresh stem barks were harvested after identification by a botanist from the plant growing at Bafoussam on the 3rd of January 2017. The identified plant was authenticated at the National Herbarium of Cameroon by comparison with a sample having the voucher reference number 444042/HNC. The barks were dried under shade at room temperature for a period of three weeks in order to avoid solar radiations from altering the API. These barks were spread on plastic bags while avoiding their stacking. Every day we turned these barks upside down so as to favour a homogenous drying process. The dried barks were ground in a clean electric grinding machine in such a way to obtain a fined powder which was stored in an airtight container.
Plant extract preparation
Three types of extraction procedures were used in order to evaluate the in vivo activity and selected the extract with the best activity since there were no studies with respect the evaluation of the antiulcer activity of the bark of Ficus thonningii Blume. These methods of extraction were:
Extraction by Maceration, Infusion and decoction
In this process, the coarsely powdered crude plant was placed in a stoppered container with the solvent (distilled water, ethanol and hydroethanolic solution 50:50) and allowed to stand at room temperature for a period of 48 hours with frequent agitation until the soluble matter has dissolved. The mixture was then strained, the marc (the damp solid material) was pressed, and the combined liquids were clarified by filtration using Whatman paper.
By infusion, fresh infusion was prepared by mixing the crude plant or part of it for a short period of time specifically 10 to 15 minutes with initially boiling water [11] and by decoction, the crude plant was boiled in a specified volume of water for a defined time generally 10 to 15 minutes; it was then cooled and filtered. This procedure is suitable for extracting water-soluble, heat-stable constituents. The starting ratio of crude plant to water was fixed, 1:4 or 1:16; the volume was then brought down to one-fourth its original volume by boiling during the extraction procedure and the concentrated extract was filtered [11].
Yield determination of the extract
The best activity was shown with the hydro-ethanolic maceration hence after 48 hours the macerate was filtered with Whatman No. 3 filtered paper and the collected filtrate was evaporated in an oven at 50 °C. This extract was weighed in order to determine the yield obtained from the initial powder quantity and then stored in an air-tight container for subsequent experimental tests.
Animal testing
The animals used were white albino rats of the wistar strain (Rattus norvegicus) aged between two and three months. These animals had an average weight of 178.2 ± 22.09 g for the antiulcer activity and 125.5 ± 10.14 g and 119.8 ± 6.50 g respectively for the males and the females used in the assessment of acute toxicity. They were raised in the animal house of the FMBS YDE 1 under favourable conditions for their growth and development. The diet consisted of a mixture of corn meal (45%), wheat flour (20%), fish meal (20%), soybean meal (10%), palm kernel (5%), bone flour for calcium intake (0.98%), cooking salt (0.5%) and vitamin complex (0.5%). Two to three times a month, a vitamin complex (Olivitasol, Cedex, France) was added in their water to drink.
Animal identification was done by cage card and corresponding bold marker body markings and they were maintained in the animal house of FMBS. For animal selection, the animals were subjected to a gross observation to ensure that the selected rats were in a good health. Rats were randomly selected with res pect to body weight for final allotment to the study. The animal environment was made up of natural air conditioned rooms with optimal air changes per hour, relative humidity, temperature and illumination cycles set to 12 h light and 12 hours dark. The animals were accommodated in groups housed in cages with stainless steel grill top, together with facilities for food and water bottle and bedding of clean paddy husk.
For administration of the test substance, the plant extract was administered by oral gavage to each rat with 1 ml of the ulcerogenic substance, using an intubation needle fitted onto a syringe of appropriate size. The dose administered to individual rat was calculated according to its body weight recorded on the day of test substance administration. The anti-ulcer reference drug used was omeprazole (OMIZEC) 20 mg batch number 260044 bought in a community pharmacy in YDE specifically on the 02 January 2017.
Acute toxicity [108]
The OECD 420 Guideline for the testing of chemicals (toxicity Oral - predetermined dose method) makes it possible to classify substances in order of toxicity. It was adopted in 2001 [16-19].
Groups of animals of a single sex receive predetermined doses of 5, 50, 300 and 2000 mg / kg according to a sequential procedure. Exceptionally, an additional dose of 5000 mg / kg may be considered. The initial dose is chosen on the basis of an orientation study such as that which is likely to cause toxic effects, but without causing severe toxic effects or death. Other groups of animals receive higher or lower doses depending on the absence or presence of toxic effects or mortality. The procedure is continued until the dose that causes evident toxic effect or the death of a single animal. The procedure is also interrupted when the highest dose does not give rise to any observed effect or the lowest dose gives no mortality.
Twenty rats, ten males and ten females with an average weight of 125.5 ± 10.14 g and 119.8 ± 6.50 g were used for this study. These animals were divided into two groups of five for each sex: the test group and the control group. Twelve hours before the study, all the animals were deprived of food, but no water. They were then weighed just before administration of the extract.
Preparation and administration of the extract
We prepared a 200 mg / ml concentration solution. 20 mL of solution was obtained from 4000 mg of extract and distilled water (sufficient for 20 mL). Then, the mixture was homogenized using a magnetic stirrer. From this solution, a dose of 2000 mg / kg of aqueous extract was administered to the male and female test groups according to their weight, while the control groups received distilled water. The animals were again deprived of food for four hours [11].
Quantification of Toxicity Biochemical Parameters
Evaluation of the renal activity
Creatinine quantification (CHRONOLAB KIT): The assay is based on the reaction of creatinine with sodium picrate as described by JAFFÉ. Creatinine reacts with alkaline picrate forming a red complex. The time interval chosen for measurements avoids interferences from other serum constituents. The intensity of the colour formed is proportional to the creatinine concentration in the sample [16-19].
Quantification of ALAT (CHRONOLAB KIT): Alanine aminotransferase (ALT) or Glutamate pyruvate transaminase (GPT) catalyzes the reversible transfer of an amino group from alanine to α-ketoglutarate forming glutamate and pyruvate. The pyruvate produced is reduced to lactate by lactate dehydrogenase (LDH) and NADH [20-23].
The rate of decrease in concentration of NADH, measured photo metrically, is proportional to the catalytic concentration of ALT present in the sample.
Quantification of ASAT (CHRONOLAB KIT): Aspartate aminotransferase (AST) formerly called glutamate oxaloacetate (GOT) catalyses the reversible transfer of an amino group from aspartate to α-ketoglutarate forming glutamate and oxalacetate. The oxalacetate produced was reduced to malate by malate dehydrogenase (MDH) and NADH [23]. The rate of decrease in concentration of NADH, measured photometrically, is proportional to the catalytic concentration of ASAT present in the sample.
Statistical Analysis
The results were expressed in terms of mean ± standard deviation. The comparisons between the groups were analyzed using one-way analysis of variance, the ANOVA test followed by Turkey's Kramer post hoc test using the GraphPad Instat version 5.0 software. A P-value of less than 0.05 was considered statistically significant.
Results
Extraction yield
The extraction yield of the hydro-ethanolic extract (50:50) of the bark of F. thonningii Blume was 17%.
Acute toxicity evaluation
Observation of the behaviour of the rats after the administration of the plant extract: Exposure of the rats to extract at dose 2000 mg/kg of body weight showed that none of the animals died during the acute toxicity study; hence the LD50 was more than 2000 mg/Kg. There was no change in behaviour in appearance, neuromuscular movement and reaction on opening of the cage (Table 1).
Table 1: Observation of the behaviour of the rats after the administration of the plant extract.
| Observations of test groups (males and females) | Day 1 (30 minutes after the induction) | Day 1 to day 14 | |
|---|---|---|---|
| Appearance | Cleaning | ++ | - |
| Piloerection | - | - | |
| Abnormal posture | - | - | |
| Hypo-activity | - | - | |
| Hypotonia | - | - | |
| Neuromuscular | Convulsion | - | - |
| Tremors | - | - | |
| Myoclonia | - | - | |
| Behaviour at the opening of the cage | Vocalization | - | - |
| Aggressiveness | - | - | |
| Hypo-reactivity | - | - |
++ Excessive, + normal, - not observe
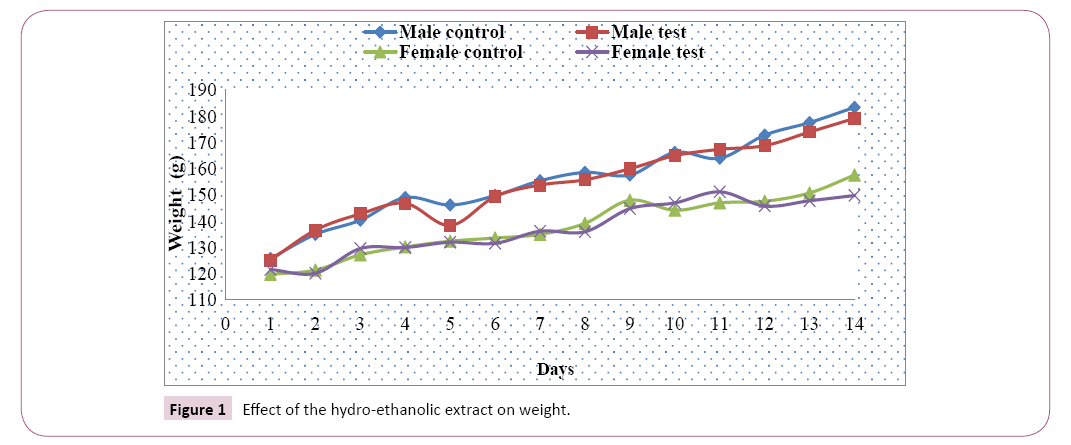
Weight analysis: The average weight of the male and female test rats and those of the control groups increased gradually from the first to the fourteenth day of exposure, and on the eleventh day with a slight decrease with the male test group recorded from the fifth day. The weight variation between test and control groups was greater in males than in females (Figure 1).
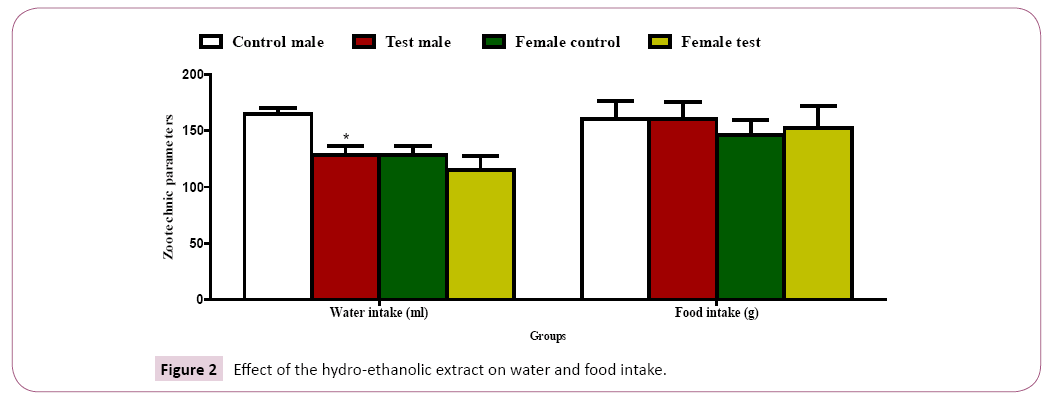
Water and food intake: A significant decrease (P˂ 0.05) in water intake was observed in the male test group as compared to the male control group with a non-significant decrease in water intake in the female test group compared to control groups (Figure 2). A non-significant increase (P˂ 0.05) in food intake was recorded in the test groups as compared to the control groups (Figure 2).
Weight of the different organs: There was a significant variation in the weight of the organs of sacrificed rats (heart, lung, liver, kidney, testis ovary, adrenal gland, brain, spleen and pancreas), between the test and control groups (Table 2).
Table 2: Effect of the hydro-ethanolic extract on the weight of the organs.
| Organs | Male control | Male test | Female control | Female test | |
|---|---|---|---|---|---|
| Heart | 0,58 ± 0,03 | 0,60 ± 0,06 | 0,48 ± 0,06 | 0,54 ± 0,08 | |
| Lungs | 1,17 ± 0,06 | 1,35 ± 0,34 | 1,03 ± 0,12 | 1,08 ± 0,18 | |
| Liver | 7,21 ± 0,66 | 7,22 ± 0,63 | 5,93 ± 0,62 | 6,36 ± 0,75 | |
| Kidneys | Left | 0,58 ± 0,03 | 0,59 ± 0,06 | 0,54 ± 0,04 | 0,57 ± 0,06 |
| Right | 0,58 ± 0,04 | 0,60 ± 0,06 | 0,54 ± 0,04 | 0,57 ± 0,04 | |
| Testis/Ovaries | Left | 0,96 ± 0,33 | 0,89 ± 0,33 | 0,05 ± 0,01 | 0,06 ± 0,01 |
| Right | 0,94 ± 0,33 | 0,89 ± 0,33 | 0,05 ± 0,01 | 0,07 ± 0,01 | |
| Adrenal glands | Left | 0,046 ± 0,005 | 0,03 ± 0,004 | 0,03 ± 0,004 | 0,04 ± 0,01 |
| Right | 0,044 ± 0,005 | 0,03 ± 0,004 | 0,03 ± 0,004 | 0,03 ± 0,01 | |
| Brain | 1,50 ± 0,16 | 1,52 ± 0,25 | 1,44 ± 0,12 | 1,4 ± 0,09 | |
| Spleen | 0,73 ± 0,21 | 0,59 ± 0,13 | 0,49 ± 0,05 | 0,58 ± 0,16 | |
| Pancreas | 1,24 ± 0,27 | 1,39 ± 0,36 | 1,55 ± 0,51 | 1,05 ± 0,43 | |
Values are means ± standard error of three replicate samples.
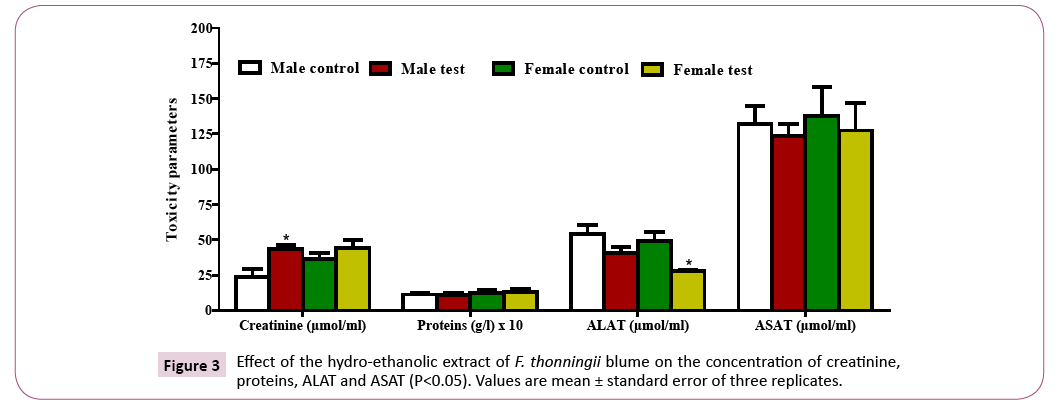
Quantification of biochemical parameters: There was an increase in the creatinine concentration in both the male (significant) and female (nonsignificant) test groups as compared to the control groups. The protein level decreased non-significantly in the male test groups and increased non-significantly in the female test groups as compared to the control groups (Figure 3). A decrease in the ALAT concentration was observed in both the male test (non-significant) and female test (significant) groups with respect to the control groups. There was a non-significant decrease in the ASAT in the test groups as compared to the control groups (Figure 4).
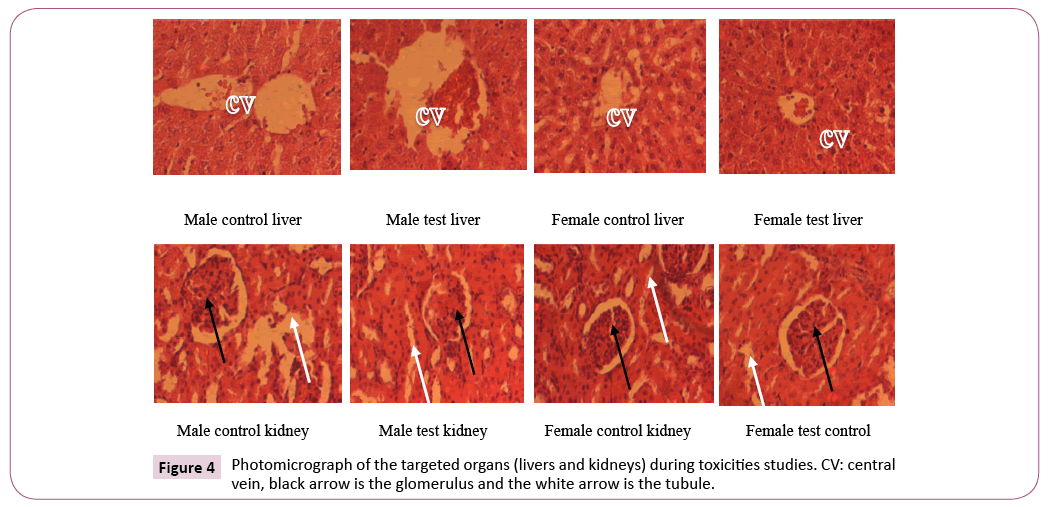
Histological analysis of the kidneys and liver exposed to plant extract treatment: There was no significant architectural change in the histological analysis of the targeted organs for the liver and kidneys.
ASAT/ALAT Ratio: This ASAT/ALAT ratio was significantly high ration compared to the normal range 4.58 ± 1.52 in female test rats followed by the male test rats 3.14 ± 0.93 (Table 3).
Table 3: Determination of ASAT/ALAT.
| Male control | Male test | Female control | Female test | |
|---|---|---|---|---|
| ASAT/ALAT | 2.75 ± 1.39 | 3.14 ± 0.93 | 3.06 ± 1.50 | 4.58 ± 1.52 |
Discussion
The genus Ficus of the Moraceae family is among the largest genera of angiosperms, and about 60 are present in Cameroun [9,17]. Earlier studies on the phytochemical screening showed that the hydro-ethanolic stem bark of F. thonningii Blume contained various biologically active compounds called phytochemicals, which were naturally produced by the plant as protection against biotic and abiotic stresses. The main groups of phytochemicals isolated from the prepared extract solution included; polyphenols, saponins, alkaloids, flavonoids, catechic tannins, coumarins, quinones, phlobotanins, anthocyanins which corroborates with the work done by Dangarembizi et al. in 2013 on the leaves of F. thonningii [10,18] and Usman et al. in 2010 [15]. Most of these phytochemicals have an effect on the gastric mucosa which could be responsible of the plant antiulcer activity.
Acute toxicity study based on the OECD 420 Guidelines on the hydroethanolic extract of F. thonningii Blume stem bark at a fixed dose of 2000 mg/Kg demonstrated that the extract did not show any signs of toxicity and mortality. The fixed dose procedure differs from the classical method of acute toxicity testing in the way that it uses fewer animals and causes less suffering. This approach avoids using the death of animals as an endpoint and relies instead on the observation of clear signs of toxicity [21-23]. This showed that the LD50 was greater than 2000 mg/Kg. According to Dangarembizi et al. the LD50 of the aqueous F. thonningii leaf extract in adult Wistar rats administered orally was shown to be above 3000 mg/Kg body weight. Furthermore, animals has showed 100% mortality after intraperitoneal administration of 600 mg/Kg body weight of the same extract and the LD50 intraperitoneal was reported to be 584 mg/Kg [10]. In another acute toxicity test, the median lethal dose of ethyl acetate extracts of F. thonningii administered orally was shown to be above 5000 mg/Kg body weight in adult Wistar rats [2,24].
From toxicity studies the males test groups showed a decrease in the water intake as compared to the control groups, this could be due to hormonal effects, whereas there was no change in general behavior or other physiological activities in the ethanolic extract of Garcinia. mangostana Linn. [19,20].
Biochemical tests can be used to diagnose any toxic effects of drugs and phytochemicals on the liver, heart and kidney [25]. Biochemical measurements can also detect any acid-base imbalance in the respiratory and metabolic systems, abnormalities in lipid metabolism and various endocrine systems as well as other nutritional or metabolic disorders [2,4,26]. The results of the liver functions tests showed that the conjugating ability of the liver was intact and there was no hepatocellular damage induced by the administration of the F. thonningii stem bark extract, as revealed by the low levels of ASAT and ALAT, hence showing that this plant has a hepatic-protective effect on the liver. A decrease in the ALAT concentration was observed in both the male test (non-significant) and female test (significant) groups with respect to the control groups. ASAT/ALAT. ratio was significantly high 4.58 +1.52 in female test rats followed by the male test rats. The AST/ALT ratio is the ratio between the concentration of the enzyme aspartate transaminase (AST) and alanine transferase (ALT) in the blood of the human or animal in this case wistar rat [23,27]. It is measured with the blood test and is sometimes useful in medical diagnostics to differentiate between causes of liver damage or hepatotoxicity. Most causes of liver cell injury are associated with a greater increase in ALT than AST; however, an AST to ALT ratio of 2:1 or greater is suggestive of alcoholic liver disease, particularly in the setting of an elevated gamma glutamyl transferase [11,27].
The AST to ALT ratio can also occasionally be elevated in a liver disease pattern in patients with non- alcoholic steatohepatitis [28], and it is frequently elevated in an alcoholic liver disease pattern in patients with hepatitis C who have developed cirrhosis. In addition, patients with Wilson’s disease or cirrhosis due to viral hepatitis may have an AST that is greater than the ALT though ratio is not typically greater than two. When the AST is higher than ALT, a muscle source of these enzymes should be considered. For example, muscle inflammation due to dermatomyositis may cause AST>ALT. This is a good reminder that AST and ALT are not good measures of liver function because they do not reliably reflect the synthetic ability of the liver and they may come from tissues other than liver (such as muscle) [23].
The aspartate amino transferase/alanine amino transferase (ASAT/ ALAT) ratio is increased in cirrhosis. Some studies indicate that the ratio may provide prognostic information as well. A high ASAT/ALAT ratio is associated with increased mortality in cirrhosis. In non-alcoholic patients the ratio may provide prognostic information independently of classical risk factors [13,29].
Transaminase levels rapidly return to normal when the cause of the disease hepatocyte is suppressed. The half-life of ASAT is shorter than that of ALT. The liver contains more ALT than ASAT. ALT is considered to be a more specific to inflammation of the liver, since AST is also present in other organs, such as the heart or skeletal muscles [9,30]. In the case of acute liver injury, ALT and AST may be used as a general measure of the degree of inflammation or lesion to the liver. This is not the case when it is a chronic liver disease because enzymes may be present at a completely normal level even in the presence of cirrhosis (healing of the liver). ALAT concentrations increase in liver damage, muscle and trauma. It is used in association with ASAT in the diagnosis of myocardial infarction because its concentration remains within normal limits, in the presence of high ASAT rates [11,31].
Creatinine is the result of the degradation of creatine, a component of the muscles. It can be transformed into ATP, which is a source of energy for the cells. The concentration of creatinine in the blood depends on the kidney's elimination muscular mass [23,32-34]. Its evaluation makes it possible to appreciate a dysfunction of the filtration kidney. The creatinine concentration is low in young subjects and in cases of myopathy with significant muscular atrophy. This concentration increases in the elderly, exercise, high-protein diet, prolonged fasting, renal insufficiency. The Creatinine is excreted by the kidneys. In case of renal insufficiency, it is retained in the blood as well as urea, and uric acid. A high level of creatinine may be indicative of renal insufficiency [27,32,33].
Traditional and conventional medicine must collaborate in a spirit of complementarity and mutual respect, to best respond to health problems of the populations: their common objective. The weaknesses and drifts of medicine often criticized by health professionals and the public because of many cases of complications in patients constitute major obstacles to its upgrading. It is important to accelerate scientific studies already traditional practices in order to make therapeutic opportunities that combine scientifically proven efficacy with cultural acceptability [9,23,34].
In toxicity studies, renal functions is likely to be affected when high doses of test compounds are being given since kidneys serve as the main organ of elimination for many drugs and their metabolites [12,32]. Determination of serum electrolytes, creatinine and urea are critical as they are the important markers of kidney function [29,31-35]. In this study, there was a significant increase in the creatinine levels in both the female and males groups with a significant increase in the males group indicating that the renal function was negatively affected by this plant.
Conclusion
This study showed that the highest dose administration of 2000 mg/kg extract to rats did not cause any lethality to the animals and no observed modification of animal behavior. This was an indication that the extract lethal dose LD50 would be above 2000 mg/kg, a relatively safe dose for administration.
Targeted organ toxicity indicated positive effect in the liver (ASAT and ALAT) but negative effects in the kidneys (creatinine). The results of the liver functions tests showed that the conjugating ability of the liver was intact and there was no hepatocellular damage induced by the administration of the F. thonningii stem bark extract, as revealed by the low levels of ASAT and ALAT, hence showing that this plant has a hepatic-protective effect on the liver.
References
- Zapata JC, Zepeda GS, Montano LA, Vazquez BE, Jesus VJ, et al. (2006) The association of helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic ulcer disease. Can J Gastroenterol 20: 277-280.
- Kumar, Abbas, Fuasto (2008) Robins and Cotran Pathologic Basis of Disease. (7thedn), Elsevier Publications pp: 797-847.
- Ndjitoyap NEC, Tzeuton C, Njoya O, Tagni SM, Kamdoum M (1998) Tolerance acceptabilite de l’endoscopie digestive haute: Analyse prospective de 530 examens. Acta endoscopia 3: 226.
- Togola A, Karabinta K, Dénou A, Haidara M, Sanogo R, et al. (2014) Protective effect of opiliaceltidifolia leaves against ethanol-induced ulcer in rats. Int J Biol Chem Sci 8: 2416-2423.
- Rachael D, Kennedy H, Erlwanger DM, Eliton C (2013) Phytochemistry, pharmacology and ethnomedicinal uses of Ficus thonningii (Blume Moraceae): A review. Afr J Tradit Complement Altern Med 10: 203-212.
- Usman H, Abdulrahman FI, Usman A (2009) Qualitative phytochemical screening and in vitro antimicrobial effects of methanol stem bark extract of Ficus thonningii (Moraceae). Afr. J. Traditional, Complementary and Alternative Medicines 6: 289-295.
- Wallace JL (2008) Prosaglandins, NSAIDS, and Gastric Mucosal Protection: Why doesn’t the stomach digest itself? Physiol Rev 1547-1565.
- Lichtenberger LM (1995) The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol 57: 565-583.
- Kato S, Aihara E, Yoshii K, Takeuchi (2005) Dual action of prostaglandin E2 on gastric acid secretion through different EP-receptor subtypes in the rat. Am J Physiol Gastrointest Liver Physiol 289: G 64-G69.
- Odebiyi O and Sofowora E (1978) Phytochemical screening. Nigeria medical plants 41-234.
- Sofowora A (1996) Medicinal plants and traditional medicine from Africa. Karthala, p: 378.
- Brzozowski I, Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, et al. (2002) Role of prostaglandin’s, nitric oxide sensory nerves and gastrinin acceleration of ulcer healing by melantonin and its precursor, L-trytophan: J Pineal Res 32: 149-162.
- Alcaraz MJ, Hoult JR (1985) Actions of favonoides and the novel anti-inflammatory flavones hypolaetin-8-glucoside, on prostaglandine biosynthesis and inactivation. Biochem Pharmacol 34: 2477-2482.
- Bronner C and Landry Y (1985) Kinetics of the inhibitory effect of flavonoids on histamine secretion from mast cells. Agents Actions 16: 147-151.
- Morikawa T, Li N, Nagatomo A, Matsuba H, Li X, et al. (2006) Triterpene saponins with gastroprotective effects from tea seed (the seeds of Camellia sinensis). J Nat prod 69: 185-190.
- Sun H, Fang WWS, Wang WZ, Hu C (2006) Structure activity relationships of oleanane and ursane-type triterpenoids. Botanical Studies 47: 339-368.
- Theoduloz C, Carrion IB, Pertino MW, Valenzuela D, Schmeda-Hirshmann G (2012) Potential gastroprotective effect of novel cyperenoic acid/ quinine derivatives in human cell cultures. Planta Medica 78: 1807-1812.
- Kishore DV, Pinto J, Mini KV (2011) Anti-ulcer activity of methanolic and aqueous extracts of leaves of Sapindus trifoliatus Linn. Int J Pharm Sci Rev Res 6: 25-27.
- OECD / OCDE 420 adopted 17 December 2001: OECD Guidelines for the testing of chemicals acute oral toxicity-method by acute toxic class. Pp: 312.
- Benjamin KN, Joseph FM, Mesfin BD, Joseph A (1994) Antiulcerative properties and acute toxicity profile of some African medicinal plant extracts: Journal of Ethnopharmacology 42: 13-18.
- Miller TA, Henagan JM (1984) Indomethacin decreases resistance of gastric barrier to disruption by alcohol. Dig. Dis. Sci 29: 141-149.
- Da Silva AR, Reginato FZ, Guex CG, Figueredo KC, Da C Araldi IC, et al. (2016) Acute and sub-chronic (28 days) oral toxicity evaluation of tincture Baccharis trimera (Less). Backer in male and female rodent animals. Regul Toxicol Pharmacol 74: 170-177.
- Gole MK, Dasgupta S (2002) Role of plant metabolites in toxic liver injury. Asia Pac J Clin Nutr 11: 48-50.
- Mitra S, Sur RK (1997) Hepatoprotection with Glycosmis pentaphylla (Retz). Indian J Exp Biol 35: 1306-1309.
- Wilsdorf G, Heckel R, Juhrke J (1991) Orienting studies in Wistar rats for the evaluation of knotgrass (Polygonum convolvulus) seed. Arch Exp. Veterinarmed 45: 123-129.
- Haukeland JW, Schreiner LT, Lorgen I, Frigstad SO, Bang C, et al. (2008) ASAT/ALAT ratio provides prognostic information independently of Child-Pugh class, gender and age in non-alcoholic cirrhosis. Scand J Gastroenterol 43: 1241-1278.
- Emmanuel EH, Mainen JM, Ramadhani SO, Nondo DT, Dennis TM, et al. (2012) A study of antimicrobial activity, acute toxicity and cytoprotective effect of a polyherbal extract in a rat ethanol-HCL gastric ulcer model. BMC Res Notes 5: 546.
- Moses AJ, Olayiwola OB, Olapeju AA, Sunday AN (2016) Evaluation of therapeutic potentials of plant extracts against against poultry bacteria threatening public health BMC Complement Altern Med. 16: 417.
- Mainen JM, Ramadhani SO, Nondo EE, Haule RO, Mahunnah LA, et al. (2014) Antimicrobial activity, acute toxicity and cytoprotective effect of Crassocephalum vitellinum (Benth). S. Moore extract in a rat ethanol-HCL gastric ulcer model BMC Res Notes 7: 91.
- Nahla S, Maryam H, Nawal A, Sareh K, Elham B, et al. (2017) The antiulcer effect of Cibotium barometz leaves in rats with experimentally induced acute gastric ulcer. Drug Des Devel Ther. 11: 995-1009.
- Margaret OS, Lilian A, Abidemi JA, Johnson AO, Oluwole BF (2012) Effect of Flabellaria paniculata Cav. Extracts on gastric ulcer in rats. BMC Complement Altern Med. 12: 168.
- Jude EO, Ette OE, John AU, Jackson O (2012) Antiplasmodial and antiulcer activities of Melanthera scadens. Asian Pac J Trop Biomed. 2: 16-20.
- Lilly BA, Aarrthy MA, Kantha DA, Sathesh KA, Kalaivani AK (2014) In vivo anti-ulcer, anti-stress, anti-allergic, and functional properties of Gymnema sylvestre R.Br. BMC Complement Altern Med. 14: 70.
- Noraziah N, Suzy MS, Shahram G, Maryam H (2014) Anti-ulcerogenic effect of methanolic extracts from Enicoshellum pulchrum (King) Hethanol-induced acute gastric lesion in animal models. PLoS One. 9: e111925
- Mehran F, Hamed K, Hairin T, Hapipah M, Mahmood AA (2014) Cytotoxic activity and in vivo effect of Syngonium podophyllum and Eichornia crassipes leaf extracts on isoniazid, induced oxidative stress and Hepatic markers. Biomed Res Int, pp: 452-459.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences