ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Evaluation of the Anticancer Activity of Bioactive Fraction G Extracted from Pavetta crassipes in Malignant Brain Tumor Cell Lines
Wilcox RM1, Huseman ED1, Lin S1, Darkwah BO1, Emeje MO2, Gamaniel KS2, Orisadipe A2, Enwerem N3,Kefas BA4, Gryka RJ1,Simpson DS1, Amos S1*
1Department of Pharmaceutical Sciences, School of Pharmacy, Cedarville University, Cedarville, USA
2National Institute for Pharmaceutical Research and Development, Abuja, Nigeria
3Division of Nursing and Allied Health, Howard University, USA
4Pharmacy Department, University of Virginia, Charlottesville, USA
- *Corresponding Author:
- Samson Amos
Department of Pharmaceutical Sciences
School of Pharmacy, Cedarville University,USA
Tel: 9377667401
E-mail: samos@cedarville.edu
Received date: August 04, 2017; Accepted date: August 12, 2017; Published date: August 14, 2017
Citation: Wilcox RM, Huseman ED, Lin S, Darkwah BO, Emeje MO, et al. (2017) Evaluation of the Anticancer Activity of Bioactive Fraction G Extracted from Pavetta crassipes in Malignant Brain Tumor Cell Lines. Am J Phytomedicine Clin Ther. Vol. 5 No. 2:16. doi:10.21767/2321-2748.100329
Abstract
Objective: Natural products have served as sources of lead compounds that are commonly used in the treatment of human diseases including cancer. Pavetta crassipes has been widely demonstrated to have ethnopharmacological potential in the management of malaria, gastrointestinal conditions, central nervous system behavioral disorders, hypertension, and cancer. The goal of our study was to evaluate the biological and molecular effects of Fraction G, obtained from the plant Pavetta crassipes, on glioblastoma invasive growth and survival.
Methodology: The antiproliferative effects of Fraction G, obtained from Pavetta crassipes, was evaluated using the trypan blue exclusion, (3-(4, 5-Dimethylthiazol- 2yl)-2, 5-Diphenyltetrazolium Bromide; MTT), and lactate dehydrogenase (LDH) assays. Flow cytometry and Western blotting analyses were carried out to examine the effects of Fraction G on cell cycle check-points and its effects on epidermal growth factor receptor-mediated signaling of AKT and MAPK pathways.
Results: In this paper, we report that the Fraction G obtained from the plant Pavetta crassipes induced a reduction in glioma cell viability and proliferation as well as induced an increase in apoptosis as evidenced by cleaved PARP, increased caspase 3/7 activity, and cell cycle arrest in the G0/G1 check point. Furthermore, we report that Fraction G inhibited the phosphorylation of AKT and MAPK following EGF treatment.
Conclusion: Taken together, our results demonstrate that Fraction G has potent inhibitory effects on pathways involved in glioblastoma proliferation and survival.
Keywords
Glioblastoma; Cancer; Pavetta crassipes; Natural products; Ethnopharmacology; Cell proliferation; Apoptosis
Introduction
Glioblastoma multiforme (GBM) is the most common and deadly type of malignant brain tumor that affects adults. The patient survival rates are low, with less than 5% of patients surviving 5 years [1,2]. The average life expectancy of patients with GBM is only slightly over 1 year, even with current treatment options [3]. According to the World Health Organization (WHO), GBM is classified into four different tumor grades with class IV being the most aggressive and also the most common type of tumor [4]. GBM can also be categorized as primary or secondary tumors. Even though primary GBM are rapid growing while secondary tumors tend to arise slower, they are almost indistinguishable, resistant to current available therapies, and have a poor prognosis [5]. Dysregulated expression of several important growth factors, survival and cell cycle regulators have been implicated as major contributing factors to GBM’s invasiveness, rapid growth, and resistance to chemotherapy. Such molecular targets include the Epidermal Growth Factor Receptor (EGFR), Platelet Derived Growth Factor Receptor (PDGFR), and the Vascular Endothelial Growth Factor (VEGF) [6-8]. Overexpression of EGFR occurs in about half of primary glioblastoma cases and correlates with decreased overall survival of GBM patients. Most brain tumors that overexpress EGFR also overexpress the active mutant variant, with a frequency of ~40–50%. The mutant form has been shown to confer a less favorable prognosis for GBM patients [9]. Once dysregulated and activated, it activates downstream protein targets, such as PI3K/AKT/mTOR and Ras-Raf-MEK-ERK. These signaling pathways are known to promote tumor growth and survival [10,11]. Although several treatment options exist, a whole host of problems including cost, drug resistance and toxicity, and the inability to surgically remove all cancerous cells pose a significant barrier to current available therapy and strategies. The need for newer effective, and less toxic therapeutic agents and strategies cannot be over emphasized. Greater than 50% of drugs currently used as chemotherapeutic agents are of natural product origin [11] and some of these include Camptotheca alkaloids, Vinca alkaloids, Taxus diterpenes, and Podophyllum lignans. Natural products of plant and animal sources are known to serve as an important source of new chemical entities and drugs with potential therapeutic benefits [12]. The research into plants that are employed to treat a number of diseases including “cancer” in traditional ethnomedicine is therefore one of the productive and logical strategies in the search for new therapeutic agents [13]. The leaves of the Pavetta crassipes K. Schum (Rubiaceae) plant are used for food as well as medicinally in African countries to treat a wide variety of diseases and illnesses, such as respiratory, cardiovascular, central nervous system and cancer [14-17]. In this study, we seek to determine and evaluate the biological effects of Pavetta crassipes bioassay guided fractions on malignant glioblastoma cells and the molecular signaling pathways perturbed by Fraction G.
Materials and Methods
Plant material
The leaves of P. crassipes K. Schum (Rubiaceae) were harvested fresh from a horticultural garden in Abuja, FCT, Nigeria, and were air-dried. The plant was botanically identified by Dr. Grace Ugbabe, and a voucher sample (number 4745) was deposited in the department of Medicinal and Traditional Medicine, National Institute for Pharmaceutical Research and Development (NIPRD), Abuja, Nigeria.
Extraction of plant material
The air-dried leaves were finely powdered, and a 500 g portion of the plant material was macerated at room temperature with 2 L of methanol (MeOH) for 24 hours. The mixture was filtered and the filtrate evaporated (40°C) in vacuo until MeOH was almost removed. The methanolic extract was dried in a vacuum desiccator to yield a dry powder of 75 g.
Bioassay guided fractionation of P. crassipes extracts
Ten grams of the methanolic extract was loaded on a flash chromatography column packed with 200 g of silica gel (230-400 mesh size). The column was successively eluted with petroleum ether 100%, petroleum ether/ethyl acetate 7:3; petroleum ether/ ethyl acetate 3:7, ethyl acetate 100%, and ethyl acetate methanol 9:1. Thirty-nine (39) fractions of 50 mL each were collected. Fractions were monitored with thin layer chromatography (TLC). Fractions showing similar compounds on the TLC were combined. Five (5) pooled fractions labeled fraction A, fraction C, fraction E, fraction G and fraction J were collected and sent for biological screening. Fraction G, eluted with 100% ethyl acetate, was found to be biologically active. Fraction G was further analyzed using Ultra High Performance Liquid Chromatography (UHPLC).
UHPLC chromatography of fraction G
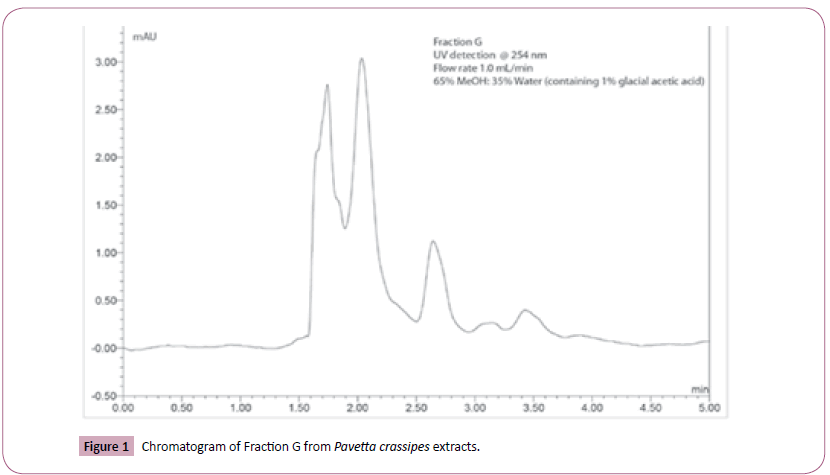
A Dionex Ultimate 3000 UHPLC instrument equipped with a quaternary analytical pump, an autosampler with integrated column compartment, a diode array detector, and an automated fraction collector was used. Columns utilized included an Acclaim 120 C18 guard column (4.6 × 10 mm, 5 μm silica) and an Acclaim 120 C18 analytical column (4.6 × 150 mm, 5 μm silica). The mobile phase was composed of 65% methanol and 35% water (containing 1% glacial acetic acid). The sample was dissolved in the mobile phase at a concentration of 1 mg/mL. The analysis was performed at a temperature of 30°C, flow rate of 1.0 mL/min and injection volume of 10 μL. Peaks were monitored at 223, 230, 254 and 365 nm.
Cell lines
Human glioblastoma cell lines (U251 MG, U1242 MG) and Normal Human Astrocytes (NHAs) were kind gifts from Dr. Isa Hussaini, University of Virginia, Charlottesville, USA. The cells were cultured in MEM-alpha (Life Technologies, Fisher Scientific, Pittsburgh, PA), supplemented with 10% FBS (Biowest, Kansas, MO), 1% penicillin and streptomycin (Fisher Scientific, Pittsburgh, PA). Prior to experimental studies and assays, cultures were grown to 70-80% confluency. These cells were serum starved and treated with Fraction G in the presence or absence of EGF (25 ng/ mL).
Chemicals and reagents
EGF and anti-tubulin antibody (DM1A) were purchased from Sigma Aldrich (St. Louis, MO), phospho-specific and total antibodies directed against ERK ½, and AKT, were obtained from Cell Signaling Technologies (Beverly, MA), antibody directed against PARP was obtained from Trevigen (Gaithesburg, MD). MTT (3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide) was purchased from Promega (Madison, WI). 0.4% Trypan blue stain was obtained from Gibco Life Technologies (Grand Island, NY). Pierce LDH cytotoxicity assay kit was obtained from Thermo Scientific (Rockford, IL).
Cell counts using Trypan blue exclusion assay
GBM cell lines U251 MG and U1242 MG were seeded at 1 × 105 cells per well in a 6 well plate in the presence or absence of Fraction G (0-20 μg/mL) for 0-72 h. Treated and untreated cells were trypsinized and harvested every 24 h. 100 μL of cell suspension was pipetted into a microcentrifuge tube and mixed with 100 μL of Trypan blue dye. 20 μL of cell suspension was added to a hemocytometer slide and the cells were counted under a microscope [18]. In another set of experiments, we tested the effects of Fraction G on non-malignant, normal human astrocytes (NHA). NHAs were grown on glass slides in a 6 well plate. After reaching 70% confluency, the cells were treated with DMSO (control) and Fraction G (10 μg/mL) for 48 h. The cells were washed with PBS and photographed using a NIKON H600L Eclipse, Japan fluorescence microscope.
Assessment of cell viability
This test was carried out to assess the effects of fraction G on glioma cell proliferation using the MTT assay. The MTT assay is based on the ability of cells to metabolize a yellow tetrazolium salt to a formazan product that can be detected using a spectrophotometer. Briefly, GBM cells were seeded at 5 × 103 cells in a 96-well plate and incubated for 24 h. The cells were then treated with various concentrations of Fraction G (0-20 μg/mL) for 48 and 72 h. Following Fraction G exposure, 20 μL of MTT reagent was added to the 96-wells containing cells and culture media (100 μL) and the plates were incubated at 37 °C in a humidified atmosphere containing 5% carbon dioxide for 4 h. At the end of the 4 h incubation period, the cell solubilization and stop reagent was added at a volume of 100 μL for 60 min., kept in the dark at room temperature and the optical density was measured using Promega Glomax Multidetection System (Madison, WI). The optical density was used to calculate the rate of cell proliferation after exposure to the different concentrations of Fraction G compared to the control wells. All experiments were performed in triplicate and the mean ± SEM for each experiment was calculated and plotted as a bar graph [19,20].
Cell cycle analysis by flow cytometry
The flow cytometric analysis of U251 glioma cells was performed using the Becton Dickinson FACScalibur (BD Biosciences, San Jose, CA) equipped with CellQuest software. U251 MG cells were plated in 10 cm plates. After 24 h the cells were serum starved and treated with varying concentrations of Fraction G (0-20 μg/mL) for 72 h. After the 72 h treatment, the floating and adherent cells were pooled together. The cell suspension was then centrifuged at 800 x g. Cell pellets were resuspended, washed with PBS, fixed in ice-cold 70% ethanol and stored at -20°C. The fixed cells were washed with PBS and stained with a solution consisting of PBS with 20 μg/mL of propidium iodide from Sigma, (St. Louis, MO), 0.1% Triton X-100 and 200 μg/mL RNAse A (Sigma, St. Louis, MO), kept in the dark for 1 h. The stained cells were analyzed using the BD FACScalibur equipped with CellQuest software. For each sample 20,000 counts were analyzed. Cells were gated using the Cellquest software to estimate the proportion of cells distributed as a percentage in the different cell cycle compartments [21].
Detection of apoptotic cells
Living and dead glioma cells were detected using fluorescence microscopy. In this assay, GBM cells were cultured on coverslips and treated with varying concentrations of Fraction G (0, 5-20 μg/mL) for 72 h. At the end of the treatment time point, cells were washed with PBS and stained with the Hoechst 33342 (10 μg) for live cells and PI (10 μg) for dead cells. Hoechst 33342 dye penetrates the membrane and therefore, has the potential to stain live cells. This stain is known to bind the adenine-thymineregions of the DNA. The fluorescence of Hoechst dye is very sensitive to DNA changes in cells. This dye can detect changes of nuclear compromise and damage. Hoechst 33342 dye emits blue fluorescence when bound to double-stranded DNA. This dye is often used to distinguish condensed nuclei in apoptotic cells. The propidium iodide dye is an impermeant dye that is often excluded from the living and viable cells. PI dye is known to penetrate cells with compromised or damaged membrane. The stained cells were placed in the dark for 10 min at room temperature. Images of cells were photographed using the NIKON H600L Eclipse, Japan fluorescence microscope [22,23].
Lactate Dehydrogenase (LDH) assay
The release of lactate dehydrogenase (LDH) is indicative of compromised membrane integrity and cell injury. The assay protocol outlined by Trevor et al. [24] was used in this study with some modifications. GBM cells (U251 MG and U1242 MG) were treated with Fraction G for 48 h. After treatment, cellular amount of LDH was measured by adding lysis buffer (1% Triton) to the cells. The amount of LDH content released by the cells was measured using Promega Glomax Multi-detection system microplate reader at a wavelength of 490 nm. The data obtained are represented as mean ± SEM of three independent experiments.
Measurement of caspase 3/7 activity
Caspase activity was detected as a means to evaluate apoptosis in cells treated with fraction G. Cells were assayed for Caspase 3/7 activity 48 h after fraction G treatment using Caspase 3/7 Glo substrate solution as recommended by the manufacturer (Promega, Madison, WI). Luminescence was measured using a Glomax multidetector system (Promega, Madison, WI). Luminescence readings were corrected by cell number and also compared to protein concentration via BCA (Thermo Scientific (Rockford, IL). Values shown are normalized to a percent maximal activity. In another set of experiments, a broad spectrum caspase inhibitor was used to rescue the effect of fraction G on upregulation of caspase cleavage. Glioma cell lines were pretreated with the caspase inhibitor Ac-DEVD-CHO (100 μM) for 4 hours and then treated with fraction G for 48 h. After a 48 h time point, the cells were counted using the hemocytometer.
Western blot analysis
Immunoblotting analyses were carried out as previously reported [25,26]. After the different treatments, cells were washed in ice cold PBS and extracted with 1% Triton X-100 cell lysis buffer. Cells were centrifuged at 14,000 x g for 10 min at 4°C. Protein concentrations of the supernatant were determined by the BCA protein assay (Bio-Rad). Proteins were subjected to electrophoresis and separated by SDS-PAGE on 10% polyacrylamide gels and then electroblotted onto nitrocellulose. The nitrocellulose membranes were incubated with primary antibody overnight at 4°C. These membranes were washed three times with phosphate buffered saline with tween 20 (PBST) and incubated with a peroxidase conjugated secondary antibody (Jackson ImmunoResearch Lab, West Grove, PA) at room temperature. After the secondary antibody, the membranes were washed and protein visualization was carried out using the enhanced chemiluminescence (ECL) reagents from Fisher Scientific (Milwaukee, WI) as described by the manufacturer. In sets of experiments investigating PARP cleavage, glioma cell lines were treated with different concentrations of fraction G, floating cells as well as the adherent cells were pooled together and centrifuged at 800 × g. The media was decanted and the cell pellet was extracted with RIPA buffer. The cell suspension was then sonicated and centrifuged at 14,000 × g for 10 min at 4 °C. Proteins (20 μg/lane) were separated by SDS-PAGE on 10% or 12% polyacrylamide gels, then transferred onto nitrocellulose and reacted with antibodies directed against PARP. Experiments were repeated three times for reproducibility. Densitometry and ImageQuant were carried out using the Alpha Innotech FluorChem2 (Cell Biosciences, Santa Clara, CA).
Statistical analysis
Data are expressed as mean ± SEM. All statistical analyses were performed with GraphPad Prism 7 software using the one-way analysis of variance followed by the Bonferroni test. A value of p<0.05 was considered to be statistically significant.
Results
HPLC analysis of fraction G
The HPLC finger print data revealed that Fraction G was a mixture of several compounds as seen in Figure 1.
Effect of fraction G from Pavetta crassipes leaves on glioma cell proliferation
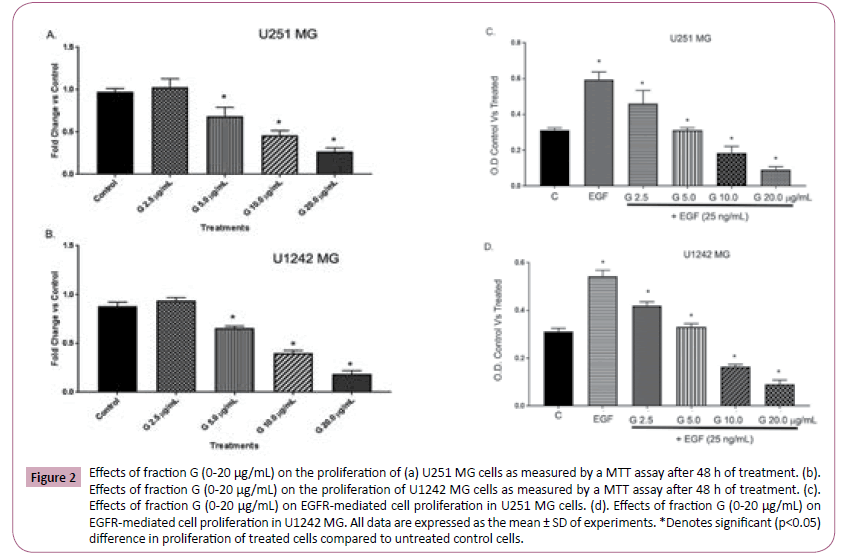
We employed the MTT assay to determine the effects of Fraction G on GBM cell proliferation and viability. The MTT assay is based on the cells ability to convert tetrazolium salt to insoluble formazan. A decrease in cellular accumulation of formazan indicates a damage in cells (loss of viable cells) or cytostatic activity. Our results revealed that fraction G induces a dose-dependent decrease in the formation of formazan by the glioma cell lines tested (U251 MG (Figure 2A) and U1242 MG (Figure 2B)). EGF is known to stimulate pathways that increase cell proliferation, survival and migration in glioblastoma. We tested the effects of Fraction G on EGF induced cell proliferation and viability using the MTT assay in GBM cells. The addition of 25 ng/mL EGF induced a robust increase in GBM cell proliferation. Fraction G concentration-dependently abrogated EGF-mediated increase in cell proliferation in U251 (Figure 2C) and U1242 (Figure 2D).
Figure 2: Effects of fraction G (0-20 μg/mL) on the proliferation of (a) U251 MG cells as measured by a MTT assay after 48 h of treatment. (b).Effects of fraction G (0-20 μg/mL) on the proliferation of U1242 MG cells as measured by a MTT assay after 48 h of treatment. (c).Effects of fraction G (0-20 μg/mL) on EGFR-mediated cell proliferation in U251 MG cells. (d). Effects of fraction G (0-20 μg/mL) on EGFR-mediated cell proliferation in U1242 MG. All data are expressed as the mean ± SD of experiments. *Denotes significant (p<0.05) difference in proliferation of treated cells compared to untreated control cells.
Effect of fraction G from Pavetta crassipes leaves on glioma cell viability
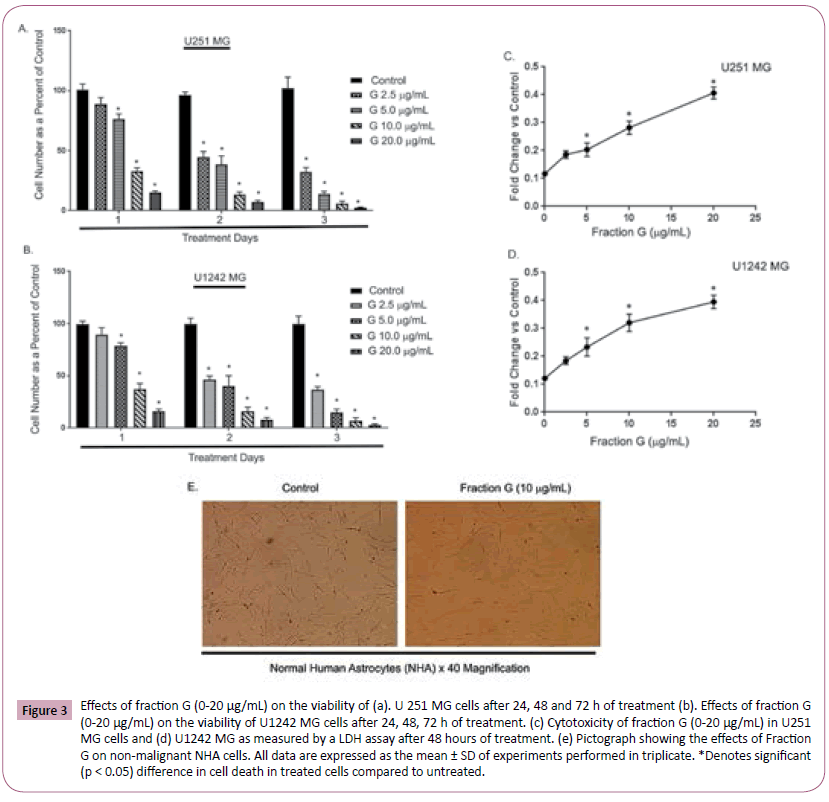
The MTT test above suggests that Fraction G decreased the viability of U1242 MG and U251 MG cells. To confirm these effects, we examined the effects of Fraction G on U1242 MG and U251 MG cell viability using the Trypan blue exclusion and lactate dehydrogenase (LDH) assays. Trypan blue exclusion is based on the principle that live cells possess intact cell membranes and will exclude trypan blue, while dying cells possess compromised cell membranes and thus will not exclude dye. We observed that both U1242 MG and U251 MG treated with Fraction G (0-20 μg/mL) showed a dose- and time-dependent increase in trypan blue positivity (Figure 3A and 3B) compared with cells treated with control medium. We also tested the ability of fraction G to cause changes in cell membrane integrity and leakage of lactate dehydrogenase (LDH) into extracellular media on U1242 MG and U251 MG. U1242 MG and U251 MG cells treated with Fraction G dose-dependently displayed an increased in the amount of lactate released into culture media after 48 h when compared to control cells (Figure 3C and 3D). Our data shows that Fraction G dose dependently decreased GBM cell viability. Futhermore, we investigated the effects of Fraction G on the viability of nonmalignant normal human astrocytes. We observed from the pictograph that treatment with fraction G (10 μg/mL) did not alter the morphology of the cells (Figure 3E).
Figure 3: Effects of fraction G (0-20 μg/mL) on the viability of (a). U 251 MG cells after 24, 48 and 72 h of treatment (b). Effects of fraction G (0-20 μg/mL) on the viability of U1242 MG cells after 24, 48, 72 h of treatment. (c) Cytotoxicity of fraction G (0-20 μg/mL) in U251 MG cells and (d) U1242 MG as measured by a LDH assay after 48 hours of treatment. (e) Pictograph showing the effects of Fraction G on non-malignant NHA cells. All data are expressed as the mean ± SD of experiments performed in triplicate. *Denotes significant (p < 0.05) difference in cell death in treated cells compared to untreated.
Effect of fraction G from Pavetta crassipes leaves on glioma cell cycle
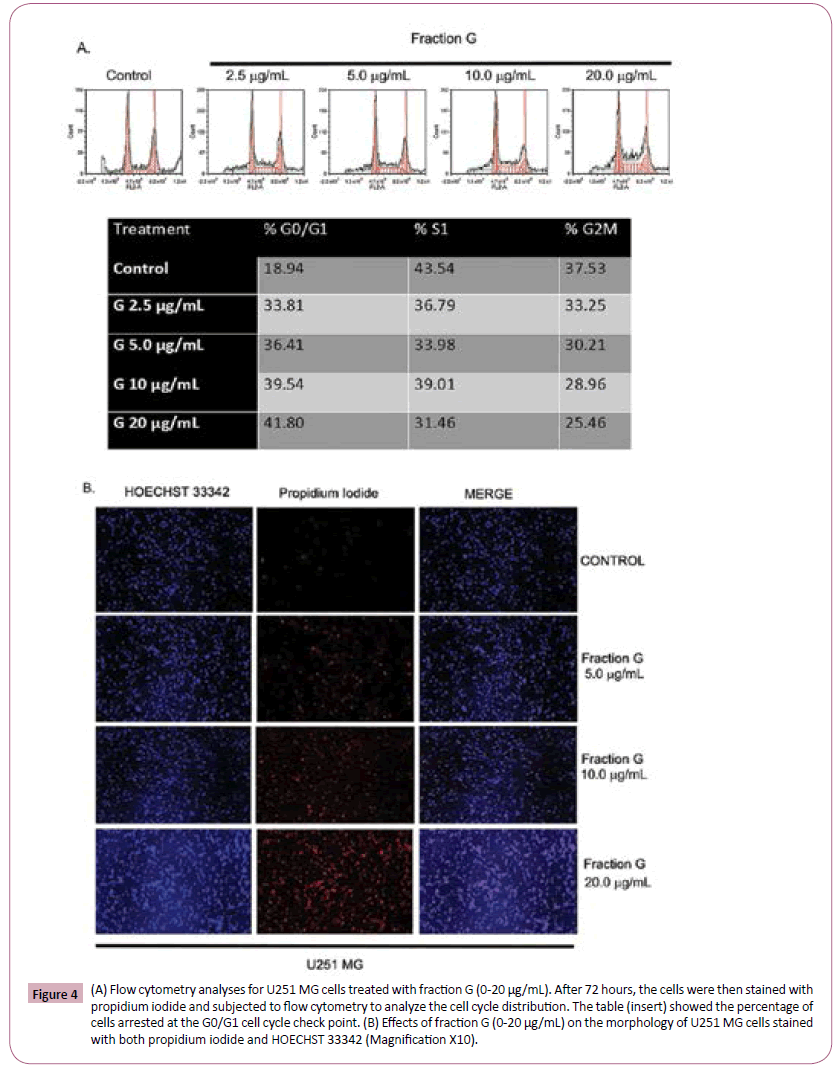
Since the MTT test does not distinguish cellular proliferation from death, we employed cellular number counts and fluorescent activated cell sorting (FACS) to ascertain the effects of Fraction G on GBM cell cycle check points. Flow cytometric analysis revealed that Fraction G significantly increased the percentage of cells arrested in G0/G1 cell cycle check point with fragmented nuclei when compared to control treated cells (Figure 4A). Our data showed that 18.94% of control cells were arrested while cells treated with Fraction G had an increase in arrested cells from 33.81% to 41.8% (Figure 4A). We further tested the effects of Fraction G on U251 MG cells and imaged both the dead and living cells using the Hoechst 33342 and propidium iodide. Our data in Figure 4B illustrates an increase in the number of cells incorporating propidium iodide with increase in the concentration of Fraction G.
Figure 4: (A) Flow cytometry analyses for U251 MG cells treated with fraction G (0-20 μg/mL). After 72 hours, the cells were then stained with propidium iodide and subjected to flow cytometry to analyze the cell cycle distribution. The table (insert) showed the percentage of cells arrested at the G0/G1 cell cycle check point. (B) Effects of fraction G (0-20 μg/mL) on the morphology of U251 MG cells stained with both propidium iodide and HOECHST 33342 (Magnification X10).
The effect of fraction G from Pavetta crassipes leaves on GBM cell death
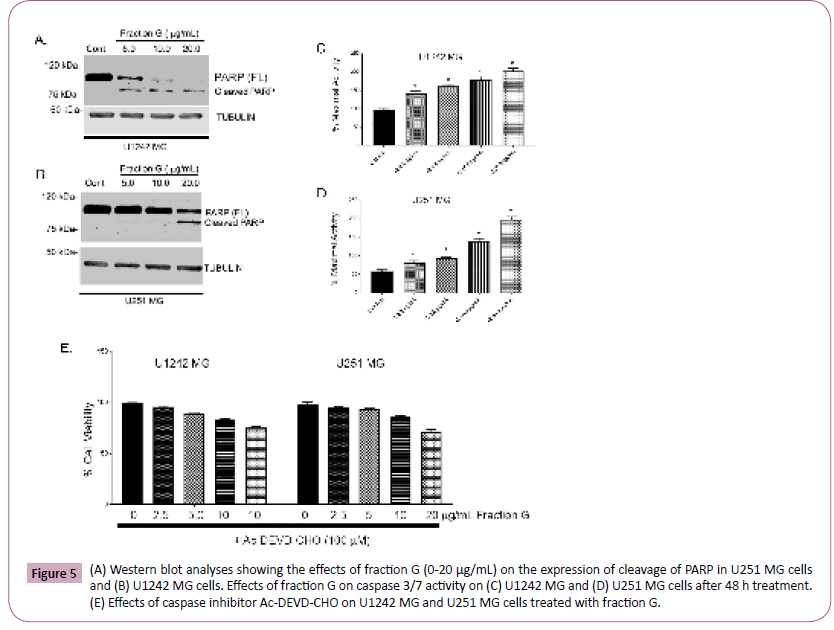
To ascertain the mechanism of cell death induced by Fraction G in U1242 MG and U251 MG cells, we measured PARP cleavage, a well-known hallmark of apoptosis (33). We observed an increase in the cleaved form of PARP from the active 116-kda DNA repair molecule to the inactive 89-kda molecule in GBM cells treated with a higher concentration of Fraction G when compared to control cells (Figure 5A and 5B). Our data shows that Fraction G induced apoptotic-dependent cell death of GBM cells. We further investigated the effects of caspase activity, we show here that fraction G induces a concentration dependent increase in caspase activity (Figure 5C and 5D). Our data also revealed that both U1242 MG cells and U251 MG cells were sensitive to Fraction G treatment. We attempt to rescue the observed cell death induced by fraction G in glioma cell lines by pretreating the cells with caspase inhibitor Ac-DEVD-CHO. Our data in Figure 5E showed that pretreatment with the caspase inhibitor abrogated the decrease in cell counts induced by fraction G.
Figure 5: (A) Western blot analyses showing the effects of fraction G (0-20 μg/mL) on the expression of cleavage of PARP in U251 MG cells and (B) U1242 MG cells. Effects of fraction G on caspase 3/7 activity on (C) U1242 MG and (D) U251 MG cells after 48 h treatment. (E) Effects of caspase inhibitor Ac-DEVD-CHO on U1242 MG and U251 MG cells treated with fraction G.
The effect of fraction G from Pavetta crassipes leaves on EGF-mediated activation of AKT and ERK ½ in GBM cells
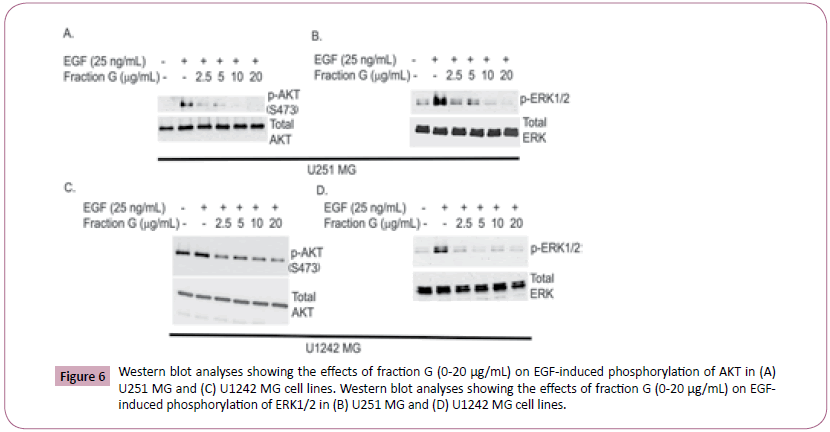
To investigate the molecular mechanisms by which Fraction G induces cell death and inhibits proliferation in GBM cells, we investigated the effects of fraction G on EGF mediated activation of MAPK and AKT. EGF binding to EGFR activates the Ras-Raf- MEK-ERK pathway which phosphorylates and activates ERK ½ downstream. AKT is activated by EGF through the PI3K/AKT/ mTOR pathway. Once activated, both AKT and ERK ½ can activate other downstream signaling proteins that reduce apoptosis and promote cell survival. Western blot analyses of U1242MG and U251 MG cells treated with Fraction G attenuated EGFRmediated phosphorylation of AKT (Figure 6A and 6C) and ERK ½ (Figure 6B and 6D) in a concentration-dependent manner. These data suggest that Fraction G interferes with EGF mediated effects on the GBM cells by inhibiting the activation of ERK ½ and AKT.
Figure 6: Western blot analyses showing the effects of fraction G (0-20 µg/mL) on EGF-induced phosphorylation of AKT in (A) U251 MG and (C) U1242 MG cell lines. Western blot analyses showing the effects of fraction G (0-20 µg/mL) on EGFinduced phosphorylation of ERK1/2 in (B) U251 MG and (D) U1242 MG cell lines.
Discussion
Medicinal plants have been used traditionally over the years to treat several disease conditions and are still being used. The resistance of cancer cells to current available chemotherapeutic agents and the toxic side effects of these agents has prompted scientists to further investigate the therapeutic potential of natural agents. Even though many extracts from plants are being investigated for their potential biological activities, it is increasingly evident that the efficacy of these products require their presence in a mixture of complex molecules rather than in a pure single state [27,28]. With this thought in mind, we sought to investigate the anticancer activity of Fraction G extract from Pavetta crassipes leaves. We have previously reported that the extract from Pavetta crassipes leaves possess some biological CNS activities [16]. In this study we demonstrated that Fraction G extract from Pavetta crassipes leaves has antitumor activity against GBM cells, via its anti-proliferation, survival and its potential to induce apoptosis. The anti-proliferative properties of Fraction G occurred via the accumulation of these cells in the G0/G1 phase of the cell cycle, while its pro-apoptotic properties will most likely be through caspase activation since we observed PARP cleavage. The ability of Fraction G to induce apoptosis as preferred cell death makes it a potential tool for GBM therapy. Various small molecules and therapeutic antibodies targeting EGFR family members have been developed and are currently used in the treatment of GBM. However, the increased use of EGFR tyrosine kinase inhibitors has led to the development of resistance to EGFR-targeting drugs due to targeted mutations and the presence of EGFR-mutated variants. EGFR signaling plays a key role in many cancers including glioblastoma. EGFR activation of the RTK/RAS/PI3K pathway aids in the regulation of cellular proliferation, angiogenesis, local tissue invasion and resistance to apoptosis [29]. AKT provides key regulation of several cell survival, growth and proliferation pathways. Additionally, changes in the AKT pathway appear to play key roles in many types of cancer. Thus this pathway has become a signaling pathway of interest to target for cancer treatment [30]. Our data revealed that Fraction G inhibited EGF mediated phosphorylation of AKT in glioma cell lines. This data agrees with several studies that have shown natural products downregulate AKT phosphorylation in gastric, breast, brain, and other cancer cell lines [8,31]. Additionally, the ERK pathway, one of several MAPK pathways, is known for its role in cell proliferation. However, it is also understood to be involved in other aspects of tumor presentation such as cellular differentiation and migration [32-34]. Here we show that Fraction G extract from Pavetta crassipes leaves inhibited EGF-mediated ERK ½ phosphorylation.
Conclusion
The present study demonstrated that Fraction G possesses in vitro biological activity against GBM cells by inhibiting cell proliferation and inducing apoptosis. Our data also demonstrate that Fraction G attenuated EGF-mediated phosphorylation of ERK ½ and AKT. Additionally, we observed that treatment with Fraction G induced PARP cleavage in two genetically different GBM cells. Fraction G extract exhibited a dose and time dependent inhibition of EGF mediated GBM cell survival and proliferation. Therefore, we conclude that Fraction G of the leaf extracts of P. crassipes possess anti-tumor effects which possibly may be mediated via the inhibition of AKT and ERK ½ signaling pathways and induction of PARP cleavage.
Acknowledgements
We thank the School of Pharmacy, Cedarville University for providing financial support for this project to Dr. Samson Amos.
Conflict of Interest
Authors declare no conflict of interest.
References
- Huse JT, Holland EC (2010) Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer 10: 319-331.
- Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359: 492-507.
- Furnari FB, Fenton T, Bachoo RM, Mukasa A (2007) Malignant astrocytic glioma: genetics biology and paths to treatment. Genes Dev 21: 2683-2710.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2007) The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol 114: 97-109.
- Ohgaki H, Kleihues P (2009) Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci 100: 2235-2241.
- Wells A (1999) EGF receptor. Int J Biochem Cell Biol 31: 637-643.
- Wang H, Xu T, Jiang Y, Xu H (2015) The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia 17: 239-255.
- Gentile MT, Ciniglia C, Reccia MG, Volpicelli F (2015) Ruta graveolens L. induces death of glioblastoma cells and neural progenitors, but not of neurons, via ERK ½ and AKT activation. PLoS ONE 10: 1-17.
- Nishikawa R, Ji XD, Harmon RC, Lazar CS (1994) A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci 91: 7727-7731.
- Holland EC, Celestino J, Dai C, Schaefer L (2000) Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet 25: 55-57.
- Gordaliza M (2007) Natural products as leads to anticancer drugs. Clin Transl Oncol 9: 767-776.
- Newman DJ, Cragg GM, Snader KM (2003) Natural products as sources of new drugs over the period 1981-2002. J Nat Prod 66: 1022-1037.
- Coseri S (2009) Natural products and their analogues as efficient anticancer drugs. Mini-Reviews in Medicinal Chemistry 9: 560-571.
- Amos S, Okwuasaba FK, Gamaniel K, Akah P (1998) Inhibitory effects of the aqueous extract of Pavetta crassipes leaves on gastrointestinal and uterine smooth muscle preparations isolated from rabbits, guinea pigs and rats. J Ethnopharmacol 61: 209-213.
- Amos S, Akah PA, Binda L, Enwerem NM (2003) Hypotensive activity of the ethanol extract of Pavetta crassipes leaves. Biol Pharm Bull 26: 1674-1680.
- Amos S, Akah PA, Enwerem N, Chindo BA (2004) Behavioral effect of Pavetta crassipes extract on rodents. Pharmacol Biochem Behav 77: 751-759.
- Abubakar MS, Musa AM, Ahmed A, Hussaini IM (2007) The perception and practice of traditional medicine in the treatment of cancers and inflammations by the Hausa and Fulani tribes of Northern Nigeria. J Ethnopharmacol 111: 625-629.
- Li Y, Zhang P, Qiu F, Chen L, Miao C, et al. (2012) Inactivation of PI3K/Akt signaling mediates proliferation inhibition and G2/M phase arrest induced by andrographolide in human glioblastoma cells. Life Sci 90: 962-967.
- Kavitha CV, Nambiar M, Ananda Kumar CS (2009) Novel derivatives of spirohydantoin induce growth inhibition followed by apoptosis in leukemia cells. Biochem Pharmacol 77: 348-363.
- Lee YC, Lin HH, Hsu CH (2010) Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol 632: 23-32.
- Denkert C, Furstenberg A, Daniel PT, Koch I (2003) Induction of G0/G1 cell cycle arrest in ovarian carcinoma cells by the anti-inflammatory drug NS-398, but not by COX-2-specific RNA interference. Oncogene 22: 8653-8661.
- Kefas BA, Cai Y, Ling Z, Heimberg H (2003) AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J Mol Endocrinol 30: 151-161.
- Hoorens A, Van de M, Kloppel G, Pipeleers D (1996) Glucose promotes survival of rat pancreatic beta cells by activating synthesis of proteins which suppress a constitutive apoptotic program. J Clin Invest 98: 1568-1574.
- Stump TA, Santee BN, Williams LP (2017) The antiproliferative and apoptotic effects of apigenin on glioblastoma cells. J Pharm Pharmacol 69: 907-916.
- Amos S, Martin PM, Polar GA, Parsons SJ (2005) Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cdelta/c-Src pathways in glioblastoma cells. J Biol Chem 280: 7729-7738.
- Hussaini IM, Karns LR, Vinton G, Carpenter JE (2000) Phorbol 12-myristate 13-acetate induces protein kinase ceta-specific proliferative response in astrocytic tumor cells. J Biol Chem 275: 22348-22354.
- Liu RH (2000) Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 78: 517-520.
- Karna P, Chagani S, Gundala SR, Rida PC (2012) Benefits of whole ginger extract in prostate cancer. Br J Nutr 107: 473-484.
- Padfield E, Ellis HP, Kurian KM (2015) Current Therapeutic Advances Targeting EGFR and EGFRvIII in Glioblastoma. Front Oncol 5: 1-5.
- Aeder SE, Martin PM, Soh JW, Hussaini IM (2004) PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene 23: 9062-9069.
- Ong CS, Zhou J, Ong CN, Shen HM (2010) Luteolin induces G1 arrest in human nasopharyngeal carcinoma cells via the Akt-GSK-3ß-Cyclin D1 pathway. Cancer Lett 298: 167-175.
- Huang C, Jacobson K, Schaller MD (2004) MAP kinases and cell migration. J Cell Sci 117: 4619-4628.
- Los M, Mozoluk M, Ferrari D, Stepczynska A, Stroh C, et al. (2002) Activation and caspase-mediated inhibition of PARP: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell 13: 978-988.
- Chazotte B (2011) Labeling nuclear DNA with hoechst 33342. Cold Spring Harb Protoc.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences