ISSN : 2393-8854
Global Journal of Research and Review
Evaluation of Free Radical Scavenging Activities of Aqueous Extracts of Fruits of Ziziphus mauritiana and Eriobotrya japonica Through in vitro Antioxidant Assays
Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar-143005, Punjab, India
Abstract
Objectives: The present study was planned to evaluate the antioxidant activities of two Indian fruits, Ber (Ziziphus mauritiana) and Loquat (Eriobotrya japonica) using standard in vitro antioxidant methods and determination of total phenolic and flavonoid contents in both the fruits. Methods: Antioxidant activities were evaluated using DPPH and chelating power assays. Total phenolic and flavonoid contents were also determined spectrophotometrically. Results: Ziziphus mauritiana extract showed higher DPPH radical scavenging activity than Eriobotrya japonica. Both fruits contain significant amount of total phenolic and flavonoid contents. Conclusion: It was observed that aqueous extracts of Ziziphus mauritiana and Eriobotrya japonica showed dose dependent response in DPPH assay, and no antioxidant activity was observed in chelating power assay.
Keywords
Ziziphus mauritiana, Eriobotrya japonica, Antioxidants, DPPH assay, Chelating power assay, Phenols.
Introduction
Human beings are subjected to oxidative stress from various pollutants, radiations, intake of oxidized foods and in vivo production of free radicals. Uncontrolled oxidation in biological systems can damage the vital cellular membranes, proteins and nucleic acids leading to various degenerative diseases. Many studies indicate that fruits are important sources of vital compounds and act as sources of antioxidants and can protect the human beings from oxidative stress related diseases [1-3]. It has been found that regular intake of fresh fruits and vegetables lowers the risk of diseases associated with oxidative stress by providing bioactive compounds like ascorbic acid, flavonoids, proteins, carbohydrates, vitamin C and polyphenols such as anthocyanins, phenolic acids, and tannins etc. [4-6]. These fruits are also referred as natural functional foods [7].
Currently, limited information is available about the antioxidant activities of major two fruits of India i.e. Ziziphus mauritiana and Eriobotrya japonica. The plant Ziziphus mauritiana belongs to family Rhamnaceae, while Eriobotrya japonica belongs to family Rosaceae. Both fruits are very nutritious and are eaten raw or cooked. Therefore, this study was planned to know the antioxidants properties of these fruits using aqueous extract and employing DPPH assay and chelating power assay.
Materials and Methods
Plant materials
Fresh fruits of Ziziphus mauritiana and Eriobotrya japonica were purchased from the local market of Amritsar, Punjab, India. Fruits were washed with tap water.

(Ziziphus mauritiana) (Eriobotrya japonica)
Preparation of extract
One gram fruit (without seed) was ground in pestle and mortar using liquid nitrogen. The powder was homogenized in 5ml distilled water and centrifuged at 4°C at 10,000 rpm for 15 min and the supernatant (aqueous extract) was used for further analysis.
Total phenolic content (TPC)
Total phenolic content of aqueous extract was determined by using method given by Singleton and Rossi [8]. Fruit extract (100μl) was diluted with water (upto 3 ml) and FC reagent (0.5 ml) was added. 2 ml sodium carbonate (20%) was added after three minutes and the absorbance of solution was measured at 650 nm using UV-Visible spectrophotometer. The results were measured in terms of mg gallic acid/100g fresh weight material.
Total flavonoid content (TFC)
Total flavonoid content of aqueous extract of fruits was determined as per method suggested by Kim [9]. 1ml sample was diluted with 4ml distilled water and mixed with 300μl NaNO2 (5%). 300μl AlCl3 (10%) was added to the solution and kept undisturbed for 5 minutes. After addition of 2ml NaOH (1M) solution was diluted with distilled water to prepare10 ml of total solution and absorbance of solution was taken at 510 nm. The results were measured in mg rutin/100g fresh weight material.
Antioxidant activities
DPPH free radical scavenging assay
The antioxidant activities of plant extracts on DPPH radical was estimated according to Blois [10]. The reaction mixture contains 200 μl different extract concentrations (25-200 mg/ml) and 2 ml of DPPH (0.1 mM in methanolic solution). The absorbance of the solution was recorded at 517 nm. The radical scavenging activity was expressed as inhibition percentage (% inhibition) and calculated as follows:
% DPPH radical scavenging = (1- AS/AC)*100.
AC = Absorbance of control, AS = Absorbance of sample solution.
Chelating power assay
The metal chelating activity of extracts was measured by the method given by Dinis [11] with few modifications. 1 ml plant extract (25-200mg/ml) was mixed with 3.5ml methanol, 0.2 ml ferrous chloride (2 mM) and 0.2 ml ferrozine (1 mM) and the solution was kept for 10 min at room temperature. The absorbance of the solution was determined at 562 nm. The decrease in absorbance of solution was taken as increase in chelating power.
Results
Total phenolic content
Amount of total phenolic content in Z. mauritiana and E. japonica was calculated from the calibration curve of gallic acid prepared by taking different concentrations of gallic acid. The equation for gallic acid is given below:
y = 0.0025x – 0.0036 (R2 = 0.9941). Where y is absorbance and x is concentration of gallic acid. From the results, it was observed that aqueous extract of Z. mauritiana and E. japonica showed significant amount of phenolic content in aqueous extracts i.e. 105.36 and 8.16 mg gallic acid/100 g fresh weight respectively.
Total flavonoid content
Total flavonoid content was determined as mg rutin/100 g fresh weight of material from the calibration curve of rutin. The curve between absorbance vs. concentration of rutin can be described by the following equation.
y = 0.0013x + 0.0004 (R2 = 0.9902). Where, y is absorbance and x is concentration of rutin. Total flavonoid content of Z. mauriana was found to be 116.31 mg and of E. japonica it was found to be 20.92 mg rutin/100 g fresh weight. From the results it was observed that aqueous extract of Z. mauriana and E.japonica showed significant amount of phenolic and flavonoid content.
DPPH assay
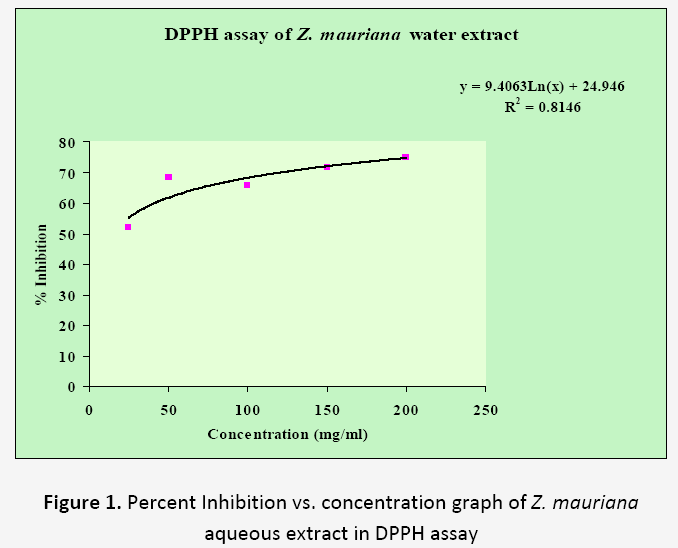
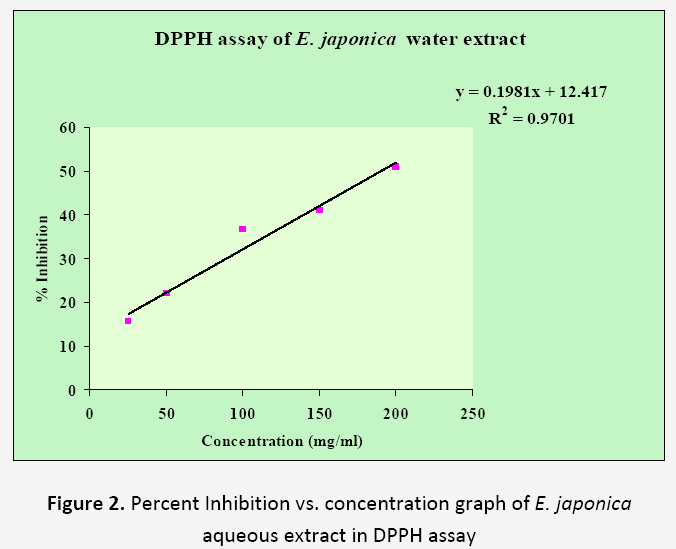
In DPPH assay, the hydrogen donating ability of the fruit extracts was determined by converting the DPPH radical to non radical by reduction process. The DPPH radical scavenging activity of aqueous extracts of fruits is shown in Table 1. Significant DPPH radical scavenging activity was found at all the tested concentrations in both plants extracts. The dose response relationship was found upto 200 mg/ml concentration. The Z. mauritiana showed 74.89 % activity and E. japonica showed 51.07 % activity in this assay. The R2 values for Z. mauritiana and E. japonica were found to be 0.8146 and 0.9701 respectively (Figure 1 and 2). From the results, it is clear that both fruit extracts showed significant amount of hydrogen donating activity. Z. mauritiana showed more activity in DPPH assay than E. japonica.
Table 1. Percent Inhibition vs. concentration values of Z. mauritiana and E. japonica fruits in DPPH assays
| S. No. | Concentration (mg/ml) | % Inhibition (Mean ± S.D.) | |
|---|---|---|---|
| Z. mauritiana | E. japonica | ||
| 1. | 25 | 51.90±0.08 | 15.60 ±0.07 |
| 2. | 50 | 68.04±0.00 | 21.86±0.07 |
| 3. | 100 | 65.61±0.20 | 36.61±0.00 |
| 4. | 150 | 71.57±0.20 | 40.97±0.00 |
| 5. | 200 | 74.89±0.15 | 51.07±0.19 |
Chelating power assay
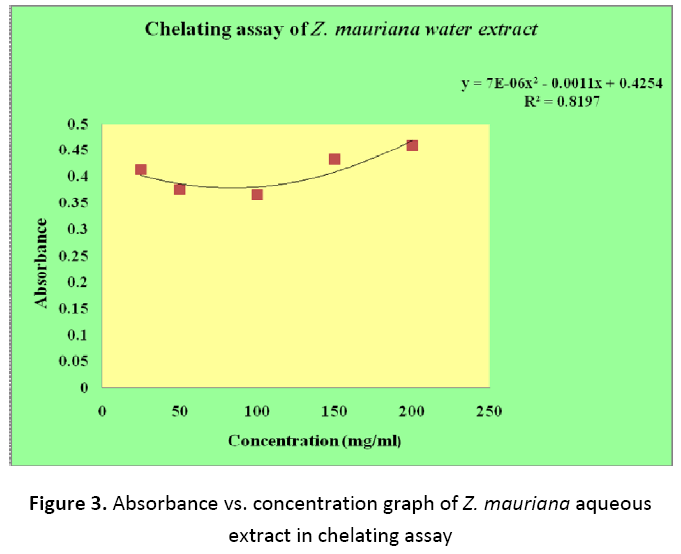
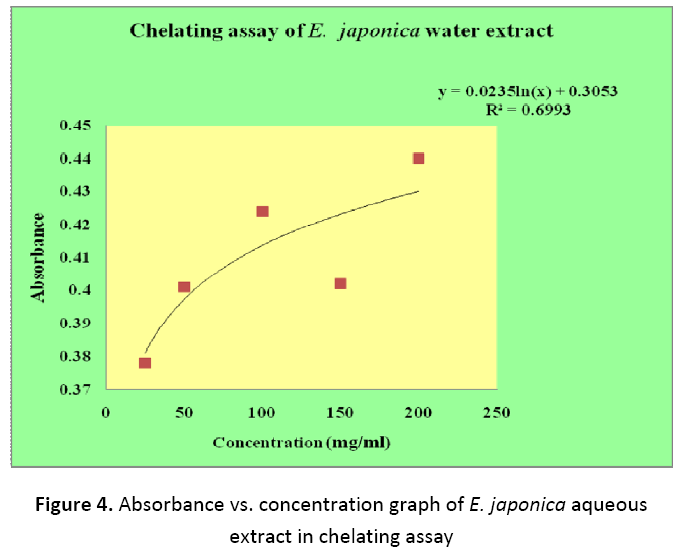
In chelating power assay, the ability of fruit extracts to chelate metal ion especially Fe2+ ion was observed. Antioxidants prevent the oxidative stress by chelating metal ions that are involved in free radical generation in biological systems. In this assay, Ferrozine was used as it form complex with Fe2+ and in the presence of other complexing agents in extract its colour intensity is reduced which is measured spectrophotometrically. As illustrated in Table 2 it was found that Z. mauritiana and E. japonica showed no significant chelating ability. With increase in concentration of extract the absorbance increased in both the plant extracts, which indicates absence of chelating ability of extracts (Figure 3 and 4).
Table 2. Absorbance vs. concentration values of Z. mauritiana and E. japonica fruits in chelating assays
| S. No. | Concentration (mg/ml) | Absorbance at 562 nm | |
|---|---|---|---|
| Z. mauritiana | E. japonica | ||
| 1. | 25 | 0.412 | 0.378 |
| 2. | 50 | 0.375 | 0.401 |
| 3. | 100 | 0.365 | 0.424 |
| 4. | 150 | 0.431 | 0.402 |
| 5. | 200 | 0.459 | 0.440 |
Discussion
Many fruits are known for their medicinal properties and daily consumption of fruits in our diet can protect us from oxidative stress disorders. Fruits provide us antioxidants in the form of phenolic compounds, ascorbic acid, carotenoids, tocopherols etc. [12-14]. It is well known that phenolic compounds including flavonoids of plant origin are mostly responsible for radical scavenging [15-18]. From the results, it is clear that Z. mauritiana and E. japonica are rich in phenolic and flavonoid content. The activity of both fruit aqueous extracts in DPPH assay may be due to the presence of total phenols and flavonoids. Phenols neutralize the free radicals by donating hydrogen atoms [19]. As shown in the results, in DPPH assay the maximum percent inhibition in Z. mauritiana was found to be 74.89%, which may be due to the presence of more phenolic content i.e. 105.36 mg gallic acid/100 g fresh weight as compared to E. japonica which showed 51.07 % inhibition activity and having less phenolic content 8.16 mg gallic acid/100 g fresh weight. Transition metals have been proposed as the catalysts for the initial formation of radicals and chelating compounds present in the plant extracts chelate the transition metals in the living systems and inhibit the generation of free radicals and protect the vital biomolecules form the free radical mediated oxidative stress. In the present study, both fruit extracts did not showed any chelating activity.
Conclusion
From the results, it is concluded that Z. mauritiana and E. japonica are rich in phenols and flavonoids and their antioxidant activities may be due to their hydrogen donating abilities instead of metal chelation.
References
- Esterbauer H, Gebicki J, Puhl H, Gunther J. (1992). The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radical Biology and Medicine. 3, 341-390.
- Kinsella JE, Frankel E, German B, Kanner JU. (1993). Possible mechanisms for the protective role of antioxidants in wine and plant foods. Food Technology. 47, 85-89.
- Sun C L, Yuan JM, Lee M J, Yang CS, Gao YT, Ross RK, Yu M C. (2002). Urinary tea polyphenols in relation to gastric and esophageal cancers: a prospective study of men in Shanghai, China. Carcinogenesis. 23, 1497-1503.
- Kris-etherton P, Hecker K, Bonanome A, Coval S, Binkoski A, Hilpreet K. (2002). Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. American Journal of Medicine. 113, 71-78.
- Nichenametla SN, Taruscio TG, Barney DL, Exon JH. (2006). A review of the effects and mechanism of polyphenolics in cancer. Critical Reviews in Food Science and Nutrition. 46, 161-183.
- Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. (2009). Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pakistan Journal of Pharmaceutical Sciences. 22, 277-281.
- Kondakova V, Tsvetkov I, Batchvarova R, Badjakov I, Dshambazova T, Slavov S. (2009). Phenol compounds-qualitative index in small fruits. Biotechnology and Biotechnological Equipment. 23, 1444-1448.
- Singleton VL, Rossi JA. (1997). Calorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16, 144- 158.
- Kim JH, Kim SJ, Park HR, Chang Nam K, Lee SC. (2009). The different antioxidant and anticancer activities depending on the color of mushroom. Journal of Medicinal Plant. Research. 3, 1016-1020.4
- Blois M S. (1958). Antioxidant determinations by the use of a stable free radical. Nature. 26, 1199-1200.
- Dinis TCP, Madeira VMC, Almeida LM. (1994). Action of phenolic derivatives (acetoaminophen, salicylate, and 5- aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavaengers. Archives of Biochemistry and. Biophysics. 315, 161-169.
- Buening MK, Chang RL, Huang MT, Fortner JG, Wood AW, Conney AH. (1981). Activation and inhibition of benzo (α) pyrene metabolism in human liver microsomes by naturally occurring flavonoids. Cancer Research. 41, 67-72.
- Nagabhushan M, Bhide SV. (1987). Antimutagenicity and anticarcinogenicity of turmeric (Curcuma longa). NutritionGrowth Cancer. 4, 83-90.
- Amonkar AJ, Padma PR, Bhide SV. (1989). Protective effect of hydroxychavicol, a phenolic component of betel leaf, against the tobacco-specific carcinogens. Mutation Research. 210, 249-253.
- Hee Kee K, Kyeong H K, Moon YH, Hyun PK. (1991). Effect of antimutagenic flavonoid, galangin, on benzo (α) pyrene metabolism in mice. Korea Biochemistry Journal. 24,141-147.
- Halliwell B, Guttridge JMC, Cross CE. (1992). Free radicals, antioxidants and human diseases: Where are we now? Journal of Laboratory and Clinical Medicine. 119, 598-620.
- Huang MT, Deschner EE, Newmark HL, Wang ZY, Ferraro TA, Conney AH. (1992). Effect of dietary curcumin and ascorbyl palmitate onaxoxymethanol-induced colonic epithelial cell proliferation and focal areas of dysplasia. Cancer Letters. 64, 117- 121.
- Sukumaran K, Kuttan R. (1995). Inhibition of tobacco-induced mutagenesis by eugenol and plant extracts. Mutation Research. 343, 25-31.
- Pietta P, Simonetti P, Mauri P. (1998). Antioxidant activity of selected medicinal plants. Journal of Agriculture and Food Chemistry. 46, 4487-4490.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences