ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Enzymolysis of By-Product Derived from Sheep Placenta to Production of Highly Active Antioxidant Peptide
1School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China
2The Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, Wuxi 214122, China
- *Corresponding Author:
- Li Ming
School of Chemical and Material Engineering
Jiangnan University
Wuxi 214122, China.
Tel: +8613382888861
E-mail: leeming@jiangnan.edu.cn
Received Date: November 26, 2016; Accepted Date: January 20, 2017; Published Date: January 26, 2017
Citation: Ming L, Huimin J, Guangqun C, et al. Enzymolysis of By-Product Derived from Sheep Placenta to Production of Highly Active Antioxidant Peptide. Synth Catal. 2017, 2:1.
Abstract
Title: A highly active antioxidant peptide from enzymatic hydrolysates of sheep placenta by-product protein was purified and identified.
Background: Bioactive peptides, as products of hydrolysis of food proteins, become the focus of current research. They exert various biological roles, one of the most crucial of which is the antioxidant activity. Sheep placenta has long been used in traditional Chinese medicine for the treatment of physiological abnormalities in human organs, and recent studies have demonstrated that it is a rich source of biological and therapeutic compounds. Although numerous activities of sheep placental peptides have been reported so far, little information is known about antioxidant peptides from sheep placenta by-product protein. In this study, we for the first time reported highly active antioxidant peptide from sheep placenta by-product protein hydrolysate.
Methods and findings: Herein, four different proteases were employed for the hydrolysis of sheep placenta by-product, respectively. Of the various hydrolysates, papain hydrolysate exhibited the highest 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and the optimum hydrolysis conditions were achieved as the substrate concentration of 33 mg/mL, pH 6.4, temperature 55°C, enzyme dosage 4900 U/g, and hydrolysis time 120 min using response surface methodology. Then, the papain hydrolysate was purified sequentially by DA201-C macroporous resin, ultrafiltration, Sephadex G-10 gel filtration, and reversedphase high performance liquid chromatography. The sequence of the peptide with the highest antioxidant activity was identified to be Glu-Pro-Val-Ser-His-Phe (molecular weight of 679.59 Da). The IC50 value of the peptide on scavenging DPPH radical was 0.074 mg/mL, lower than that of glutathione.
Conclusions: The enzymatic hydrolysates from sheep placenta by-product possess a potent biological activity.
Keywords
Sheep placenta by-product; Enzymatic hydrolysis; Antioxidant peptide; Surface methodology
Introduction
Oxygen is an essential element for life to perform biological functions. However, it generates various kinds of Reactive Oxygen Species (ROS) such as hydroxyl radicals, superoxide anion radicals, and non-free radical such as singlet oxygen and hydrogen peroxide [1]. The excess ROS could modify small cellular molecules, proteins and DNA, as well as play a main role in the occurrence of diseases including diabetes mellitus, neurological disorders, cardiovascular diseases, and Alzheimer’s disease [2-4]. In foods, the oxidation of fatty acids and lipids result in subsequent development of undesirable deterioration of quality and potentially toxic reaction products. Therefore, it is important to inhibit the formation of ROS and oxidation occurring in the living body and foodstuffs [5]. At present, some synthetic antioxidants such as Butylated Hydroxyanisole (BHA), Butylated Hydroxytoluene (BHT) and n-propyl gallate, though exhibit strong antioxidant activities, have potential risks to human health and are restricted in food industry [6,7]. Thus, there has been a great deal of interest in finding new antioxidants from natural sources to replace synthetic ones.
In the last two decades, the enzymatic hydrolysis of protein has attracted much attention of the researchers all over the world. Many studies have described the bioactive function of some hydrolyzed peptides includes, but does not limit to antithrombotic, antihypertensive, anticancer, antimicrobial and antioxidant activities [8-11]. Particularly, peptides from many animal sources have been found to exhibit strong antioxidant activity such as chicken breast protein [12], tilapia skin gelatin [13], bovine brisket sarcoplasmic proteins [14], ovomucin [15], chicken liver [16], duck skin by-products [17] and Ostrich egg white protein [18]. The antioxidant activity of these hydrolysates has been ascribed to the cooperative effect of a number of properties including their ability to scavenge free radicals, to act as metal-chelators and oxygen quenchers [19]. These peptides usually contain 3-16 amino acid residues, and the antioxidant activity is more related to their composition, structure and hydrophobicity. Moreover, presence of proper amino acids and their correct positioning in peptide sequence play an important role in the antioxidant activity of peptides [20,21].
Sheep placenta has long been used in traditional Chinese medicine for its pharmacodynamic effects, and the latest studies have demonstrated that it is an animal recourse which is rich in biological and therapeutic components [22-23]. Normally, the water-soluble immuno-active peptides were extracted as health care ingredients after ultrafine grinding and freezing centrifugation [24], the residual precipitate was then discarded as industrial waste. In fact, the precipitate as sheep placenta by-product was a rich source of proteins, and these proteins are potentially valuable resources. However, little information is known about antioxidant peptides from sheep placenta by product protein [25-27]. In this paper, we purified an antioxidant peptide derived from enzymatic hydrolysate of sheep placenta by-product protein, and determined the amino acid sequence.
Methods
Chemicals and reagents
Sheep placenta was kindly donated by Xiaoweiyang Ltd (Wuxi, China). DPPH was purchased from Sigma Chemical Co. (St. Louis, MO). Papain, trypsin, neutral protease and alkaline protease were purchased from Yuanye Biological Technology Co. (Shanghai, China). All other chemicals and reagents used were of analytical grade and purchased from Sinopharm Chemical Reagent Co. (Shanghai, China).
Preparation of hydrolysis material: Sheep placenta was rinsed with deionized water, then homogenized with deionized water (two times the weight of sheep placenta) using a high speed tissue homogenizer (JJ-2, Ronghua instrument manufacture Co., Jintan, China). After centrifugation at 10000 × g for 15 min at 4°C, the precipitate was collected and lyophilized. The lyophilized precipitate (sheep placenta by-product) was then grinded using a high-speed pulverizer (DFY-300, Linda machinery Co., Wenling, China), collected through 60-mesh screen and used as hydrolysis material.
Preparation of sheep placenta by-product protein hydrolysates (SPBPH): Four proteases (papain, trypsin, neutrase and alcalase) were used for the hydrolysis of sheep placenta by-product. SPBPH were hydrolyzed by pH-stat method with each protease for 150 min in a batch reactor and then heated in a boiling water bath at 100°C for 15 min to inactivate enzyme activity. The samples were then centrifuged at 10000 × g for 20 min at 4°C, and the supernatant was lyophilized, and assayed for DPPH radical scavenging activity.
Determination of the degree of hydrolysis: The degree of hydrolysis (DH) was analyzed using pH-stat method [28] described by Adler-Nissen and defined as the percentage ratio of the number of peptide bonds broken (h) to the total number of peptide bonds per unit weight (htot). It was calculated from the amount of base (NaOH) added to keep the pH constant during the hydrolysis as given below:
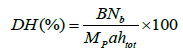 where B is the amount of NaOH consumed (mL) to keep the pH constant during the reaction, Nb is the
normality of the base, MP is the mass of protein (in grams,%N × 8.0), 1/α is the calibration factor for pH-stat, and htot is the
content of peptide bonds [28].
where B is the amount of NaOH consumed (mL) to keep the pH constant during the reaction, Nb is the
normality of the base, MP is the mass of protein (in grams,%N × 8.0), 1/α is the calibration factor for pH-stat, and htot is the
content of peptide bonds [28].
Optimization of hydrolysis conditions by response surface methodology: Response surface methodology (RSM) was applied to predict the optimal hydrolysis conditions of SPBP hydrolysis by using papain. Optimization of three variables temperature (X1), total enzyme dose (ED, X2) and pH (X3) for the DPPH radical scavenging activity (Y) was performed using Design-expert 8.0.6 software package (Stat-Ease Inc., USA). Each variable had three levels and 15 runs in all were employed. The response functions (Y) was related to the coded variables (X1, X2, X3) by a second order polynomial (Eq. 1) using the method of least squares.
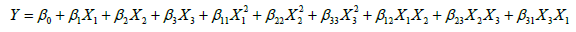
Antioxidant activity
DPPH radical scavenging activity: DPPH radical scavenging activity was measured by using the methodology by Nanjo et al. [29] with some modifications. Briefly, 2.0 mL SPHs solution was added to 2.0 mL of 0.1 mM DPPH in ethanol. The mixture was left in dark for 1 h at room temperature. The absorbance of the resulting solution was measured in different intervals at 517 nm. The DPPH radical scavenging activity (DRSA) was calculated based on the following equation, in which A, A’ and A0 was the absorbance value of the sample, the blank and the control group, respectively.
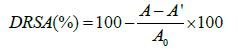
Hydroxyl radical scavenging activity: Hydroxyl radical scavenging activity was measured according to the method of Jin et al. [30].
Superoxide anion radical scavenging assay: Superoxide anion radical scavenging activity was determined using to a modified version of the method of Xie et al. [31].
Reducing power: Reducing power was measured following the method of Wu et al. [32].
Purification of antioxidant peptides and determination of amino acid sequences
Macroporous resin: The lyophilized SPBPH was dissolved in deionized water, and loaded onto DA201-C (1.4 × 35.5 cm) macroporous resin equilibrated with deionized water, and eluted with a linear gradient of ethanol solution (20%, 30%, 40%, 50%, 60%, v/v) in the same buffer at a flow rate of 200 mL/h. The different fractions were all lyophilized for the measurement of DPPH radical scavenging activity, and the contents of polysaccharide in different fractions were also determined by phenol-sulfuric acid method.
Ultrafiltration: The fraction with the highest DPPH radical scavenging activity and the lowest polysaccharide content after macroporous resin was further separated by ultrafiltration through a 1,000 molecular weight cut-off (MWCO) membrane at 0.3 Mpa, 25°C. Two fractions exhibiting DPPH radical scavenging activity were pooled, lyophilized and named as S1 (MW<1 kDa) and S2 (MW>1 kDa), respectively.
Gel filtration chromatography: The fraction with the highest DPPH radical scavenging activity (S1) after ultrafiltration was dissolved in deionized water and loaded onto a Sephadex G-10 gel filtration column (1.4×25 cm) and then eluted with deionized water at a flow rate of 1.0 mL/min. Each fraction was pooled, lyophilized and measured for antioxidative activity in DPPH radical scavenging activity.
Reversed-phase high performance liquid chromatography (RP-HPLC): The fraction with the highest DPPH radical scavenging activity (S12) after gel filtration chromatography was dissolved in ultrapure water and separated by semi-preparing RP-HPLC (Waters, USA) on a Prep C-18 column (5 μm, 1.0 × 25 cm; X bridgeTM, USA) using a linear gradient of acetonitrile (10-90% in 20 min) at a flow rate of 3.0 mL/min. The elution peaks were monitored at 220 nm, and their DPPH radical scavenging activities were measured using the same method. The active peaks were concentrated and lyophilized.
Determination of amino acid sequence: The amino acid sequence and an accurate molecular mass of the purified peptides were determined using a MALDI-Q-TOF mass spectrometer (Waters, USA) coupled with an electrospray ionization (ESI) source [13].
Statistical analysis: All analyses were run in triplicate and averaged. A one-way analysis of variance (ANOVA) was conducted for the analysis of response values obtained by the RSM model. P<0.05 indicated statistical significance [33].
Results
Preparation of SPBPH
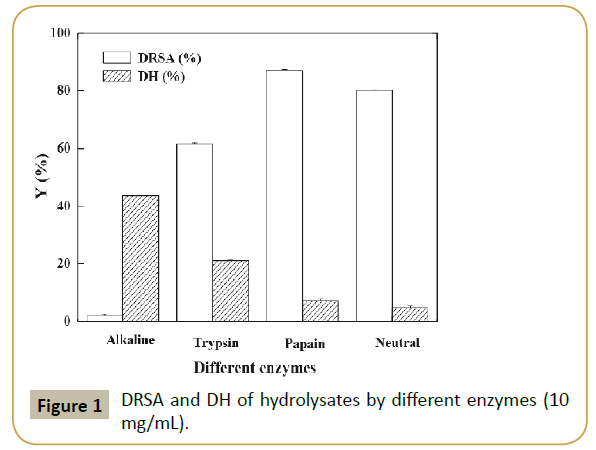
The hydrolysis of SPBP was carried out with four enzymes by pH-stat method for 150 min at optimal conditions for each enzyme. The results showed that the hydrolysis proceeded at a high rate during the initial 20 min and then slowed down. After 120 min, the procedure reached a steady state which indicated the hydrolysis was almost completed [33]. Therefore, the hydrolysis time was adopted as 120 min. In addition, DRSA of the resultant SPBPH were measured and the data were shown in Figure 1. It was shown that the DH of SPBP by alkaline protease was the highest, however, the resulting hydrolysate showed the lowest DRSA (near zero). The highest DRSA (87.04%) was observed in the papain hydrolysate and the DH of SPBP by papain was moderate. The DH reflects the percentage of peptide bonds cleaved by protease and peptides with proper degree of hydrolysis exerting important effects on their antioxidant abilities [34]. Nevertheless, DRSA was not necessarily boosted by the increase of DH. The objective of the present work was to optimize the enzymatic hydrolysis conditions for the highest antioxidant capacity of the hydrolysate; hence papain was adopted for hydrolysis.
Optimization of hydrolysis conditions by RSM
The optimal hydrolysis conditions of SPBP by papain were studied by RSM. Observed values for DRSA at different combinations of independent variables [35] are listed in Table 1. The regression equation for DRSA of SPBPH as function of three independent variables temperature, enzyme dose and pH (X1, X2 and X3) and their interactions was derived as follows:
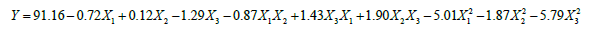
| Run no. | Temperature (X1) | Enzyme dose (X2) | pH (X3) | Y (%) | DH (%) |
|---|---|---|---|---|---|
| 1 | 50 | 5000 | 7.0 | 78.21 | 3.68 |
| 2 | 60 | 5000 | 6.0 | 79.65 | 3.19 |
| 3 | 55 | 6000 | 6.0 | 83.21 | 8.45 |
| 4 | 55 | 4000 | 7.0 | 80.02 | 4.0 |
| 5 | 55 | 6000 | 7.0 | 84.32 | 7.52 |
| 6 | 50 | 5000 | 6.0 | 83.57 | 2.0 |
| 7 | 60 | 6000 | 6.5 | 82.51 | 14.05 |
| 8 | 60 | 4000 | 6.5 | 84.34 | 4.47 |
| 9 | 55 | 5000 | 6.5 | 91.50 | 7.66 |
| 10 | 50 | 4000 | 6.5 | 84.33 | 7.66 |
| 11 | 50 | 6000 | 6.5 | 86.01 | 9.0 |
| 12 | 55 | 4000 | 6.0 | 86.47 | 6.76 |
| 13 | 55 | 5000 | 6.5 | 90.82 | 7.96 |
| 14 | 55 | 5000 | 6.5 | 91.15 | 8.14 |
| 15 | 60 | 5000 | 7.0 | 80.02 | 3.38 |
Table 1 Experimental design and results of response surface analysis.
The results for analysis of variance (ANOVA) for DRSA of SPBPH by papain demonstrated that the model is highly significant at 99% confidence level (P<0.0001), as shown in Table 2. The desirability value was close to 1 (R2 = 0.9974) and the model fitted the experimental data with an acceptable determination coefficient. Therefore, the results were suitable to obtain the optimum SPBPH with high DPPH radical scavenging activity.
| Factors | Coefficients | SS | df | MS | F | P-value | Level of significance |
|---|---|---|---|---|---|---|---|
| Model | - | 248.69 | 9 | 27.63 | 210.32 | <0.0001 | ** |
| Linear | |||||||
| X1 | -0.7 | 3.92 | 1 | 3.92 | 29.84 | 0.0023 | ** |
| X2 | 0.12 | 0.11 | 1 | 0.11 | 0.86 | 0.3966 | |
| X3 | -1.29 | 13.39 | 1 | 13.39 | 101.92 | 0.0002 | ** |
| Interactions | |||||||
| X1X2 | -0.89 | 3.06 | 1 | 3.06 | 23.31 | 0.0048 | ** |
| X1X3 | 1.43 | 8.24 | 1 | 8.24 | 62.7 | 0.0005 | ** |
| X2X3 | 1.9 | 14.4 | 1 | 14.4 | 109.62 | 0.0001 | ** |
| Quadratic | |||||||
| X12 | -5.01 | 92.66 | 1 | 92.66 | 705.31 | <0.0001 | ** |
| X22 | -1.87 | 12.94 | 1 | 12.94 | 98.5 | 0.0002 | ** |
| X32 | -5.79 | 123.66 | 1 | 123.66 | 941.23 | <0.0001 | ** |
| Lack of Fit | - | 0.43 | 3 | 0.14 | 1.23 | 0.4784 | |
| Residual | - | 0.66 | 5 | 0.13 | R2 0.9974 | ||
| Pure Error | - | 0.23 | 2 | 0.12 | Adj R2 0.9926 | ||
| Cor Total | - | 249.35 | 14 | - | |||
Table 2 Analysis of variance of Y regression equation (*P<0.05; **P<0.01; ***P<0.001).
The results of regression analysis showed that temperature and pH had a linear effect on DRSA values while as temperature, enzyme dose and pH had quadratic and interaction effect (P<0.01). For any term in the model, a larger regression coefficient and a smaller P-value would indicate a more significant effect on the respective response variables. For the DRSA in the model, pH value was the most significant factor on the DPPH radical scavenging activity.
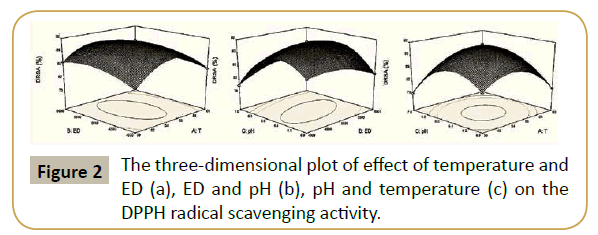
Response surface plot as a function of temperature and enzyme dose is depicted in Figure 2a. It was concluded that the increase in enzyme dose increased DPPH radical scavenging activity. This might be because more enzyme molecules could provide more chances for hydrolysis to occur. Figure 2b showed enzyme dose and pH had interaction effect on DRSA. It was found that decrease in pH with respect in enzyme dose increased DRSA to 87.05% and showed gradual decrease to 82.60% with increase in enzyme dose. Figure 2c shows response surface plot as function of pH and temperature. It was observed that, DRSA firstly increased, and then decreased with the increase of temperature, enzyme dosage and pH. The reason for the decrease of DRSA may due to denaturation and inactivation of enzymes at higher temperature and pH.
According to the data analysis, the highest DRSA of 91.27% was obtained at the following parameters: substrate concentration of 33 mg/mL, temperature at 55°C, enzyme dosage of 4900 U/g, pH 6.4 and hydrolysis time for 120 min. The statistic result was verified by carrying out the experiments at the optimum conditions in triplicate with experimental DRSA of 92.15% and DH of 7.92%, and the IC50 values of the DPPH radical scavenging activity was 1.65 mg/mL. Compared with other hydrolyzed peptides from animal sources in the literature, the DRSA of SPBPH was higher than egg white protein (71.12%) [36], Nemipterus japonicus muscle protein (45.30%) [37] and Sphyrna lewini muscle protein (21.76%) [38], but lower than reduced glutathione (95.42%), at the same concentration. The difference in DRSA may be because of the difference in animal species, difference in hydrolysis enzyme and other conditions. The hydrolysate obtained at the optimum parameters also exerted scavenging effect on hydroxyl radicals, superoxide radicals and reducing power with values of 48.88%, 93.45% and 0.436, respectively. The IC50 values of the superoxide anion radical and hydroxyl radical scavenging activities were 1.69 and 10.68 mg/mL, respectively. The superoxide anion radical scavenging activity was significantly higher than egg white protein (27.87%) [34], and Tilapia frame protein (58.50%) [39].
Isolation of antioxidant peptides and characterization of their antioxidativity
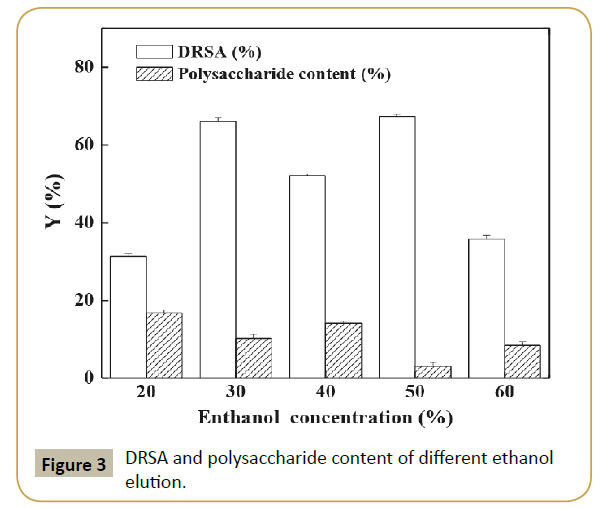
Macroporous resin: For the further purification of antioxidant peptides, it was necessary to removing polysaccharides (content was 17.35±1.03%) and gathering peptides by macroporous resin. Figure 3 shows that the elution with different concentration of ethanol had different DRSA and polysaccharide contents. Among them, 50% ethanol elution named as SPBPH-I possessed the highest DPPH radical scavenging activity (5 mg/mL, 67.34%) and the lowest polysaccharide content (3.17%). Therefore, SPBPH-I was selected for further study.
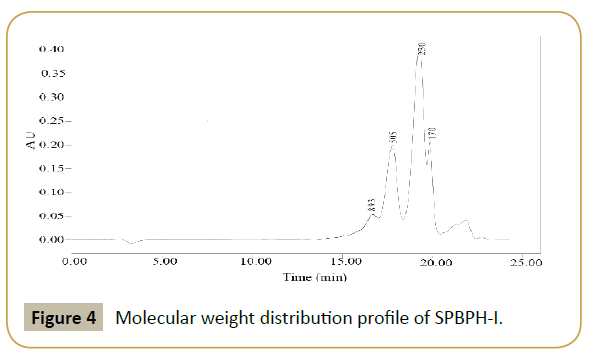
Ultrafiltration: Molecular weight is one of key factors of peptides’ bioactivity [39,40]. Using ultrafiltration to remove large peptides is reported to improve the bioactivities of the hydrolysates [41,42]. The distribution of molecular weight of SPBPH-I (Figure 4) showed that the MW of near 90% SPBPH-I was under 1 kDa, and the rest was over 10 kDa. To purify the antioxidative peptide, SPBPH-I was filtered through 1-K MWCO membrane. Two fractions were pooled, lyophilized and named as S1 (MW<1 kDa) and S2 (MW>1 kDa), respectively. The DRSA were 16.12%, 34.96% and 28.07% at 2 mg/mL for SPBPH-I, S1 and S2. Therefore, S1 was selected for further study.
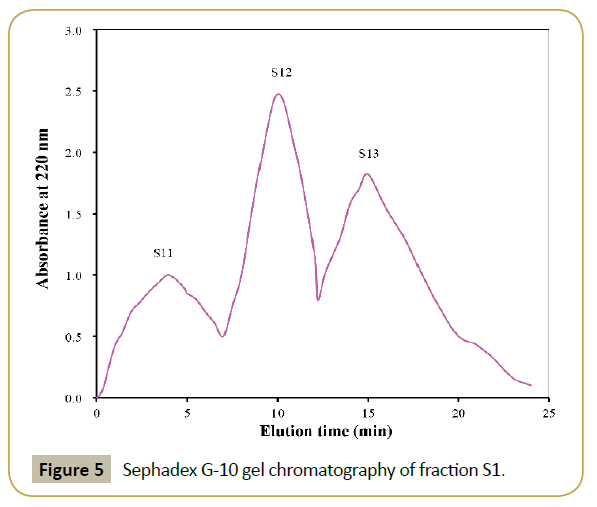
Gel filtration chromatography: The elution profile of S1 with size exclusion chromatography on Sephadex G-10 column was shown in Figure 5. Three fractions (designated as S11, S12 and S13) were separated, pooled and lyophilized. Their DRSA were 42.62%, 50.84% and 38.83% at 2 mg/mL, respectively. Fraction S12 exhibited the highest DRSA. Therefore, S12 was selected for next step.
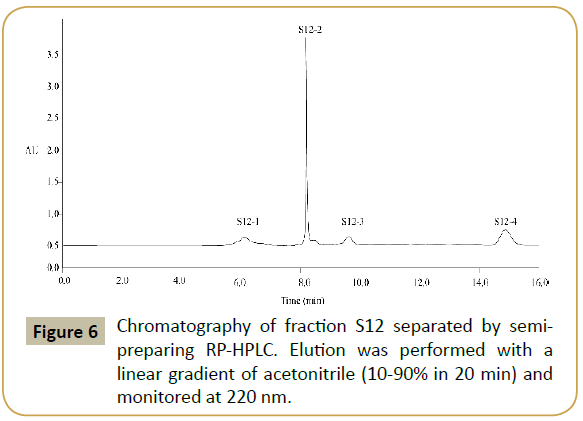
Reversed-phase high performance liquid chromatography: Fraction S12 was further separated by RP-HPLC and fractionated four potions (S12-1, S12-2, S12-3 and S12-4). The elution profile of peptides was shown in Figure 6. Each fraction was pooled, lyophilized, and measured for antioxidative activity in DPPH radical scavenging activity. It was observed that S12-1, S12-2, S12- 3 and S12-4 had varying degrees of scavenging ability, 10.23%, 32.52%, 19.48% and 22.10% at 50 μg/mL on DPPH radical. Among all fractions collected, fraction S12-2 exhibited the highest DPPH radical scavenging activity (IC50 value, 0.074 mg/mL) which was higher than that of glutathione (IC50 value, 0.18 mg/mL) as positive control.
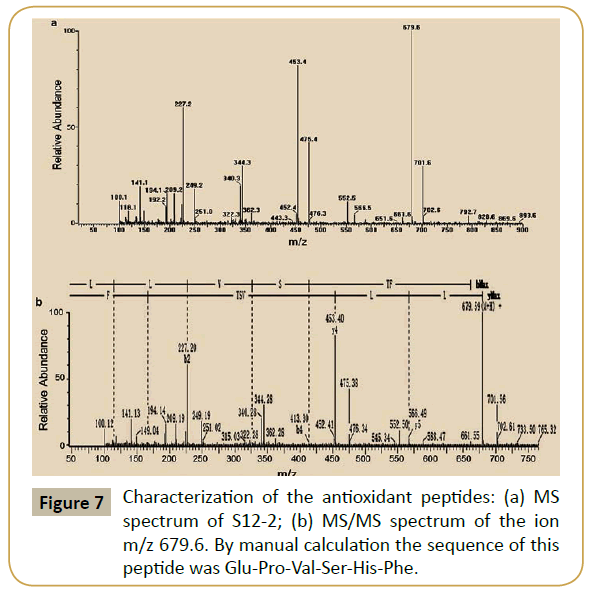
Characterization of purified peptides: The active fraction of S12-2 obtained after RP-HPLC was subjected MALDI-TOF-MS for sequencing peptide. As shown in Figure 7a, the molecular weight of the peptide was 679.6 Da. The amino acid sequence of the ion m/z 679.6 was determined, and the antioxidative peptide was composed of six amino acids, Glu-Pro-Val-Ser-His-Phe (Figure 7b).
Antioxidative peptides have possessed higher antioxidant properties than intact proteins and they can favor antioxidant cell capacity because, they can act as: i) A proton donor; ii) A radical scavenger; iii) A ferric-reducing capacity and or metal-ion chelator; iv) As a physical barrier that prevent ROS generation or their access to biological targets [43]. Food-derived peptides can also enhance antioxidant cell capacity by inducing the gene expression of proteins that are able to protect cellular components from oxidative stress-induced deterioration [44]. Antioxidant capacity of proteins and peptides has been reviewed by Elias et al. [45].
Many studies have been performed in the last two years to understand how the predominance of particular amino acids in the peptides, molecular weight as well as their hydrophobicity influences the antioxidative properties of the peptides [8]. It was reported that antioxidant peptides mostly contain below the 20 amino acid residues per molecule and the lower the molecular weight, the higher their chance to cross the intestinal barrier and exert biological effects [12]. The sequences of peptides have hydrophobic amino acid Pro that is expected to favor oxidation inhibition and promotes synergy with non-peptide antioxidants [2]. The pyrrolidine ring of Pro tends to interrupt the secondary structure of the peptide imposing conformational constraints. Hydrophobic amino acid residue Val is contribute to inhibit lipid peroxidation by increasing solubility of peptides in lipid phase and thereby facilitating better interaction with radical species; the presence of peptides in the water-lipid interface facilitates the scavenging of generate free radicals [46]. Hydrophobic amino acid residue Phe is able to scavenge hydroxyl radicals to form more stable para-, meta- or ortho-substituted hydroxylated derivatives and the rings enable the chelation of pro-oxidant metal ions [2]. Additionally, His have been suggested to play an important role in radical scavenging activity due to the imidazole group. The imidazole ring of His tends to participate in hydrogen atom and single electron transfer reactions in order to neutralize free radicals or bind metal ions [45]. Glu has electron-donating ability that enables the scavenging radicals and ferric reducing capacity. Glu can also act as metal-ion binders that limit the propagation of lipid peroxidation caused by transitional metal ions and change the redox cycling capacity [8]. Thus, it could be presumed that the observed higher radical scavenging activity of the purified peptide could be attributed to the presence of these amino acids.
Discussion
In this study, sheep placenta by-product protein was hydrolyzed using papain and RSM was used to determine optimal hydrolysis conditions for obtaining antioxidant peptide. It was shown that the second-order polynomial model was sufficient to well describe and predict the responses of DRSA of hydrolysate. The suggested hydrolysis conditions was found as substrate concentration of 33 mg/mL, pH 6.4, temperature 55°C, enzyme dosage 4900 U/g, and time 120 min. Moreover, the peptide purified from sheep placenta by-product protein hydrolysates has important amino acids with potency to scavenge free radical and could to be used as natural antioxidants in functional foods or nutraceuticals. However, further detailed clinical research studies on the purified peptides are needed in regard of in vivo antioxidant activities.
References
- Guelcin I (2009) Antioxidant activity of L-adrenaline: a structure-activity insight. Chem Biol Interact 179: 71-80.
- Moskovitz J, Yim MB, Chock PB (2002) Free radicals and disease. Arch Biochem Biophys 397: 354-359.
- Lee HP, Zhu XW, Casadesus G, Castellani RJ, Nunomura A, et al. (2010) Antioxidant approaches for the treatment of Alzheimer's disease. Expert Rev Neurother 10: 1201-1208.
- Pantavos A, Ruiter R, Feskens EF, de Keyser CE, Hofman A, et al. (2015) Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: the Rotterdam study. Int J Cancer 136: 2178-2186.
- Halliwell B, Murcia MA, Chirico S, Aruoma OI (1995) Free-radicals and antioxidants in food and in-vivo-what they do and how they work. Crit Rev Food Sci Nutr 35: 7-20.
- Barlow S, Schlatter J (2010) Risk assessment of carcinogens in food. Toxicol Appl Pharm 243: 180-190.
- Kaur C, Kapoor HC (2001) Antioxidants in fruits and vegetables-the millennium's health. Int J Food Sci Technol 36: 703-725.
- Mine Y, Li-Chan ECY, Jiang B (2010) Bioactive proteins and peptides as functional foods and nutraceuticals. Wiley Blackwell Publishing, Hoboken, pp: 5-11.
- Erdmann K, Cheung BWY, Schroder H (2008) The possible roles of food derived bioactive peptides in reducing the risk of cardiovascular diseases. J Nutr Biochem 19: 643-654.
- Vercruysse L, Van Camp J, Smagghe G (2005) ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: a review. J Agric Food Chem 53: 8106-8115.
- Hook VY, Burton D, Yasothornsrikul S, Hastings RH, Deftos LJ (2001) Proteolysis of ProPTHrP (1–141) by ‘‘prohormone Thiol Protease’’ at multibasic residues generates PTHrP-related peptides: implications for PTHrP peptide production in lung cancer cells. Biochem Biophys Res Commun 285: 932-938.
- Sun YY, Pan DD, Guo YX, Li JJ (2012) Purification of chicken breast protein hydrolysate and analysis of its antioxidant activity. Food Chem Toxicol 50: 3397-3404.
- Zhang YF, Duan X, Zhuang YL (2012) Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 38: 13-21.
- Di Bernardini R, Mullen AM, Bolton D, Kerry J, O'Neill E, et al. (2012) Assessment of the angiotensin-I-converting enzyme (ACE-I) inhibitory and antioxidant activities of hydrolysates of bovine brisket sarcoplasmic proteins produced by papain and characterisation of associated bioactive peptidic fractions. Meat Sci 90: 226-235.
- Chang OK, Ha GE, Han GS, Seol KH, Kim HW, et al. (2013) Novel antioxidant peptide derived from the ultrafiltrate of ovomucin hydrolysate. J Agr Food Chem 61: 7294-7300.
- Chou CH, Wang SY, Lin YT, Chen YC (2014) Antioxidant activities of chicken liver hydrolysates by pepsin treatment. Int J Food Sci Technol 49: 1654-1662.
- Lee SJ, Kim YS, Hwang JW, Kim EK, Moon SH, et al. (2012) Purification and characterization of a novel antioxidative peptide from duck skin by-products that protects liver against oxidative damage. Food Res Int 49: 285-295.
- Tanzadehpanah H, Asoodeh A, Chamani J (2012) An antioxidant peptide derived from Ostrich (Struthio camelus) egg white protein hydrolysates. Food Res Int 49: 105-111.
- Moure A, Dominguez H, Parajo JC (2006) Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem 41: 447-456.
- Chen HM, Muramoto K, Yamauchi F, Nokihara K (1996) Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J Agr Food Chem 44: 2619-2623.
- Sarmadia BH, Ismaila A (2010) Antioxidative peptides from food proteins: a review. Peptides 31: 1949-1956.
- EI Amiri B, Remy B, Sousa NM, Joris B, Ottiers G, et al. (2003) Isolation and partial characterization of three pregnancy-associated glycoproteins from the ewe placenta. Mol Reprod Dev 64: 199-206.
- Onuaguluchi G, Ghasi S (1996) The pharmacological basis for the use of dried sheep placenta in traditional obstetric practice in Nigeria. J Ethnopharmacol 54: 27-36.
- Liu LX, Ren XH, Tang HJ, Tang ZY, He CM, et al. (2013) Separation and purification of small-molecule peptides with immunomodulating activity from goat placenta. Chinese Food Sci 34: 273-276.
- Zhu BW, Ge RH (2003) Preparation of active peptide from sheep placenta by bienzyme hydrolysis. J Chinese Food Ferment Indust 29: 41-45.
- Teng DK, Fang Y, Song XY, Gao YX (2011) Optimization of enzymatic hydrolysis parameters for antioxidant capacity of peptide from goat placenta. Food and Bioproducts Processing 89: 202-208.
- Hou YC, Zhou JJ, Liu WW, Cheng YX, Wu L, et al. (2015) Fermentation process and bioactivity of peptides prepared from fermented goat placenta residues. Transactions of the Chinese Society of Agricultural Engineering 31: 311-316.
- Adler-Nissen J (1986) Enzymic hydrolysis of food proteins. Elsevier, pp: 39-41.
- Nanjo F, Goto K, Seto R, Suzuki M, Sakai M, et al. (1996) Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic Bio Med 21: 895-902.
- Jin M, Cai YX, Li JR, Zhao H (1996) 1,10-phenanthroline-Fe2+ oxidative assay of hydroxyl radical produced by H2O2/Fe2+. Prog Biochem Biophys 23: 553-555.
- Xie ZJ, Huang JR, Xu XM, Jin ZY (2008) Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem 111: 370-376.
- Wu HC, Chen HM, Shiau CY (2003) Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res Int 36: 949-957.
- Naqash SY, Nazeer RA (2012) Optimization of enzymatic hydrolysis conditions for the production of antioxidant peptides from muscles of Nemipterus japonicus and Exocoetus volitans using response surface methodology. Amino Acids 43: 337-345.
- Li B, Chen F, Wang X, Ji BP, Wu YN (2007) Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization mass spectrometry. Food Chem 102: 1135-1143.
- Wijesinghe WA, Jeon YJ (2012) Enzyme-assistant extraction (EAE) of bioactive components: a useful approach for recovery of industrially important metabolites from seaweeds: A review. Fitoterapia 83: 6-12.
- Chen C, Chi YJ, Zhao MY, Lv L (2012) Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids 43: 457-466.
- Wang B, Li ZR, Chi CF, Zhang QH, Luo HY (2012) Preparation and evaluation of antioxidant peptides from ethanol-soluble proteins hydrolysate of Sphyrna lewini muscle. Peptides 36: 240-250.
- Fan J, He JT, Zhuang YL, Sun LP (2012) Purification and identification of antioxidant peptides from enzymatic hydrolysates of Tilapia (Oreochromis niloticus) frame protein. Molecules 17: 12836-12850.
- Guo H, Kouzuma Y, Yonekura M (2009) Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem 113: 238-245.
- You SJ, Wu JP (2011) Angiotensin-I converting enzyme inhibitory and antioxidant activities of egg protein hydrolysates produced with gastrointestinal and nongastrointestinal Enzymes. J Food Sci 76: 801-807.
- Davalos A, Miguel M, Bartolome B, Lopez-Fandino R (2004) Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J Food Prot 67: 1939-1944.
- Mendis E, Rajapakse N, Kim SK (2005) Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J Agr Food Chem 53: 581-587.
- Freitas AC, Andrade JC, Silva FM, Rocha-Santos TAP, Duarte AC, et al. (2013) Antioxidative peptides: trends and perspectives for future research. Curr Med Chem 20: 4575-4594.
- Udenigwe CC, Aluko RE (2012) Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci 77: 11-24.
- Elias RJ, Kellerby SS, Decker EA (2008) Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr 48: 430-441.
- Ranathunga S, Rajapakse N, Kim SK (2006) Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur Food Res Technol 222: 310-315.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences