ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Evaluation of Integrated Management of Common Bacterial Blight of Common Bean in Central Rift Valley of Ethiopia
Ararsa L1*, Fikre L2 and Getachew A3
1College of Agriculture and Environmental Sciences, Arsi University, Asella, Ethiopia
2College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia
3Ethiopian Institute of Agricultural Research, Malkassa Agricultural Research Center, Adama, Ethiopia
- *Corresponding Author:
- Ararsa L
College of Agriculture and Environmental
Sciences, Arsi University, Asella, Ethiopia
Tel: +2510913245504
E-mail: ararsaleta@gmail.com
Received date: January 02, 2018; Accepted date: January 27, 2018; Published date: January 29, 2018
Citation: Ararsa L, Fikre L, Getachew A (2018) Evaluation of Integrated Management of Common Bacterial Blight of Common Bean in Central Rift Valley of Ethiopia. Am J Phytomed Clin Ther Vol. 6 No. 1:3. doi:10.21767/2321-2748.100339
Abstract
Common bacterial blight is the most destructive bean diseases resulting in seed yield and quality losses worldwide. Recommended control measures include varietal resistance, production and use of ‘‘clean’’ seed, antibiotic seed treatments, foliar spray with copper hydroxide and intercropping. However, none of the above mentioned management methods is satisfactory when applied alone. Therefore the current study aim to evaluate integrated disease management through seed treatment, intercropping and Bacticide (Copper hydroxide 77% WP) spray. Streptomycin at the rate of 50,000 ppm and garlic and moringa extracts of 10-1 dilution were used for seed dressing
Keywords
Common bacterial blight; Integrated disease management; Plant extract
Introduction
Common bean (Phaseolus vulgaris L.) is one of the most important pulse crops in Ethiopia. The main production areas include eastern Ethiopia, the south and the south west, the west and the Rift Valley. The Rift Valley area accounts for more than half of the country’s bean production, mainly of the white pea bean type that is grown for export [1]. Currently, Ethiopia is one of the most important beans producing country in the world. The report by central statistical agency, CSA [2] indicates that the country produces 3,878,023.01 Qts in 2011/12 main cropping season and the estimate production for 2012/13 is 4,127,345.88 Qts. The report reveals that although the area under production increase from year to year the productivity is declining. The main reasons for low productivity of common bean in Ethiopia include luck of certified seed [3] and disease, insect pest and weeds [1]. Among the many diseases affecting bean plants, common bacterial blight (CBB) is the most destructive bean diseases [4,5]. CBB may be highly destructive during extended periods of warm and humid weather, resulting in yield and seed quality losses.
These conditions commonly occur in Central Rift Valley during flowering to seed setting growth period and the disease is highly distributed and most severe during this period and farmers considered as it is a major production constraint which limits the productivity and market value of their bean.
Seed transmission plays a significant role in the development of an epidemic common bacterial blight [6] and seed inoculum management considered as the primary management option. Recommended control measures include production and use of ‘‘clean’’ seed from regions supposed to be disease free [7], antibiotic seed treatments [8,9] foliar spray of bactericides such as copper sulphate and copper hydroxide [7-10] intercropping [11,12] and varietal resistance [13]. However, lack of high level of resistance in common bean and susceptibility of the resistant cultivars to the virulent races (pathotypes) in another area were the constraints in use of disease resistance as CBB management option [14]. Although Besides the use of pathogen-free seeds, insignificant pathogen levels can also be attained by the use of seed treatments with the antibiotics such as streptomycin sulphate can control CBB in bean [8,9] concerns of a potential buildup of antibiotic resistance in the soil micro-flora [15] reduce the use this antibiotic. Moreover, chemical control of CBB is often inefficient and expensive [16,17]. Therefore, an investigation of affordable and environmentally friendly methods in controlling Xap and integration of all possible CBB management strategies would be important. Garahushoma [18] reported 20% (v/v) garlic extract seed treatment was significantly reduce levels of bacterial seed-borne pathogens in beans without interfering with seed viability and germination. Another report by Raghavendra [19] revealed aqueous, methanol and ethanol extracts of Acacia nilotica showed significant antibacterial activity against Xanthomonas axonopodis pv. malvacearum, X. a. pv. phaseoli and X. campestris pv. vesicatoria. Hence, the aim of the current study was to evaluate the effectiveness of integrate disease management through seed treatment, intercropping and chemical spray for the management of CBB in common bean.
Materials and Methods
Description of the study area
The experiment was carried out during 2015 main growing seasons at two sites in the central rift valley area namely Melkassa Agricultural Research Center (Melkassa) and Arsi Negele Agricultural Research Substation (Arsi Negele). Melkassa is located 99 km southeast of Addis Ababa in the semi-arid region of Central Rift Valley at 8°24’ N latitude, 39° 12’ E longitude and the altitude of the area is 1550 masl. The ten years (2003 to 2012) average weather data show that the area receives an average of 915.7 mm annual rainfall and the maximum and minimum annual mean temperatures are 28.9°C and 13.8°C, respectively. The soil type of the site is Andosol which is cultivated for long period of time [20]. Arsi Negele is also one of the sub-centers of MARC and located to 228 km south of Addis Ababa at 7° 25’ N latitude, 38° 31’ E longitude and an elevation of 1900 masl. The past ten years (2003 to 2012) data shows the area receives an average annual rainfall of 881.2 mm and the maximum and minimum annual mean temperatures of 27°C and 10.6°C, respectively. The soil type of the site is Nitosol [20].
Experimental material and treatments
Plant extract and streptomycin seed treatments, bean- maize intercropping and cupper hydroxide 77% WP (bacticide) spray were evaluated for their potential to reduce bean common bacterial blight epidemics and yield loss in bean cultivar Awash-1. Garlic cloves and ginger powder used in the experiment were purchased from market in Adama and moringa leave were collated from Melkassa Agriculture Research Center compound. Plant extraction was done in Melkassa Agricultural Research Center food science laboratory and the in vitro evaluation experiment was conducted in Plant Pathology laboratory of the center.
Plant extraction
Aqueous extraction: Garlic cloves were peeled and washed with distilled water, then the cloves were cut into small pieces, and the pieces were ground to a thick paste. Hundred grams of the paste were transferred into a beaker and filled up to 500 ml with SDW. The mixture was stirred thoroughly with a spatula to obtain a homogeneous suspension which was then covered with an aluminium foil and left to stand for 24 hours at room temperature. In a laminar air flow hood, sterile Whatman filter paper cones were used in a sterile funnel to separate out the debris from the crude garlic extract into a sterile glass jar and stored at 4°C until used. Fifty grams of ginger and moringa powder were each dissolved in 500 ml of SDW. The mixture was stirred thoroughly with a spatula to obtain a homogeneous suspension which was then covered with an aluminium foil and left to stand for 24 hours at room temperature. In a laminar air flow hood, sterile Whatman filter paper cones were used in a sterile funnel to separate out the debris from the crude extract into a sterile glass jar and stored at 4°C until used.
Petroleum ether extraction: Dried powder of moringa leaf and ginger rhizome and garlic paste was continuously refluxed with petroleum ether at 60°C for 3 h using soxhlet apparatus. The extracts were concentrated under reduced pressure in a rotary evaporator and stored in air-tight containers at 4°C until used.
In vitro antibacterial assay: Two ten-fold serial dilutions (10-1 and 10-2) and undiluted aqueous and petroleum ether extracts of each plant extract were prepared. The blank discs of 5 mm diameter were punched from filter paper of uniform thickness and sterilized by heat. The blank discs were separately soaked with each of extract. Xap inoculum was grown in nutrient broth, incubated at 28°C for 24 hrs. One ml of the broth culture of the bacterium was spread over the nutrient agar taken in glass Petri dishes aseptically. The extract soaked discs and the control (SDW and petroleum ether soaked) disc were placed on the inoculated nutrient agar in the Petri dishes and incubated at 28°C. After 5 days incubation the zones of inhibition of bacterial growth around the discs were measured.
Seed treatment: An infected bean seed lot confirmed by the direct plating procedure having 8% infection was used as planting material for the experiment. Based on the inhibition zone result, petroleum ether extract of garlic and moringa extract were used as seed treatment for further field trials. Streptomycin at the rate of 50,000 ppm and 10-1 dilution of petroleum ether extract of garlic and moringa extracts were used for seed dressings. Seeds were dressed by thoroughly mixing them in each solution at the rate of 50 ml/kg seed. All dressed seed samples were spread out and dry under shade.
Experimental design and management
The field experiment was carried out in the 2015 main growing season at two sites (Melkassa and Arsi Negele) of Melkassa Agricultural Research Center trial sites. Four seed treatments, two cropping system and two spray treatments were laid out in 4 × 2 × 2 factorial with randomized complete block design (RCBD) and each treatment replicate three times. Untreated seed, monocropping and unsprayed plot used as control plot. Each block and plots laid at 1 m and 0.5 m spacing respectively. Each plot has an area of 3.2 m*2 m and contains eight rows of bean in the case of sole cropping and four rows of bean and four rows of maize in the case of inter cropping. Planting was done on July 15, 2015 at Arsi Negele and July 18, 2015 at Melkassa. Bean planted at the spacing of 0.4 m and 0.1 m between rows and plants respectively, while maize planted at the spacing of 0.4 m between rows and 0.2 m between plants. Copper hydroxide 77% WP (bacticide) spray was made three times at 14 days interval starting from 35DAP. Weeding and cultivation was done manually for all treatments. No fertilizer was applied for all treatments.
Data collection
Disease incidence was determined as a number of plants affected per plot and expressing as percentage. Disease severity was assessed as the modified CIAT 0-9 scales [21], where 0=no infection, 1=1%, 2=2-5%, 3=6-10%, 4=11-15%, 5=16-30%, 6=31-50%, 7=51-75%, 8=75-85% and 9=>85% lesion area on the infected leaves. The severity grade was converted in to Percentage Severity Index (PSI) with the formula:
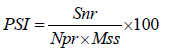
Where Snr=the sum of numerical ratings, Npr=number of plant rated, Mss=the maximum score of the scale. Incidence was determined by checking primary leaves of each plant 21 days after sowing. Then after, records were taken at 35, 49, 63 and 77 days after sowing. Disease severity was assessed on 10 randomly selected and per tagged plants per plot. The Area Under Disease Progress Curve (AUDPC) was calculated according to Shaner and Finney [22], by the formula:
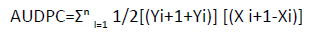
Where Yi=disease severity score at time i, and Xi=time of scoring (days after planting). Disease progress rate was computed from logistic model of disease severity as r=ln[(1/1-x)-(1/1-y)]/(ti-tf). Mean number of pods per plant was computed as number of pods of 10 plants randomly taken from the middle rows, and computing the average. The mean number of seed per pod was computed as average number of seeds from randomly sampled 10 pods. Grain yield per plot was measured as the weight of seed yield from the sex middle rows at 12% moisture content. Hundred seed weight was measured as weight of 100 randomly sampled seeds. Percent seed discoloration was determined as percentage of number of diseased seeds from 100 randomly sampled seeds. Relative yield loss percentage was computed as the yield difference of the basic treatment (treatment plots with all treatment combination) and the lower treatments by the formula:
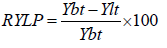
where RYLP is relative yield loss percentage, Ybt yield of basic treatment and Ylt yield from the lower treatment
Data analysis
All disease and yield and yield component (seed yield, relative yield loss percentage, No of pod per plant, No of seed per pod, hundred seed weight and seed discoloration percentage) data were subjected to analysis of variance (ANOVA) procedure with SAS 9.2 statistical analysis software. When there is treatment differences mean separation tests were performed using least significant difference (LSD).
Discussion
CBB can be managed using different disease management strategies including resistant varieties [23-25] cultural practices [26,27] seed treatment [18,19] and foliar chemical spray [9,10,28,29]. The present study evaluated the integrated effect of seed treatment with streptomycin and plant extracts, intercropping and foliar application of copper hydroxide 77% WP (bacticide) on disease development, seed yield and yield components. The result reveled that seed treatment, chemical spray and intercropping showed good potential in reducing diseases incidence, severity and yield loss in bean due to common bacterial blight and increase seed yield and yield components at both locations. Chemicals have been recommended as a seed treatment and foliar protectants to control CBB before it cause severe damage [30]. Streptomycin has given marginal control of CBB by reducing initial inoculum from the external surface of the seeds [8]. In the present study, seed treatment by streptomycin, garlic extract and moringa extract combined by copper hydroxide (bacticide) spray reduce disease incidence both at Arsi Negele and Melkassa over the other treatments. Interaction effect of treated seed with bacticide spray significantly reduce final disease incidence in untreated unsprayed plot from 89.98% to 54.17% at Arsi Negele and from 67.84% to 42.07% at Melkassa. Final percent severity index (PSI) were also reduced by the same treatment from 62.41% to 33.70% at Arsi Negele and from 48.70% 25.93% at Melkassa Seed treatment combined with chemical spray also improve pod per plant (PPPlt), seed per pod (SPP), at both location and promote seed yield and reduce seed discoloration (SDP) and relative yield loss (RYLP) at Arsi Negele. At Melkassa seed treatment alone increased seed yield and reduce relative yield loss and SDP. Spray of copper-based chemicals such as copper-hydroxide (Kocide-101) is among the chemicals used for foliar application, so as to reduce the dissemination of bacterial cells from diseased plant to the healthy one. The authors indicate the result of two-year study at Colorado suggested that application of copper-hydroxide (Kocide-101) at weekly interval might be effective and immediate means of reducing losses due to CBB in commercial common bean production [31]. In the present study, foliar sprays of copper hydroxide (bacticide) three times at 14 days interval starting from 35 days after planting (DAP) reduced final disease incidence, PSI, seed discoloration percentage and relative yield loss and increased yield and yield components at both locations. Selamawit [29] also reported similar results which indicated spray of copper-based chemical at 5 days interval increased yield over unsprayed one. Schwartz [32] also reported applying copper hydroxide contact bactericides early in the seasons every 7 to 10 days intervals during cool, moist weather can decrease establishment of bacterial pathogens (Figures 1-4). This would reduce the effect of the disease on the photosynthetically active leaves so that appropriate amount of manufactured assimilates reached the developing seeds that contribute to the yield improvement. A report by Balaz [33] showed satisfactory results in X. campestris pv. phaseoli control has been obtained by using copper-based compounds. Interaction effect of seed treatment and chemical spray had pronounced effect in reducing all disease parameters and increasing yield and yield component at Arsi Negele while significantly reduce all disease parameters but only improve the pod per plant and seed per pod at Melkassa (Tables 1 and 2). The difference in effectiveness of the treatments between the two locations might be related with climatic factors variation, which contribute more on disease development and yield potential of the crop. Planting streptomycin treated seed accompanied with bacticide spray significantly reduces final disease severity by 28.71% and 22.77% respectively at Arsi Negele and Melkassa (Tables 3-9). Here the seed treatment combined by chemical spray bring in up to 0.95 t/ ha yield advantage over untreated and unspray treatment at Arsi Negele (Tables 10 and 11) while seed treatment result in up to 0.7 t/ha yield advantage over untreated plot at Melkassa (Tables 12 and 13). Relative yield losses were also significantly reduced by the treatments applied over untreated plots. At Melkassa, where yield reduction where relatively higher, seed treatment resulted up to 31.8% yield loss reduction over untreated plot while at Arsi Negele seed treatment combined with bacticide spray resulted in up to 33.68% yield loss reduction over untreated and unspray control plot. These results are in agreement with Tumsa [9] finding in which a combination of streptomycin seed treatment with once and twice spray of Kocide-101 significantly reduce CBB epidemics and improve bean yield and yield components. Sintayehu and Amare [34] also report seed treatment with streptomycin integrated with biofumigation and foliar sprays of kocide-101 at two weeks interval were significantly reducing CBB epidemics and increasing yield and yield components. Belachew [35] also reported that combined application of mancozeb seed treatment and cultural practice, planting on the ridge reduce CBB incidence and PSI and increase yield and yield component both in susceptible and tolerant varieties. In the current study, common bean-maize intercropping were also significantly reduce CBB incidence, severity, AUDPC and relative yield loss at both locations as compared with sole common bean cropping system. The yield and yield component were also increased in intercropping over sole cropping. This can be because of the interception of inoculum movement from diseased plant to the health plant by the intercropped maize reduces disease incidence, severity and progress rate. Fininsa [10] in his field experiment conducted at Haramaya University experimental field station found that in maize bean intercropping systems, both relative and predicted seed yield and 100 seed weight losses to CBB were generally less than in pure stand. Kassahun [36] also report that common bean-sorghum (2:1 ratio) intercropping were significantly reduce CBB progress, AUDPC and relative seed yield and hundred seed weight loss at Eastern Amhara region as compared with sole common bean cropping system. In general, higher significant variation were observed in all disease and yield parameters including seed yield within seed treatments than other treatment factors. This is because the main predisposing factor for transmission of the diseases is infected seeds and seed treatment plays a significant role in reducing development of common bacterial blight by reducing the initial inoculum of the pathogen [34,37] and improve yield and yield components. Schwartz [32] report seed treatment with antibiotic has been recommended to disinfect external contamination of seed by CBB pathogen. Garahushoma [18] reported that a 20% (v/v) extract of garlic was significant in reducing levels of bacterial seed-borne pathogens on beans without affecting the germination of the crop. Goss [38,39] reported they were able to achieve control of Xanthomonas campestris pv campestris black rot disease of cabbage plants with leaf, seed and bark extracts of moringa.
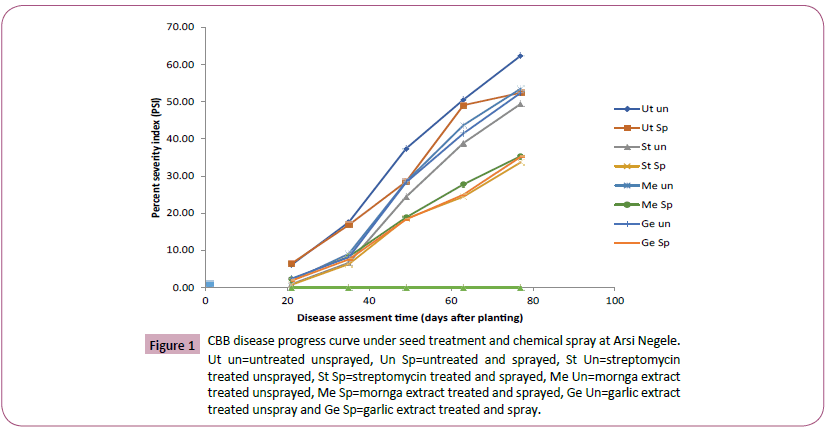
Figure 1: CBB disease progress curve under seed treatment and chemical spray at Arsi Negele. Ut un=untreated unsprayed, Un Sp=untreated and sprayed, St Un=streptomycin treated unsprayed, St Sp=streptomycin treated and sprayed, Me Un=mornga extract treated unsprayed, Me Sp=mornga extract treated and sprayed, Ge Un=garlic extract treated unspray and Ge Sp=garlic extract treated and spray.
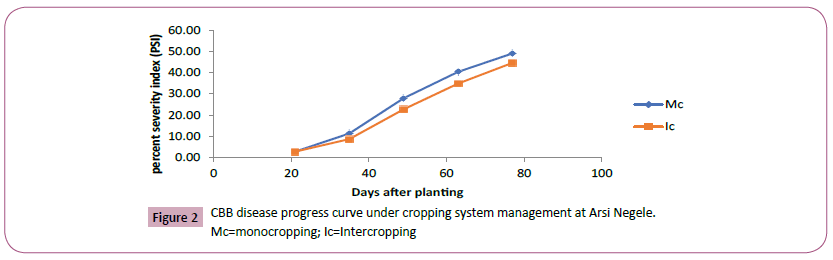
Figure 2: CBB disease progress curve under cropping system management at Arsi Negele.
Mc=monocropping; Ic=Intercropping
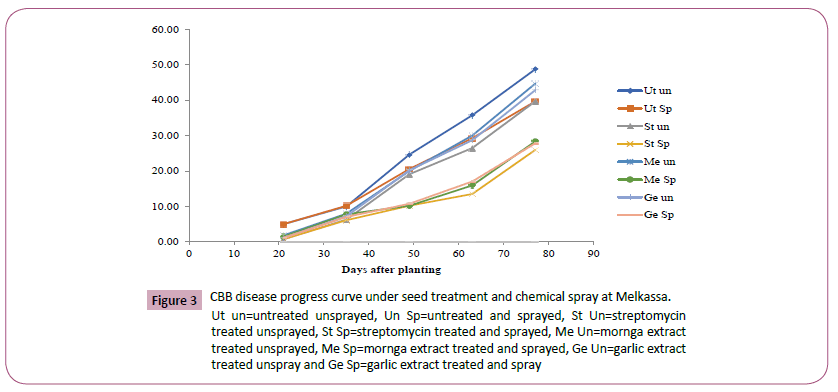
Figure 3: CBB disease progress curve under seed treatment and chemical spray at Melkassa.
Ut un=untreated unsprayed, Un Sp=untreated and sprayed, St Un=streptomycin treated unsprayed, St Sp=streptomycin treated and sprayed, Me Un=mornga extract treated unsprayed, Me Sp=mornga extract treated and sprayed, Ge Un=garlic extract treated unspray and Ge Sp=garlic extract treated and spray
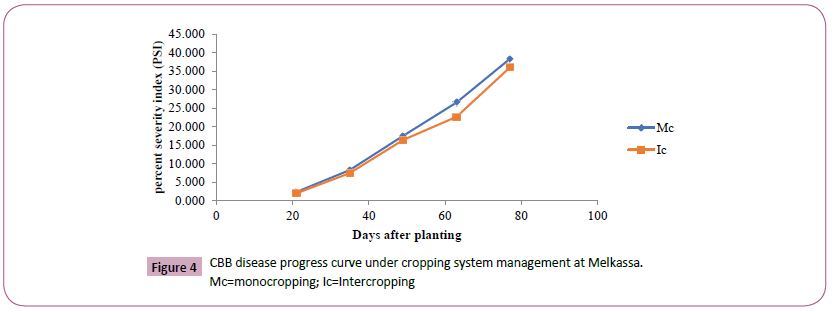
Figure 4: CBB disease progress curve under cropping system management at Melkassa.
Mc=monocropping; Ic=Intercropping
| Seed treatment | Spray | Arsi Negele | Melkassa | ||
|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||
| ut | un | 11.06a | 89.98a | 10.00a | 67.84a |
| ut | sp | 11.19a | 77.24c | 9.58a | 57.36b |
| st | un | 0.93d | 76.92c | 0.99c | 59.50b |
| st | sp | 0.92d | 54.17d | 1.01c | 42.07c |
| Me | un | 3.91b | 83.37b | 2.28b | 62.81ab |
| Me | sp | 2.62bc | 59.33d | 2.03bc | 45.06c |
| Ge | un | 3.68bc | 80.70bc | 1.31bc | 61.99ab |
| Ge | sp | 2.54c | 56.08d | 1.34bc | 43.92c |
| CV% | 24.83 | 6.91 | 27.57 | 11.24 | |
| LSD | 1.34 | 5.83 | 1.15 | 7.23 | |
Means with the same letter across the column are not significantly different. Ut=untreated, st=streptomycin, Me=mornga extract, Ge=garlic extract, un=unspray and Sp=spray
Table 1: Effect of seed treatment and chemical spray on incidence of CBB of bean at different days after planting at Arsi Negele and Melkassa.
| Cropping System | Arsi Negele | Melkassa | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| MC | 4.37a | 75.77a | 3.60a | 59.78a |
| IC | 4.84a | 68.68b | 3.53a | 50.35b |
| CV% | 23.86 | 4.35 | 27.54 | 5.51 |
| LSD (0.05) | ns | 1.85 | ns | 1.79 |
Means labeled with the same letter across the column are not significantly different Mc=monocropping, Ic=intercropping
Table 2: Effect of cropping system on incidence of CBB of bean at different days after planting at Arsi Negele and Melkassa.
| Seed treatment | Spray | Arsi Negele | Melkassa | ||
|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||
| ut | un | 6.11a | 62.41a | 5.00a | 48.70a |
| ut | sp | 6.49a | 52.41bc | 5.00a | 39.63c |
| st | un | 0.93cd | 49.45c | 0.74c | 39.63c |
| st | sp | 0.74d | 33.70d | 0.74c | 25.93d |
| Me | un | 1.85bc | 53.52b | 1.85b | 44.63b |
| Me | sp | 2.22b | 35.37d | 1.48bc | 28.33d |
| Ge | un | 2.41b | 52.41bc | 1.11bc | 42.79bc |
| Ge | sp | 1.85bc | 35.19d | 1.11bc | 27.79d |
| CV% | 32.11 | 7.29 | 34.71 | 7.7 | |
| LSD | 1.05 | 3.99 | 0.86 | 3.35 | |
Means labeled with the same letter across the column are not significantly different Ut=untreated, st=streptomycin, Me=mornga extract, Ge=garlic extract, un=unspray and Sp=spray.
Table 3: Effect of seed treatments and chemical spray on PSI of CBB of bean at different days after planting at Arsi Negele and Melkassa.
| Cropping System | Arsi Negele | Melkassa | ||
|---|---|---|---|---|
| 21DAP | 77DAP | 21DAP | 77DAP | |
| MC | 2.82a | 49.03a | 2.31a | 38.33a |
| IC | 2.82a | 44.58b | 1.94a | 36.02b |
| CV% | 31.02 | 5.45 | 32.75 | 7.18 |
| LSD (0.05) | ns | 1.5 | ns | 1.57 |
Means with the same letter across the column are not significantly different. Mc=mono cropping, Ic=intercropping
Table 4: Effect of cropping system on PSI of CBB of bean at different days after planting at Arsi Negele and Melkassa.
| Seed treatment | Spray | Arsi Negele | Melkassa |
|---|---|---|---|
| ut | un | 1957.41a | 1361.11a |
| ut | sp | 1734.45b | 1152.41b |
| st | un | 1334.59d | 1008.52c |
| st | sp | 930.74e | 604.08d |
| Me | un | 1525.74c | 1136.85b |
| Me | sp | 1033.15e | 683.15d |
| Ge | un | 1475.19cd | 1092.78bc |
| Ge | sp | 972.22e | 689.63d |
| CV% | 10.08 | 8.77 | |
| LSD | 161.35 | 99.03 | |
Means labeled with the same letter across the column are not significantly different, Ut=untreated, st=streptomycin, Me=mornga extract, Ge=garlic extract, un=unspray and Sp=spray
Table 5: Effect of seed treatment and chemical spray on AUDPC of CBB of bean at Arsi Negele and Melkassa.
| CS | SPR | Arsi Negele | Melkassa |
|---|---|---|---|
| Mc | un | 1686.50a | 1219.17a |
| Mc | sp | 1271.70bc | 813.43b |
| Ic | un | 1459.00ab | 1080.46a |
| Ic | sp | 1063.00c | 751.20b |
| CV% | 22.69 | 20.61 | |
| LSD | 256.16 | 164.05 | |
Means labeled with the same letter across the column are not significantly different. Mc=mono cropping, Ic=intercropping, un=unspray and Sp=spray
Table 6: Effect of cropping system and chemical spray on AUDPC of CBB of bean at Arsi Negele Melkassa.
| Seed Treatment | Arsi Negele | Melkassa |
|---|---|---|
| Ut | 0.0447c | 0.0466a |
| St | 0.0563a | 0.0472a |
| Me | 0.0506b | 0.0451a |
| Ge | 0.0533ab | 0.0464a |
| CV% | 9.56 | 9.94 |
| LSD (0.05) | 0.0041 | ns |
Means labeled with the same letter across the column are not significantly different.
Table 7: Effect of seed treatment on disease progress rate of CBB of bean at Arsi Negele and Melkassa.
| Cropping System | Arsi Negele | Melkassa |
|---|---|---|
| MC | 0.0493b | 0.0461a |
| IC | 0.0532a | 0.0466a |
| CV% | 9.56 | 9.94 |
| LSD (0.05) | 0.0029 | 0.0027 |
Means labelled with the same letter across the column are not significantly different. Mc=monocropping, Ic=intercropping.
Table 8: Effect of cropping system on disease progress rate of CBB of bean at Arsi Negele and Melkassa.
| Spray | Arsi Negele | Melkassa |
|---|---|---|
| Un | 0.0580a | 0.0533a |
| Spr | 0.0445b | 0.0339b |
| CV% | 9.56 | 9.94 |
| LSD (0.05) | 0.0029 | 0.0027 |
Means labelled with the same letter across the column are not significantly different. Un=unspray, Sp=spray
Table 9: Effect of chemical spray on disease progress rate of CBB of bean at Arsi Negele and Melkassa.
| ST | SPR | Arsi Negele | Melkassa | |||||
|---|---|---|---|---|---|---|---|---|
| PPPlt | SPP | SDP | Yield | RYLP | PPPlt | SPP | ||
| ut | un | 12.97f | 3.50e | 18.50a | 1.81g | 36.27a | 10.88e | 2.60e |
| ut | sp | 15.92d | 4.33cd | 9.00c | 2.30de | 18.84cd | 12.40d | 3.47d |
| st | un | 16.38c | 4.42bc | 10.83b | 2.35d | 17.00d | 15.60bc | 3.82bc |
| st | sp | 18.08a | 4.70d | 5.50e | 2.76a | 2.57g | 16.08a | 4.00a |
| Me | un | 15.45e | 4.27d | 10.83b | 2.12f | 25.31b | 15.47c | 3.48d |
| Me | sp | 16.97b | 4.52b | 7.50d | 2.50c | 11.82e | 15.67abc | 3.70c |
| Ge | un | 15.77de | 4.40bc | 9.83bc | 2.26e | 20.14c | 15.57bc | 3.67c |
| Ge | sp | 17.38b | 4.68a | 7.17d | 2.62b | 7.42f | 16.02ab | 3.88ab |
| CV% | 2.44 | 2.5 | 12.19 | 2.61 | 12.52 | 2.63 | 4.26 | |
| LSD | 0.640 | 0.127 | 1.410 | 0.071 | 2.550 | 0.452 | 0.178 | |
Means labeled with the same letter across the column are not significantly different. Ut=untreated, st=streptomycin, Me=mornga extract, Ge=garlic extract, un=unspray and Sp=spray
Table 10: Effect of CBB on yield and yield components of bean under seed treatments and chemical spray at Arsi Negele and Melkassa.
| Cropping System | Arsi Negele | Melkassa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPPlt | SPP | HsWt | SDP | Yield | RYLP | PPPlt | SPP | HsWt | SDP | Yield | RYLP | |
| MC | 15.87b | 4.35a | 15.92b | 10.17a | 2.30b | 18.65a | 14.50b | 3.48b | 16.25a | 8.54a | 1.69b | 22.98a |
| IC | 16.36a | 4.360a | 16.33a | 9.63a | 2.37a | 16.19b | 14.92a | 3.68a | 16.54a | 8.21a | 1.81a | 17.63b |
| CV% | 1.78 | 2.27 | 4.22 | 9.90 | 1.95 | 9.40 | 2.14 | 2.90 | 4.46 | 10.18 | 1.81 | 6.88 |
| LSD (0.05) | 0.169 | ns | 0.401 | ns | 0.027 | 0.966 | 0.186 | 0.061 | ns | ns | 0.019 | 0.820 |
Means labeled with the same letter across the column are not significantly different. Mc=monocropping, Ic=intercropping
Table 11: Effect of CBB on bean yield and yield components under cropping system management at Arsi Negele and Melkassa.
| Seed Treatment | Arsi Negele | Melkassa | |||
|---|---|---|---|---|---|
| HsWt | HsWt | SDP | Yield | RYLP | |
| UT | 14.92d | 15.17c | 10.50a | 1.31d | 40.31a |
| ST | 17.25a | 17.25a | 7.17c | 2.01a | 8.51d |
| ME | 15.75c | 16.42b | 8.00b | 1.75c | 20.19b |
| GE | 16.58b | 16.75ab | 7.83bc | 1.93b | 12.21c |
| CV% | 4.22 | 4.46 | 10.18 | 1.81 | 6.88 |
| LSD (0.05) | 0.570 | 0.610 | 0.710 | 0.026 | 1.160 |
Means labeled with the same letter across the column are not significantly different.
Table 12: Effect of CBB on bean yield and yield components under seed treatment at Arsi Negele and Melkassa.
| Chemical Spray | Arsi Negele | Melkassa | |||
|---|---|---|---|---|---|
| HsWt | HsWt | SDP | Yield | RYLP | |
| Un | 15.67b | 16.00b | 10.83a | 1.62b | 26.03a |
| Sp | 16.58a | 16.79a | 5.92b | 1.87a | 14.18b |
| CV% | 4.22 | 4.46 | 10.18 | 1.81 | 6.88 |
| LSD (0.05) | 0.401 | 0.430 | 0.500 | 0.019 | 0.820 |
Means labeled with the same letter across the column are not significantly different.
Table 13: Effect of CBB on yield and yield components of bean under chemical spray management at Arsi Negele and Melkassa.
Conclusions and Recommendations
In this study that aim to evaluate integrated effect of seed treatment, intercropping and copper hydroxide spray treatments in reducing CBB epidemics and their contribution to yield and yield components. The result of disease, yield and yield component data revels that all treatment main factors and integration of seed treatment with bacticide spray significantly reduce the level of disease epidemic and amount of yield loss attributed to CBB. Intercropping common bean with maize has significantly reduced CBB development and increase yield and yield component compared with sole planting but there was no interaction effect with the other management options. In the intercropping, common bacterial blight disease epidemics were reduced because the maize may be used as physical barrier against bacterial inoculum from reaching to common bean. Seed treatment with streptomycin and the plant extracts were also reduce CBB development and increase bean yield and yield component over untreated control at both locations. Foliar spray of bacticide applications significantly reduce disease incidence, severity, AUDPC and disease progress and improve yield and yield components over unsprayed plots both at Arsi Negele and Melkassa. However, seed treatment combined with bacticide foliar sprays had pronounced effect in reducing CBB epidemics and improving yield and yield components and avoiding yield losses. Therefore use of treated seeds with streptomycin, garlic extract and moringa leaf extract combined with bacticide foliar spray is the best CBB management option for bean producers. Moreover, considering the potential of garlic and moringa extract investigated hear and the risk of development of resistance against chemical pesticide and its deleterious effects on life supporting system investigation of alternative plant extracts for management of CBB should be continue. Analysis and identification of the chemical constitute of the crude plant extracts and formulation and industrialization of the active ingredient also need due research attention to bring effective and environmentally safe disease management strategy.
References
- Ali K, Kenneni G, Ahmed S, Malhotra R, Beniwal S (2006) Food and Forage Legumes of Ethiopia: Progress and Prospects.
- CSA (2012) Report on area and crop production forecast for major crops for 2012/13 Meher season.
- Gurmu F (2007) Participatory Varietal Selection of Haricot Beans (Phaseolus vulgaris L.) Varieties in Umbullo Wacho and Beresa Watersheds in The Southern Region. Operational Research and Capacity Building for Food Security and Sustainable Livelihoods: Proceedings of Irish Aid Supported Operational Research Project Review Workshop.
- Fininsa C (2001) Epidemiology of Bean Bacterial Blight and Maize Rust in Intercropping. Doctoral Thesis, Swedish University of Agricultural Science, Uppsala.
- Abiy T, Fekede A, Chemeda F (2006) Lowland pulse diseases research in Ethiopia. Proceedings of Food and Forage Legumes of Ethiopia: Progress and Prospects, pp: 228-237.
- Ali K, Gemechu A, Seid K (2003) The workshop on Food and Forage Legume. International Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria.
- Yu ZH, Stall RE, Vallejos CE (1998) Detection of genes for resistance to Common Bacterial Blight of Beans. Crop Sci 38: 1290-1296.
- Schwartz HF, Steadman JR, Hall R (2005) Compendium of bean diseases. 2nd edn. St Paul Minn. APS Press, American Phytopathological Society.
- McMullen MP, Lamey HA (2000) Seed treatment for disease control. Disease management guide. Northern Dakota State University, p: 447.
- Tumsa K (2007) Integrated management of common bacterial blight of common bean through host resistance and chemical applications in the central rift valley, Ethiopia. MSc Thesis, Haramaya University, Haramaya, Ethiopia.
- Fininsa C (2003) Relationship between common bacterial blight severity and common bean yield loss in pure stand and maize common bean intercropping. International Journal of Pest Management 49: 177-185.
- Fininsa C, Yuen J (2002) Temporal progression of bean common bacterial blight (Xanthomonas campestris pv. phaseoli) in sole and intercropping systems. European Journal of Plant Pathology 108: 485-495.
- Fikre L (2004) Effect of intercropping and cultivar mixtures on common bean diseases and yield. Pest Management Journal of Ethiopia 8: 71-81.
- Mutlu N, Miklas P, Reiser J, Coyne D (2005) Backcross breeding for improving resistance to common bacterial blight in pinto beans (Paseolus vulgaris L.). Plant Breeding 124: 282-287.
- CIAT (Centro International de Agricultura Tropical) (1999) Annual report. International center for Tropical Agriculture CIAT California, Colombia, p: 54.
- Hagedorn DJ, Inglis DA (1986) Handbook of Bean Diseases. Madison, Wisconsin.
- Dursun A, Figen MD, Ahin F (2002) Identification of resistance to common bacterial blight disease on common bean genotypes grown in Turkey. Journal of American Society of Horticultural Sciences 108: 1573-8469.
- Popovic T (2008) Detection of phytopathogenic bacteria on bean seed and cultivar susceptibility. University of Novi Sad, Serbia.
- Garahushoma C (2002) Effectiveness of Different Botanical Extracts in Controlling Bacterial and Fungal Seed-Borne Pathogens in Farm-Retained Bean Seed. BSc Thesis, University of Zimbabwe.
- Raghavendra MP, Satish S, Raveesha KA (2006) In vitro evaluation of anti-bacterial spectrum and phytochemical analysis of Acacia nilotica. Journal of Agricultural Technology 2: 77-88.
- MARC (Melkassa Agricultural Research Center) (1997) Melkassa Agricultural Research Center Profile, Melkassa.
- CIAT (Centro International de Agricultura Tropical) (1998) Annual report. International Centre for Tropical Agriculture, CIAT, California, Colombia, p: 39.
- Finney R, Shanner G (1977) The effect of nitrogen fertilization on the expression of slow mildewing resistance in Knox wheat. Phytopathology 67: 1051-1056
- Kiryakov I, Genchev D (2000) Resistance of bean cultivars and lines to X. campestris pv. phaseoli (Smith) Dye. Bulgarian Journal of Agricultural Science 6: 525-528.
- Miklas PN, Coyne DP, Grafton KF, Mutlu N, Reiser J, et al. (2003) A major QTL for common bacterial blight resistance derives from the common bean great northern landrace cultivar Montana No. 5. Euphytica 131: 137-146.
- Asensio MC, Asensio C, Singh SP (2006) Gamete selection for resistance to common and halo bacterial blights in dry bean intergene pool populations. Crop Science 46: 131-135.
- Cafati CR, Saettler AW (1980) Effect of host on multiplication and distribution of bean common blight bacteria. Phytopathology 70: 675-679.
- Osdaghi E, Shams M, Alizadeh A, Lak MR (2010) Study on common bean seed lots for contamination with Xanthomonas axonopodis pv. phaseoli by BIO-PCR technique. Journal of Agricultural Technology 6: 503-513.
- Webster DM, Temple SR, Schwart HF (1983) Bacterial blights of snap beans and their control. Plant Disease 67: 935-940.
- Selamawit C (2004) Occurrence of common bacterial blight strains and its effect on quality of common bean seeds in Ethiopia. MSc Thesis, School of Graduate Studies, Haramaya University, Ethiopia, pp: 56-60.
- Schwartz HF, Galvez GE (1980) Common bean production problems: disease, insect, soil and climatic constraints of Phaseolus vulgaris. Centro Internacional de Agricultura Tropical (CIAT), Columbia, p: 424.
- Gilbertson RL, Maxwell DP (1992) Common bacterial blight of bean. In: Chaube HS, Kumar J (eds.), Plant diseases of international importance. Diseases of Vegetables and Oil Seed Crop 2: 18-39.
- Schwartz HF (2004) Bacterial diseases of beans. Disease fact sheet. Colorado State University 2: 913.
- Balaz J, Popovi T, Vasi M, Nikoli Z (2008) Razrada metoda za dokazivanje Xanthomonas axonopodis pv. phaseoli na semenu pasulja. Pesticidi i Fitomedicina 23: 89-98.
- Sintayehu F, Amare A (2016) Integrated management of common bacterial blight (Xanthomonas campestris pv. phaseoli) and its effect on seed yield of common bean (Phaseolus vulgaris L.). International Journal of Life Sciences 4: 336-348.
- Balachew K, Gebremariam M, Alemu K (2015) Integrated Management of Common Bacterial Blight (Xanthomonas axonopodis pv. phaseoli) of Common Bean (Phaseolus vulgaris) in Kaffa, Southwest Ethiopia. Malaysian Journal of Medical and Biological Research 2: 147-152.
- Kassahun A (2008) Reaction of common bean cultivars to Xanthomonas axonopodis pv. phaseoli strains and integrated management of common bacterial blight in Eastern Amhara Region, Ethiopia. MSc Thesis, Haramaya University, Haramaya, Ethiopia.
- Schaad NW, Cheong S, Tamaki S, Hatziloukas E, Panopoulos NJ (1995) A combined biological and enzymatic amplification (bpcr) technique to detect Pseudomonas syringae pv phaseolicola in bean seed extracts. Phytopathol 85: 243-248.
- Goss M, Mafongoya P, Gubba A, Tesfay S (2017) Black Rot (Xanthomonas campestris pv. campestris) Control in Field Grown Cabbage (Brassica oleracea var. Sugar Loaf) with Moringa oleifera Extracts. International Journal of Plant & Soil Science 18: 1-11.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences