ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Ethnobotanical Notes and Phytopharmacologiques on Solanum nigrum Linn. (Family: Solanaceae)
Albouchi F1,2, Attia M1, Hanana M3 and Hamrouni L2*
1Faculty of Sciences, University of Carthage, Bizerte, Tunisia
2Laboratory of Management and Valorization of Forest Resources, National Institute of Research in Rural Engineering Waters and Forests, University of Carthage, Tunisia
3Laboratory of Molecular Physiology of Plants, Biotechnology Center of Borj, Hammam, Tunisia
- *Corresponding Author:
- Hamrouni L
Laboratory of Management and Valorization
of Forest Resources, National Institute of
Research in Rural Engineering Waters and
Forests, University of Carthage, Tunisia
Tel: +21621269226
E-mail: hamrounilam@yahoo.fr
Received date: December 14, 2017; Accepted date: February 15, 2018; Published date: February 19, 2018
Citation: Albouchi F, Attia M, Hanana M, Hamrouni L (2018) Ethnobotanical Notes and Phytopharmacologiques on Solanum nigrum Linn. (Family: Solanaceae). Am J Phytomed Clin Ther Vol.6 No.1:5 doi: 10.21767/2321-2748.100341
Abstract
Solanum nigrum Linn. (Family: Solanaceae) is commonly known as tit begun, phuti begun, gurki, gurkamai and kakmachi, black nightshade, duscle, Garden Nightshade, hound’s berry, petty Morel, wonder Berry, small-fruited black nightshade. It is a species of the Solanum genus, native to Eurasia and introduced in the Americas, Australia, Asia and South Africa. Parts of this plant can be highly toxic to livestock and humans, and it is considered a weed. Nonetheless, ripe berries and cooked leaves are used as food in some locales; and plant parts are used as a traditional medicine. This paper reviews the literature on the morphology, distribution, ecology, habitat requirements, physiology and impacts, biological and pharmacological activity.
Keywords
Solanum nigrum Linn; Taxonomy; Physiology; Biology
Introduction
The genus Solanum is the biggest and the most complex of the family Solanaceae, it is composed of more than 1500 species, many of which are also economically important throughout their cosmopolitan distribution. This family includes fruits and vegetables such like potato (Solanum tuberosum L.), tomato, peppers and ornamental plants such as petunia and other medical plants like Atropa belladonna L. (deadly nightshade), Datura stramonium L. (Jimson weed), and Hyoscyamus niger L. (black henbane), eggplant (S. melongena L.) and naranjilla (quitoense S. Lam.). Too the species cultivated for their drug use include bittersweet like (S. dulcamara L.) and S. viarum Dun both used as sources of corticosteroids [1,2]. Although the species are distributed throughout the world, they often occur in warm temperate and tropical regions with centers of diversity occurring in the southern hemisphere, especially in South America. Other centers of speciation occur in Australia and Africa, with relatively few and less diverse species are found in Europe and Asia [3]. S. nigrum species are amongst the most common and popular leafy vegetables in the warm humid zones of Africa, and arguably the most important group of traditional leafy vegetables on the African continent after Amaranthus [4].
Taxonomy and morphology
Taxonomy: The taxonomic complexity of species associated with the section Solanum has long been accepted [5-10]. Various classical, experimental and numerical studies have demonstrated that the complexity is attributable to a number of causes [11-13]. Among these causes there are phenotypic plasticity [14,15], genetic variation, Polyploidy, natural hybridization, discordant variation [16]. S. nigrum (solanaceae) is one of 1500 species withen the genus Solanum. Different from S. nigrum; S. americanum Miller; S. chenopodioides Lam.; S. physalifolium Rusby var. nitidibaccatum (Bitt.); S. retroflexum Dunal in; S. sarrachoides Sendtn; S. scabrum Miller [17]. Synonyms of S. nigrum (black nightshade, European Black Nightshade or locally just "black nightshade", Duscle, Garden Nightshade, Hound's Berry, Petty Morel, Wonder Berry, Small-fruited black nightshade or popolo). The name of the genus is thought to derive from the solamen latin, and refer to the appeasement or sedative effects associated with many species of Solanum.
Taxonomic position: Kingdom: plantae, Order: solanales, Family: Solanaceae, Genus species: solanum nigrum.
Morphology
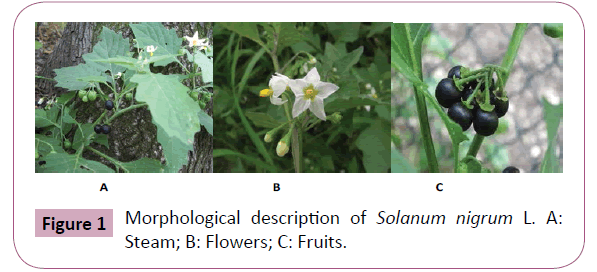
S. nigrum L commonly as Black nightshade is a dicot weed in the Solanaceae family. It is an annual herbaceous plant of 30-100 cm heigh, pubescent with simple hairs. Stems are often angular, pubescent-pubescent. The opposite leaves, with whole limb, oval and diamond shape are slightly cogged, the bases are cuneate, 4-10 and 3-7 cm wide, pubescent, entire or coarsely dentate, the apex is obtuse. Inflorescences are extra-axillary umbels, the calyx cup-shaped, the corolla is white, 8-10 mm long, the lobes ovateoblong, pubescent abaxially, ciliate spreading. Filaments are 1-1.5 mm long, anthers oblong, 2.5-3.5 mm. Fruiting 2 pedicels are strongly deflexed. The fruiting calyx is applied to the berry. The flowers are sometimes white veined with purple, with a flat corolla; they are grouped by 3 or 5 along the stem. The fruits are dull black, globose, 8-10 mm in diameter. The fruits are spherical berry 8-10 mm in diameter, green and greenish yellow to black laying maturity (Figure 1). The fruits are toxic, so they wall are not richer toxin when they are degraded to maturity. Their alkaloid content varies depending on the location of the plant where it grows and depending on the season [18].
Distribution and habitat
The black nightshade is widely distributed in various habitats throughout the world, from tropical to temperate regions and from sea level to altitudes exceeding 3500 m [19]. It is a rather common species in wet woods, near river, wasteland, old field, ditches roadside and cultivated land. Their wide tolerance of habitat types, their ability to flower while still young and their prolific seed production all contribute to the success of these species as widespread weeds [15]. They are generally found in disturbed habitats, such as roadsides, often on arable land especially the edges of cultivated fields and plantations, in hedgerows, on railway cuttings, quaysides and rubbish tips, in areas around buildings and houses, under trees, on forest and grassland margins, as garden weeds, on shingle beaches, riverbanks and in gullies. The species mainly colonize moist environments, only occurring in areas of low rainfall when the land is subject to irrigation. This plant grows well in fertile soils, especially those rich in nitrogen or phosphorus [20].
Reproduction
Reproduction is principally by seed, but the use of shoot cuttings as propagules, especially during the rainy season, has also been reported, though plants propagated in this way yield less than those propagated by seed [21]. Plantlets have also been regenerated from 'S. nigrum' mesophyll chloroplasts [22]. The species belonging to the Solanum genus are predominantly self-pollinating or autogamous though natural out- and cross breeding can and does occur [23]. Salisbury reported that the average production of each plant of S. nigrum is about 240 berries in Britain, the old plants can produce more than 3,600 seeds where each berry contains about 40 seeds. With over 90% of these seeds germinate during the following season [23]. Therefore, the potential for harm of this species as a weed is clearly seen. Similar data are recorded by other authors solanum nigrum can be achieved a 178000 seeds/plant [20].
Ecology
Water stress: S. nigrum species are intolerant to water stress. An annual rainfall of 500-1200 mm is needed for optimum growth [21]. They grow on a wide range of soil types, but thrive best on fertile soils, especially those high in nitrogen and phosphorous [24].
Contaminated soils: S. nigrum L is considered to be a potential plant for restoring Cd contaminated soils. In a post-culture experiment, it would accumulate Cd up to 103.8 and 124.6 mg/kg DW in stem and leaves respectively, without phototoxic symptoms and reduction growth [25]. However S. nigrum has a potential to purify Cd contaminated soils [26]. Through to the high tolerance of S. nigrum to cadmium, the diversity of endophytic bacteria associated with Cd hyperaccumulator plant S. nigrum L. grown in mine tailings has been determined [27] however, among these endophytic bacterial we find: Proteobacteria (61.93%; 19 OTUs), other clones belonged to Bacteroidetes (12.18%; 5 OTUs), Firmicutes (2.03%; 2 OTUs), Actinobacteria (18.78%; 10 OTUs) and uncultured bacteria (5.08%; 4 OTUs). The dominant genera were Sphingomonas and Pseudomonas, which accounted for 24.37% and 12.18% of total clones respectively. Also he shows that Pseudomonas sp. LK9 would improve the nutrition supplies of N, Fe and P enhance soil heavy metal availability and affect host mediate of MWOAs secretion in soils, resulting in a significant increase of shoot biomass and metal uptake of S. nigrum growing in multi-metal -contaminated soils.
Pests and diseases: Black aphids can cause a reduction in the harvest, also they can infest the inside of the leaves causing leaf curling and infestation sum of heights of plants prevents their development. Flea beetles also attack plants resulting significant damage, in addition Zonocerus variegatus induce big damage to fruit wall [28]. In West Africa during the winter viral epidemic ''compensation vain yellow" S. nigrum attack by viruses transmitted by whiteflies (Bemisia tabaci (Genn.)) inducing leaf yellowing. Fungal diseases do not seem a big problem for this species. For this, they can be infected by Cladospurium oxysporum Berk. M. A. and Curtis. This pathogen can be controlled by regular spraying with a suitable fungicide [28].
Principal uses
The traditional uses: S. nigrum has been extensively used traditionally to treat various ailments such as pain, inflammation and fever. This plant is also used in the Oriental systems of medicine for various purposes - as an antitumorigenic, antioxidant [29], diuretic and antipyretic agent, anti-inflammatory [30], hepatoprotective [31], so antibacterial and antiulcer. Various compounds have been identified which are responsible for diverse activities. The leaves contain rich amount of calcium, iron, phosphorus, carbohydrates, protein, fat, crude fiber, and minerals. This herbal plant is used as medicine for asthma, vomiting of blood, reducing blood glucose level and bilious matter phlegmatic rheumatism and ulcer [32]. Also he has been used for treating liver diseases [33]. The juice of the berries is used as antidiarrheal, ophthalmic, hydrophobicity, and for dropsy, heart disease, diuretic. Seeds are useful in vertigo. The roots are helpful in ophthalmopathy, rhinopathy and hepatitis. It was reported earlier that the aerial parts of S. nigrum is believed to provide its anti-ulcer action of acid and peptic suppression of aspirin induced in rats ulcerogenesis [34].
Toxicity and deleterious properties
Toxicity: In ancient literature, S. nigrum species are considered noxious and poisonous to animals and humans. Indeed, the toxicity is widely attributed by the alkaloid solanin. The effects of solanine poisoning in the human beings are reported as nausea, vomiting, diarrhea, abdominal cramps, headache, dizziness, speech loss, fever, sweating and tachycardia, reduced heart rate, pupil dilation, blindness, mental confusion, convulsions, coma and death [35,36]. These effects usually appear about eight hours after ingestion. In animals, ingestion can cause rapid heartbeat and breathing, thus dark color diarrhea followed by constipation, lack of rumination, drowsiness and dry mouth in cattle, low body temperature, swelling, in coordination, tremors and staggering movements [37]. However, the degree of toxicity depends of the quantity of solanine. S. nigrum tolerates a large concentration of nitrate nitrogen (NO3-N) or it may cause animals toxicity by NO3-N. In addition, acute toxicity by nitrate can lead to death and the chronic toxicity results in a decrease in milk production, abortion, muscle tremors, staggering gait, rapid heartbeat, frequent urination, labored breathing, followed by collapse and coma, with or without convulsions. However, the degree of nitrates toxicity can cause adverse effects on human health and animal production is uncertain, while actual effects observed after ingestion of plants nightshade could be due either to the solanine or NO3-N or both [37]. Nevertheless, a chemical investigation of various members of the S. nigrum reported the presence of potentially toxic alkaloids in unripe fruit, with ripe berries and vegetative parts nailing these compounds. Thus, these plants are probably toxic to livestock that could consume the entire plant. However, these plants are used as fodder for animals without any adverse effect in some areas [38]. So the evolution of toxic levels of these alkaloids depends on their growth as a result of climatic and regional conditions, and even the age of the plants concerned [36,39]. The boiling bodies S. nigrum destroyed all the inherent toxicity of these species, or most ethnobotanical reports of their use as vegetables refer to the kitchen or cooking requires the rejection of liquid boiling. Other similar contributions show the toxicity of immature berries. However, drying does not destroy the toxic alkaloid solanine kind (glycosidal of alkaloids that are responsible for the bitter taste often associated with this species) [40].
Deleterious properties: S. nigrum is included among the worst weeds in the world, Harmful to agriculture and horticulture in most regions of the world (grains, garlic, barley, potato, etc.) [20,39]. S. nigrum is in competition with cultures for humidity, light and nutrients; also it can contaminate the culture through coloring liberated by walls berries which greatly reduces the quality and economic viability of the culture producers [41,42]. Black nightshade is associated with a wide spectrum of nematodes and microorganisms potentially destructive [43].
Among these pathogens we find Nematodes like Rotylenchus similis, R. similis; Aphelenchoides ritzembozi; Heterodera marioni, Meloidogyne spp.; Pratylenchus neglectus; Meloidogyne hapla; Ditylenchus destructor; Rotylenchus reniformis; Ditylenchus phyllobis, Nothangirina phyllobia; Meloidogyne incognita; Tylenchorhynchus claytoni; Heterodera schachtii [39]. Pathogenic bacteria: Xanthomonas vesicatoria; Pseudomonas solanacearum; Pseudomonas tabaci.
Fungal pathogens: Colletotrichum atramentarium; Cercospora nigrescens, Diporotheca rhizophila, Alternaria solani; Fhytopthora infestans, Macrophoma de subconica, Erysiphe cichoracearum, Rhizoctonia solani, la rouille (Puccinia subtriata), la septoriose solanina, brûlure sud (Sclerotium rolfsii), la verticilliose (Verticillium albo-atrum), rhizoctone violet (Rhizoctonia crocorum) et le charbon blanc (Entyloma de australe).
Chemical composition of S. nigrum
S. nigrum is so rich on secondary metabolites such as the total alkaloids, steroid alkaloids, and steroid saponins glycoprotein, which showed anti-tumor activity. In addition flavonoids, tannins, saponins, glycosides, proteins, carbohydrates, coumarins and phytosterol [44]. Immature berries of S. nigrum contents had large solasodine but decreases fruit ripening [45]. Recently studies show 4 new steroidal glycosides alkaloids solamargine, solasonine, α and b solanigrinechez isolated by berries of S. nigrum. Chemical analysis of the methanol extract of root and stem shows a steroidal genin saturated identified as tigogenin (glycoside and two spirosestanol furostanol glycosides) by mixed melting point and IR spectroscopy [46]. Among the new compounds recently experienced solanigrosides-CH and degalactotigonin two steroidal saponins (called Nigrumnins I and II), two new disaccharides (BDthevetopyranosyl ethyl-(1-4)-BD-oleandropyranoside and BDthevetopyranosyl ethyl-(1 -4)-a-Doleandropyranoside) identified by spectroscopic methods. The seeds of S. nigrum have a high fat content and are considered an important source of linoleic acid [47] and contain solanine, protein, oleic acid, palmitic acid and stearic acid and sitosterol [48]. The leaves are a rich source of riboflavin. They also contain nicotinic acid and vitamin C, beta-carotene, citric acid, protein, lipid, steroid alkaloids glycol, solasonine and solamargine acid. Fruits contain saponins and alkaloids steroidal glycol, solanine, solamargine, solasonine a-b, and solanigrine aglycone, solasodine, Genin steroidal trigogenin (Table 1). All parts and immature green contain steroidal glycosides, as glycoalkaloids. In the genus Solanum are important both ecologically and commercially. In the genus Solanum are important both ecologically and commercially. They are widely considered defensive allelochemicals of plants against pathogens and predators. In economic terms, they are used in place of steroid sapogenin diosgenin as raw material for the industrial production of corticosteroids. The main steroid alkaloids are Solanine and solasonine [49-55].
| Class of compound | Compound Name | References |
|---|---|---|
| Steroide Glycoalcaloides | Solasodine | [49] |
| 12β,27-dihydroxysolasodine | [50,51] | |
| 23-O-acetyl-12β- Hydroxysolasodine | [52] | |
| N- Methylsolasodine | [50] | |
| Solasonine(-Solanigrine) | [53,54] | |
| α-Solamargine (-Solanigrine) | [49,54,55] | |
| Solanigridine | [49] | |
| β-Solamargine | [55] | |
| Solanine (α,β) | [56] | |
| Tomatidenol | [50] | |
| Solanaviol | [51] | |
| Solanocapsine | [50] | |
| Solasodi-3,5-ene | [57] | |
| Saponine | Diosgenin | [58] |
| Tigogenin | [52] | |
| Desgalactotigonin | [59] | |
| Nigrumnin I | [60] | |
| Nigrumnin II | [60] | |
| Sterols | Cholestrol | [61] |
| Campesterol | [61] | |
| Stigmasterol | [61] | |
| β-Sitosterol | [61,72] | |
| Flavonoides | Quercetin-3-glucosyl-(16) galactoside | [62] |
| Quercetin-3-gentiobioside, | [62] | |
| Quercetin-3-galactoside | [62] | |
| Quercetin-3-glucoside | [62] | |
| Quercetin 3-O-(2Gal- rhamnosyl)-glucosyl(16)- galactoside | [62] | |
| Isoquercitrin | [62] | |
| Quercetin3-O- rhamnosyl (12)-galactoside | [62] | |
| Carotenoides | β-Carotene | [63] |
| Vitamines | Vitamin C | [63] |
| Acides gras | Palmitic acid | [72] |
| Palmitoleic acid | [72] | |
| Stearic acid | [72] | |
| Linoleic acid | [72] | |
| Squalene | [72] | |
| Uttronin-β-Dglucopyranosyl | [64] | |
| Uttronin-β-Dxylopyranosyl | [64] | |
| Uttroside-β-D-glucosyl | [64] | |
| Tricarboxylic Acid | Citric acid | [63] |
| Carbohydrates | Fructose | [63] |
| D-glucose | [63] | |
| L-Rhamnose | [65] |
Table 1: Susceptibility pattern of Staphylococcus aureus and Pseudomonas aeruginosa compared to diffused discs with different materials and duration of smoke.
Essential oils
The volatile compounds of essential oil from wild S. nigrum have determinated by head-space/solid-phase micro-extraction (HS/SPME) and gas chromatography/mass spectrometry (GC/ MS). Essential oils extracted shows the following composition : Dillapiole (22,22%), un cadinol (16,47%), para-cymène (10,01%), (E)-1- (2,6,6-triméthyl 1,3-cyclohexadiène-1-yl)-2-butène- 1-one ou b-damascénone (9,08%), a-phellandrène (8,48%), b-pinène (5,93%), un-bisabolol acétate (4,53%), (Z,E)-4,6,8- Megastigmatriene (4,09%), Phytol (2,49%), Linalyl butanoate (2,13%), 8-méthylène-tricyclo [3.2.1.0 (2,4)] octane (2,60%) et le limonène (2,03%) [56].
Biological and pharmacological activity
The richness of the species S. nigrum biologically active compounds has enabled the species of this genus in front of the pharmaceutical scene.
Antibacterial activity: The crude extracts of leaves, seeds and roots of S. nigrum showed a potent antibacterial and antifungal activity. Thus, the sensitivity of microorganisms to Depond crude extracts of the nature of the solvent and tested microorganism [57-67]. The methanol extracts of the leaves and seeds of S. nigrum show potent activity against all microorganisms namely; Escherichia coli, Citrobacter, Shigella flexenari, Staphylococcus aureus, Pseudomonas aeruginosa and Yersinia aldovae; Cereviciae Saccharomyces, Aspergillus parasiticus, Trichophyton rubrum, Macrophomina, Fusarium solani and Candida albicans [68]. Although, the ethanol extract of the root shown as well antifungal activity against A. brassicicola [69]. The ethanol and ethyl acetate extracts of methanol leaves, the seeds and the roots were analyzed for their antifungal activity against fungal strains such as Penicillium notatum, Aspergillus Niger, and Trichoderma viridae Fuserium oxisporium or diameter d inhibition was compared to the standard antibiotics. Only extracts from seeds that have antifungal activity [70].
The anti-HCV activity: Recent research of Javed sign that methanol and chloroform extracts of seeds contains potential antiviral agents against HCV, which extracts exhibit an inhibition rate of HCV varies from 37% to 50% respectively at nontoxic doses. The chloroform extract decreases the functioning of the HCV protease in a dose dependent manner) [71,72].
The hepatoprotective activity: Some research reported that the water extract of S. nigrum have a protective effect against liver damage were evaluated in carbon tetrachloride (CCl4)- induced chronic hepatotoxicity in rats. However this study suggest that S. nigrum could protect liver against the CCl4 induced oxidative damage in rats and this hepatoprotective effect might be contributed to its modulation on detoxification enzymes and its antioxidant and free radical scavenger effects. So the Oral administration of S. nigrum significantly reduces thioacetamideinduced hepatic fibrosis in mice, probably through the reduction of TGF-1 secretion [73]. Also, the methanol extracts of S. nigrum have hepato-protective effects against liver injury induced by injection of CCl4 in rats. These effects are signed by decreased serum aspartate aminotransferase (AST), alanine amino-transferase (ALT), alkaline phosphate (ALP) and a significant decrease in bilirubin in rats treated with the extract methanolic compared to untreated group [74]. The hepatoprotective activity of the ethanol extract of S. nigrum berries has confirmed; albeit the effect of the extracts was evaluated by the assay of serum protein, Serum bilirubin and serum enzymes like Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT) and Markers for oxidative stress like Glutathione (GSH), Superoxide Dismutase (SOD), Lipid Peroxidase (LPO) and histopathological studies of liver. In the groups where hepatic injury induced by CCl4 and extract were given simultaneously, the toxic effect of CCl4 was controlled significantly (<0.05) by maintenance of structural integrity of hepatocyte cell membrane and normalisation of functional status of liver. Histology of liver sections from S. nigrum+CCl4 treated rats revealed moderate centrilobular hepatocytes degeneration, few areas of congestion with mild fatty changes. The extracts of S. nigrum possess significant hepatoprotective activity in comparison to standard drug Silymarin [75].
The antioxidant activity: The researchers have been succeeding to isolate a glycoprotein (150 KDa) from ethanolic extract of fruit of S. nigrum. This glycoprotein has scavenging activity againt DPPH, OH, O. radical. In particular this glycoprotein has a nonspecific radical scavenging activity in the DPPH assay, which was similar to that of ascorbic acid. So, in the deoxyribose assay for the OH radical, the S. nigrum glycoprotein highly removed OH radical deduced from deoxyribose degradation. However, they concluded that S. nigrum glycoprotein function as natural antioxidant [76]. Also, the antioxidant activity of S. nigrum has confirmed by highlighting the protective effects of lunasin against oxidative DNA damage. However, this lunasin peptide compound has been purified from S. nigrum, it did not scavenge generated hydroxyl radical, although the lunasin blocked the generation of hydroxyl radical by chelating Fe2+ ion [77].
Cardio-protective activity: The cardioprotective activity of the methanol extract of the fruit was evaluated with an in vitro total injury ischemia-reperfusion performed using doses of 2.5 and 5.0 mg/kg for 6 days per week for 30 days. The results indicate that the extract exhibited significant (p<0.001) of the cardioprotective activity against the ischemia-reperfusion. The activity took place independently of the dose. The methanol extract of berries S. nigrum has a cardio-protective activity [78].
Analgesic activity: Ethanol extracts of S. nigrum was evaluated for analgesic activity. The analgesic activity of the extract was evaluated for its central and peripheral pharmacological action using the hot plate Eddy and acetic acid-induced writhing respectively. The study was carried out using doses of 100, 250 and 500 mg/kg orally, or extract shows a positive result of the analgesic activity [78].
Antidiarrheal activity: The ethanol extract of dried fruits of S. nigrum was evaluated for anti-diarrheal activity. The fruit extract showed a significant increase (P<0.01 and P<0.001) anti-diarrheal activity against castor oil to induce. Diarrhea in mice in which it reduced the frequency of defecation and increase the average latency period at a dose of 250 mg/kg and 500 mg/kg body weight [78].
The cytotoxic activity: The ethanol extract of dry fruits of S. nigrum Linn. The cytotoxic activity is highlighted by the lethality test brine shrimp, the extract showed significant cytotoxicity with LC90 and LC50=63.10 μg/ml=160 μg/ml [78].
The anti-inflammatory activity: The methanolic of whole plants of S. nigrum extract were investigated for anti-inflammatory activity in experimental animal models. The MeOH extract at a dose of 100 mg/kg body weight and 200 mg/kg body weight have shown a significant anti-inflammatory dose-dependent activity. The standard drugs were indomethacin (10 mg/kg) and cyproheptadine (8 mg/kg) [79]. Ethanol extracts of S. nigrum were evaluated for anti-inflammatory activity using Carrageenan induced rat paw edema. The study was carried out using doses of 100, 250 and 500 mg/kg orally. The anti-inflammatory activity at a dose of 500 mg/kg (P<0.01) as compared to the standard drug diclofenac sodium (50 mg/kg) [80]. The effect of methanol extracts of S. nigrum fruits were studied on carrageenan induced paw edema. This extract decreased the edema induced in the hind paw with a dose (375 mg/kg body weight) showed significant anti-inflammatory [48].
Anticancer activity: Anticancer activity has also been demonstrated that ethanol extract from ripe fruits of S. nigrum inhibit the proliferation of human MCF-7 breast cancer cells and induce cell death by apoptosis [81]. The S. nigrum glycoprotein (a molecular weigh 150 KDa) has a potent cytotoscic effect on the TH-29 cells. However, additions of S. nigrum glycoprotein with OH radical contribute together to the cytotoxicity of HT-29. Also the S. nigrum glycoprotein has a strong antioxidant activity and regulator affect to NK-B, so the latter effect, in turn, induces cells death by apoptosis. In conclusion, the S. nigrum glycoprotein can be considered to have pharmacological values as a prenventive agent for colon cancer [76]. Also the research has shown that the aqueous extract of S. nigrum has been reported to demonstrate approximately 40% cell growth inhibition in HepG2 cells [82]. Recent research shows the methanol extracts of fruits were tested for their inhibitory effect on the cell line the HeLa. The percentage of viability of the cell line was performed using Trypan blue dye exclusion method. The MeOH extract where significant cytotoxicity on HeLa cell line in the range of concentration between 10 mg/ml to 0.0196 mg/ml using the SRB assay [83]. The main phytoconstitents of whole plant S. nigrum have been reported to act in various tumors, although the solamargine and solasonine inhibits growth and spread of various cancers, including breast, liver, lung and cyst cancers and leukaemia. Also the steroidal glycosides inhibit growth and spread of colon cancers and pheochromocytoma. The polysaccharides of this herb have significant inhibitory effect on growth of liver cancer by two distinct anticancer activities [84].
The anti-epileptic activity: Aqueous extract of the leaves of S. nigrum was evaluated for anti-epileptic activity in chicks, mice and rats by extract administration by intraperitoneal route. At a time of 30 min pretreatment and graded doses, animals were challenged with different types of proconvulsant. The result indicates that the aqueous extract of leaves acted significantly (P<0.05), dose-dependent protection against electrically induced seizure in chicks and rats, pentylenetetrazol induced seizure in mice and rats and seizure picrotoxin induced in mice and rats. The anti-seizure property of the extract was potentiated by amphetamine. The anticonvulsant potential of herb S. nigrum has been revealed with the aqueous extracts with solasodine and diosgenin compounds could be used for treatment of Epilepsy [85].
Hypoglycemic activity: The ethanol extract of S. nigrum leaves in rats having hyperglycemia (alloxan monohydrate) induces a reduction in the level of blood glucose significantly in a dose dependent manner from 2 h to 10 h of study, while glibenclamide standard drug has shown similar effect during the experiment. Treatment with Alloxane causes permanent destruction of β cells and impaired renal function and sulfonylurea drugs are known to lower the level of glucose in the blood by stimulating β cells to release insulin [86]. However, the ethanol extract of S. nigrum has anti-hyperglycemia effects and may suggest additional effects on the pancreas and intra-intestinal shares [87]. The aqueous extracts of S. nigrum significantly reduced glucose and other lipid parameter at doses between 200 and 400 mg/kg body weight of diabetic rats for 21 treatments. These findings show the antidiabetic potential of this plant [88]. The recent research indicated that aqueous extracts of leaf and berries having an effect significant hypoglycemic effect in dose independent manner. Moreover the stem extract hasn’t a profound effect [89].
Anti-gastritis activity and anti-ulcerogenic effects: S. nigrum is considered anti-gastritique and anti-ulcerogenic. This activity can be explained by the presence of antioxidant vitamins, minerals, tannins and other phytochemicals. With other studies specific active constituents can be identified and their mechanism of action can be elucidated. This plant has both anti-gastritic and antiulcer effects. With the combination of nutrients and antioxidant phytochemicals have great therapeutic importance as preventive functional food [90]. Thus the fruits of S. nigrum are can be considered protective gastric by their action against free radicals [91]. However, in the previous study, it was found that water extract, ethanol extract and n-butanol extract of Solanum nigrum L. could inhibit the growth of human gastric cancer MGC- 803 cells [92]. Six steroidal glycoalkaloids from S. nigrum has isolated and purified by acid extraction and alkaline precipitation. Experimental results showed that compounds solasonine, b1- solasonine, solamargine and solanigroside P have cytotoxicity to human gastric cancer MGC-803 cells [93]. Also, the aqueous leafs of S. nigrum inhibited the aspirin induced and pyloric ligated ulcer models on experimental rats. Further, this extracts also shows spasmolytic effect on ileum contraction [94]. The petroleumether extract of S. nigrum berries can inhibits parameters linked to the asthma disease. Since, it contains an antiasthmatic compound "b–sitosterol", and significantly inhibited increased leukocyte and eosinophil count due to milk allergen and showed maximum protection against mast cell degranulation by clonidine [95].
Other activity
The ethanol extract of whole plants of the S. nigrum exerted cytoprotection against gentamicin-induced toxicity on Vero cells. However, the protection of the cells from the effects of gentamicin (increasing in the cells permeability, a decrease in mitochondrial dehydrogenase activity) has been determined through the incubation of the Vero cells with the different concentrations of the dried 50% ethanol extract [96]. Also, the researchers have succeeded to synthesize a silver nanoparticle by using the aqueous extracts of S. nigrum. These nanoparticles are spherical to polyhedral in shape witn size of 50-100 nm. In larvicidal bioassay with synthesized AgNPs, highest mortality are observed at 10 ppm against An stephensi with LC 50 values of 1.33, 1.59, 1.56 ppm and LC 90 values of 3.97, 7.31, 4.76 ppm for dry leaves, fresh leaves and berries respectively. So the Antibacterial activity test reveals better results against fish pathogenic bacteria than human pathogenic bacteria. Moreover, the silver nanoparticle hasn’t any effects and anomaly in the organism as Toxorhynchites larvae (mosquito predator), Diplonychus annulatum (predatory water-bug) and Chironomus circumdatus larvae (chironomid) are also exposed to respective lethal concentrations [97].
Conclusion
Solanum nigrum have been used since time immemorial/ antiquity for the treatment of human ailments. Even today, the traditional systems of medicine continue to be widely practiced. Their use as such is recorded from the earliest times and various species, especially Solanum nigrum, are mentioned and often illustrated in all of the early Herbals, with Dioscorides being one of the first to record their medicinal properties. Since then this ‘species has continued to be widely acclaimed for its medicinal effects in every country in which the taxon is found.
References
- Edmonds JM (1978) Solanaceae in flowering plants of the world. Oxford University Press, pp: 228-229.
- Arcy WG (1991) The Solanaceae since 1976 with a review of its biogeography. In: Solanaceae III: Taxonomy, Chemistry and Evolution. Academic Press, London, pp: 75-137.
- Symon DE (1981) A revision of the genus solanum in Australia. J Adelaide Bot Gard 4: 1-367.
- Schippers S (2002) African indigenous vegetables. In: An over review of the cultivated species.
- Stebbins GL, Paddock EF (1949) The Solanum nigrum complex in Pacific North America. Madrono 10: 70-81.
- Heiser CB (1963) Estudio biosistematico de solanum (Morella). In: El Ecuador Ciencia y Naturaleza 6: 50-58.
- Symon DE (1970) Dioecious Solanums. Taxon 19: 909-910.
- Venkateswarlu J, Rao MK (1972) Breeding systems, crossability relationships and isolating mechanisms in the Solanum nigrum complex. Cytologia 37: 317-326.
- Darcy WG (1973) Solanaceae. In: Flora of Panama. Miss Bot Gard 60: 573-780
- Darcy WG (1974) Solanum and its close relatives in florida. Annals Missouri Botanical Garden 61: 819-867.
- Edmonds JM (1972) A synopsis of the taxonomy of solanum sect solanum (maurella) in South America. Kew Bull 27: 95-114.
- Edmonds JM (1977) Taxonomic studies on solanum L. Section solanum (maurella) Bot.
- Edmonds JM (1979) Biosystematics of solanum L. section solanum (maurella). In: The Biology and Taxonomy of the Solanaceae. Academic Press, London, UK, pp: 529-548.
- Baylis GTS (1958) A cytogenetical study of New Zealand forms of solanum nigrum L, S. nodiflorum Jacq and S. gracile Otto. Transaction Royal Society of New Zealand 85: 379-385.
- Henderson RJF (1974) Solanum nigrum L. (solanaceae) and related species in Australia. Contributions from the Queensland Herbarium No 16: 1-78.
- Jardine NRS (1971) Mathematical Taxonomy. John Wiley and Sons, London, UK.
- Ganapathi A, Rao GR (1986) Taxonomic status of the solanum nigrum complex found in India. Proceeding Indian Academy Science 96: 241-246.
- Lin HM, Tseng HC, Wang CJ (2008) Chemico Biological Interaction 171: 283-293.
- Edmonds JM, Glidewell SM (1977) Acrylamide gel electrophoresis of seed proteins from some solanum (section solanum) species. Plant Systematic and Evolution 127: 277-291.
- Holm LG, Plunknett DL, Pancho JV (1977) Solanum nigrum L. In: The world’s worst weeds. Distribution and Biology. University Press of Hawaii, pp: 430-434.
- Edmonds JM, Chweya JA (1997) Black nightshades solanum nigrum L. and related species. Institute of Plant Genetics and Crop Plant Research/International Plant Genetic Resources Institute, Rome.
- Wang G, Xia Z (1983) Regeneration of plantlets from solanum nigrum L. mesophyll protoplasts. Acta Botanica Sinica 25: 111-114.
- Salisbury E (1961) Black nightshade. In: Weeds and Aliens. Collins 28: 187-188.
- Muchiri SV (2004) Characterization and purification of African nightshade (Solanum nigrum complex) accessions for sustainable seed production in Kenya. MSc Dissertation, Jomo Kenyata University of Agriculture and Technology, Thika, Kenya.
- Wei SH, Zhou QX, Koval PV (2006) Flowering stage characteristics of cadmium hyperaccumulator Solanum nigrum L. and their significance to phytoremediation. Science of Total Environment 369: 441-446.
- Ji PH, Sun TH, Song YF, Ackland ML (2011) Strategies for enhancing the phytoremediation of cadmium-contaminated agricultural soils by solanum nigrum L. Environmental Pollution 159: 762-768.
- Liang C, Shenglian L, Jueliang C, Yong W (2012) Diversity of endophytic bacterial populations associated with Cd-hyperaccumulator plant solanum nigrum L. grown in mine tailings. Applied Soil Ecology 62: 24-30.
- Epenhuijsen CW (1974) Growing native vegetables in Nigeria. FAO, Rome, pp: 80-88.
- Dancey JE, Chen HX (2006) Strategies for optimizing combinations of molecularly targeted anticancer agents. Nature Reviews Drug Discovery 5: 649-659.
- Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev, pp: 564-582.
- Lin HM, Tseng HC, Wang CJ (2008) Hepatoprotective effects of solanum nigrum Linn extract against CCl(4) - induced oxidative damage in rats. Chem Biol Interact 171: 283-293.
- Jainu M, Srinivasulu C, Devi S (2006) Antiulcerogenic and ulcer healing effects of S. nigrum (L) on experimental ulcer models: Possible mechanism for the inhibition of acid formation. Journal Ethnopharmacol 104: 156-163.
- Ikeda T, Tsumagari H, Nohara T (1992) Steroidal oligoglycosides from solanum nigrum growing in Azerbaijan. Biologicheskie Nauki 3: 15-18.
- Solanum L (2010) Germplasm resources Information network. United States Department of Agriculture.
- Watt JM, Breyer BMG (1962) Solanum nigrum L. In: The medicinal and poisonous plants of southern and eastern Africa. E and S Livingstone Ltd., Edinburgh and London, pp: 996-1000.
- Cooper MR, Johnson AW (1984) Black nightshade-solanum nigrum. In poisonous plants in Britain and their effects on animals and man. HMSO London, UK, pp: 219-210.
- Weller RF, Phipps RH (1978) A review of the black nightshade (solanum nigrum L.). Protection Ecology 1: 121-139.
- Schilling EE, Anderson RN (1992) Common names and species identification in blacknight shades, Solanum sect. solanum (solanaceae). Economic Botany 46: 223-225.
- Rogers BS, Ogg AG (1981) Biology of weeds of the solanum nigrum complex (solanum section solanum) in North America. US Department of Agriculture Science and Education Administration. Agricultural Reviews and Manuals, Western Series No 23: 1-30.
- Everist SL (1974) Poisonous plants of Australia. In: Angus and Robertson, Sydney, pp: 462-475.
- Burgert KL, Bumside OC, Fenster CR (1973) Black nightshade leaves its mark. Farm, Ranch and Home Quarterly, pp: 8-10.
- Ogg AG, Rogers BS, Schilling EE (1981) Characterization of black nightshade (solanum nigrum) and related species in the United States. Weed Science Journal of the American Chemical Society 29: 27-32.
- Karschon R, Horowitz M (1985) Infraspecific variation of solanum nigrum L. and S. villosum Miller, important crop weeds in Israel. Phytoparasitica 13: 63-67.
- Hussain AOD, Pople SP (1992) Dictionary of Indian medicinal plants central Institute of medicinal and aromatic plants. Lucknow, p: 35.
- Nadkarni KM (1976) Indian Materia Medica. 3rd edn. Popular Prakashan 1: 1156.
- Ravi V, Saleem TSM, Patel SS, Raamamurthy J, Gauthaman K (2009) Anti-inflammatory effect of methanolic extract of solanum nigrum Linn berries. International Journal of Applied Research in Natural Products 2: 33-36.
- Ravi V, Saleem TSM, Maiti PP (2009) Phytochemical and pharmacological evaluation of solanum nigrum Linn. African Journal of Pharmacy and Pharmacology 3: 454-457.
- Ghani A (2003) Medicinal plants of Bangladesh. In the Asiatic Society of Bangladesh, Dhaka, p: 382.
- Schreiber K (1958) Planta Medica 6: 435-439.
- Doepke W, Sabine D, Matos N (1987) Z Chemistry 27: 64.
- Yoshida K, Yahara S, Saijo R (1987) Chemical and Pharmaceutical Bulletin 35: 1645-1648.
- Doepke W, Sabine D, Matos N (1988) Z Chemistry 28: 185.
- Tomova M (1962) Farmatsiya 12: 16-19.
- Aslanov SM (1971) Khim Prir Soedin 5: 674.
- Aslanov SM, Novruzov EN (1978) Izv Akad Nauk Az 3: 15-18.
- ElAshaal HA, Ghanem SA, Melek FR (1999) Fitoterapia 70: 407-411.
- Waclaw RB (1968) Diss Pharm Pharmacol 20: 311-318.
- Khanna P, Rathore AK (1977) Indian Journal Experimental Biology 15: 808-809.
- Ke H, Kobayashi H, Dong AJ (1999) Planta Medica 65: 35-38.
- Ikeda T, Tsumagari H, Nohara T (2000) Chemical Phramaceutical Buletin 48: 1062-1064.
- Bhatt PN, Bhatt DP (1984) Journal Experimental Botany 35: 890-896.
- Nawwar MAM, El-Mousallamy AMD, Barakat HH (1989) Phytochemistry 28: 1755-1757.
- Rzhavitin VN (1959) Priroda48: 107.
- Sharma SC, Chand R, Sati OP, Sharma AK (1983) Phytochemistry 22: 1241-1244.
- Bull PM (1958). Acta Chemica Scandinavica 12: 358.
- Avat T, Mohammad MK (2013) Chemical composition analysis of the essential oil of solanumn nigrum L. by HS/SPME method and calculation of the biochemical coefficients of the components. Arabian Journal of Chemistry.
- Kavishankar GB, Lakshmidevi N, Mahadeva MS (2011) Phytochemical analysis and antimicrobial properties of selected medicinal plants against bacteria associated with diabetic patients. International Journal of Pharmaceutical and Biology Sciences 2: 509-518.
- Britto AJD, Gracelin DHS, Kumar PR (2011) Antimicrobial activity of a few medicinal plants against gram negative bacteria. International Journal of Applied Biology and Pharmaceutical Technology 2: 457-461.
- Muto M, Mulabagal V, Huang HC (2010) Japan toxicity of black nightshade (Solanum nigrum) extracts on Alternaria brassicicola, causal agent of black leaf spot of Chinese cabbage (Brassica pekinensis). Department of International Agricultural Development, Tokyo University of Agriculture, Sakuragaoka, Setagaya-ku, Tokyo.
- Sridhar TM, Josthna P, Naidu CV (2011) Antifungal activity phytochemical analysis of solanum nigrum (L.) an important antiulcer medicinal plant. Journal of Ecobiotechnology 3: 11-15.
- Javed T, UsmanAA, Sana R (2011) In-vitro antiviral activity of solanum nigrum against Hepatitis C Virus. Virology Journal 8: 26.
- Hanna AG, Yassin FYS, Allam RI (1996) Journal of Pharmaceutical Sciences 37: 211-231.
- Heo KS, Lim KT (2004) Antioxidative effects of glycoprotein isolated from solanum nigrum L. Journal of Medicinal Food 7: 349-357.
- Elhag RAM, Badwi MAE (2011) Hepatoprotective activity of solanum nigrum extracts on chemically induced liver damage in rats. Journal of Veterinary Medicine and Animal Health 3: 45-50.
- Subash KR, Ramesh KS, Binoy V (2011) Study of Hepatoprotective activity of Solanum nigrum and Cichorium intybus. International Journal of Pharmacology 7: 504-509.
- Kung SH, Sei JL, Lim KT (2004) Cytotoxic effect of glycoprotein isolated from solanum nigrum L. through the inhibition of hydroxyl radical-induced DNA-binding activities of NF-kappa B in HT-29 cells. Environmental Toxicology and Pharmacology 17: 45-54.
- Jin BJ, Ben O (2010) Lunasin peptide purified from solanum nigrum L. protects DNA from oxidative damage by suppressing the generation of hydroxyl radical via blocking fenton reaction. Cancer Letters 293: 58-64.
- Bhatia N, Maiti PP (2011) Evaluation of cardio protective Activity of Methanolic Extract of solanum nigrum Linn. in Rats. International Journal of Drug Development and Research 3: 139-147.
- Arunachalam G, Subramanian N (2009) Evaluation of anti-inflammatory activity of methanolic extract of solanum nigrum (solanaceae). Iranian Journal of Pharmaceutical Sciences Summer 5: 151-156.
- Kaushik D, Jogpal V (2009) Evaluation of activities of solanum nigrum fruit extract. Archives of Applied Science Research 1: 43-50.
- Son YO, Kim J, Lim JC (2003) Ripe fruits of solanum nigrum L. inhibits cell growth and induces apoptosis in MCF-7 cells. Food Chemical and Toxicology 41: 1427-1428.
- Lin HM, Tseng HC, Wang C (2007) Induction of autophagy and apoptosis by the extract of solanum nigrum Linn in HepG2 Cells. Journal of Agricultural and Food Chemistry 55: 3620-3628.
- Patels S, Gheewala N, Suthar A, Shah A (2009) In-vitro cytotoxicity activity of solanum nigrum extracts against Hela cell lines and Vero cell lines. International Journal of Pharmacy and Pharmaceutical Sciences 1: 38-46.
- Pandey G, Madhuri S (2008) Some anticancer agents from plant origin. Pl Arch 8: 527-532.
- Noel NW, Joseph AA, Helen OK (2008) Antiseizure activity of the aqueous leaf extract of solanum nigrum linn (solanaceae) in experimental animals. African Health Sciences 8: 74-79.
- Pari L, Maheswari J (1999) Hypoglycaemic effect of musa sapientum L. in alloxan-induced diabetic rats. Journal of Ethnopharmacol 68: 321-325.
- Day C, Catwright T, Provost J, Bailey CJ (1990) Hypoglycaemic effect of momordica charantia extracts. Planta Medica 56: 426-429.
- Poongothai K, Ahmed KSZ, Ponmurugan P, Jayanthi M (2010) Assessment of antidiabetic and antihyperlipidemic potential of solanum nigrum and Musa paradisiaca in alloxan induced diabetic rats. Journal of Pharmacy Research 3: 2203-2205.
- Sathya MST, Palaniswamy M (2011) Pharmacognostical and hypoglycemic activity of different parts of Solanum nigrum Linn plant. International Journal of Pharmacy and Pharmaceutical Sciences 4: 0975-149.
- Maddala R, Subbulakshmi G, Sandhya K (2013) Anti gastritic and antiulcerogenic effects of solanum nigrum in laboratory animals. International Journal of Nutrition and Food Sciences 2: 266-271.
- Jainu M, Devi CSS (2004) Antioxidant effect of methanolic extracts of solanum nigrum berries on aspirin induced gastric mucosal injury. Indian Journal of Clinical Biochemistry 19: 57-61.
- Ding X, Gao SG, Li GY (2011) Study on in vitro anti-tumor effect of different solution extracts from Solanum nigrum L. Lishizhen Medicine and Materia Medica Research 22: 1244-1246.
- Ding X, Zhu F, Yang Y, Li M (2013) Purification, antitumor activity in vitro of steroidal glycoalkaloids from black nightshade (solanum nigrum L). Food Chemistry 141: 1181-1196.
- Muthukumar A, Periyasamy M, Manohar R (2013) Ulcer Protective and spasmolytic activity of aqueous extract of solanum nigrum leaves in experimental Rats. International Journal for Pharmaceutical Research Scholars 2: 2277-2283.
- Nirmal SA, Patel AP, Bhawar SB (2012) Antihistaminic and antiallergic actions of extracts of solanum nigrum berries: Possible role in the treatment of asthma. Journal of Ethnopharmacology 142: 91-97.
- Prasanth Kumar V, Shashidhara S, Kumar MM, Sridhara BY (2001) Cytoprotective role of solanum nigrum against gentamicin induced kidney cell (vero cells) damage in vitro. Fitoterapia 72: 481-486.
- Anjali R, Anupam G, Goutam C (2013) Mosquito larvicidal and antimicrobial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of solanum nigrum L. (Solanaceae: Solanales). Acta Tropica 128: 613-622.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences