ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
Enumeration of Endophytic Fungi in Irvingia gabonensis from Oban Division of The Cross River National Park, Nigeria
Ndibukke E.O*, Chukunda F. A., Ukoima N. H
Department of Forestry and Environment, Rivers State University, Nkpolu-Oroworukwo, Nigeria
Abstract
Endophhytic fungi in Irvingia gabonensis (Aubrey-Lecomte ex O. Rorke) Baill growing at Akamkpa and Oban, Cross River National Park, Nigeria were studied. Barks and leaves of the plant were collected in the months of February - March and June - September, 2019 for the dry and wet seasons sampling respectively. 384 pieces of barks and leaves of the plant were cultured on Potato Dextrose Agar (PDA). A total of 228 isolates belonging to 11 species of endophytic fungi; Hyphomycetes 10 and Zygomycetes 1 was isolated. The colonisation frequency of the leaves was 64.58% for both Akamkpa and Oban, while that of the bark was 48.96% and 59.38% for Akamkpa and Oban respectively. Acremonium sp colonized both bark and leaves more in the wet season in both locations. The presence of Acremonium spp in bark was lower when compared with their level in leaves especially in Akamkpa which was similar (P<0.05) to the level obtained in the wet season. Verticillium sp colonized only leaves in both locations but could not record any tangible (P<0.05) presence in bark in both locations. Rhizopus stolonifer colonized the bark of the plant highest in the wet season in Akamkpa. This was significantly (P<0.05) higher than those in both seasons in Oban and wet season on leaves in Oban. Geotrichum candidum could only colonize the bark significantly (P<0.05) in the wet season in Oban. Its presence during dry season was lower but not significantly (P>0.05) below the values obtained during the wet season in the same location. Simpson’s diversity index seemed high between 0.7567 and 0.8371. The ecological indices seem to suggest that I. gabonensis supports a diverse endophytic fungi assemblage in both locations.
Keywords
Endophytic fungi; Irvingia gabonensis; Oban; Akamkpa; Acremonium sp.
Introduction
The word "endophyte" refers to any plant that resides temporarily or permanently inside the living tissues of another plant. It was first used by de Bary [1]. The term is kept for the diverse group of fungi which live asymptomatic within photosynthetic tissue of plant for the all or some part their life cycle [2]. Endophytic fungi infect and live asymptomatically mostly in the aerial tissues of the host plant and are hyper diverse [3-5]. Recent studies show that endophytic fungi are found everywhere, from the tropics, temperate, xerophytic and aquatic environments [6-9]. Endophytic fungi are in symbiotic relationships with their hosts where the endophytes obtain nutrients and protection from the hosts, but contribute to effective host defense machinery against pathogens, herbivores or abiotic stress [10].
Endophytic fungi are an important source of bioactive molecules. They are probably one of the major potential sources for new, useful metabolites [11]. These bioactive metabolites have a broad range of biological activities and could be the starting materials for pharmaceuticals or lead structures for the development of pharmaceuticals [12]. A lot of bioactive compounds produced by endophytic fungi contain antimycotic, anticancer agents, antibiotics, insecticides and antiviral compounds [13,14].
Irvingia gabonensis (Aubrey-Lecomte ex O. Rorke) Baill, commonly called Wild Mango or African Mango, is a tree that can grow to a height of 15-40 metres. It is native to West Africa where it grows wild in the lowland equatorial forest of Africa [15]. The fruit pulp is edible and can be used for a fruit drink and for jam production; its kernel can be processed into flour by extraction, drying and grinding, the pounded seed is added to meat and various vegetable dishes as a sauce. Margarine and cooking oil can be obtained from the kernels [15].
I. gabonensis seed has gained some popularity as a weight loss supplement in the U.S. in recent years, though the chemical composition of the seeds has rarely been analysed [16]. The dietary fibre of the seed of I. gabonensis like other forms of water-soluble dietary fibres, are "bulk forming" laxatives. I. gabonensis seeds delay stomach emptying, leading to a more gradual absorption of dietary sugar. This effect can reduce the elevation of blood sugar levels that is common after a meal [17]. In traditional African medicine, I. gabonensis has been used as a treatment for a wide range of conditions like: diabetes, dysentery, bleeding, liver problems, and as an antimicrobial and analgesic [18]. This study was to enumerate the endophytic fungi found in Irvingia gabonensis growing at Akamkpa and Oban in the Cross River National Park, Cross River State, Nigeria.
Materials and Methods
Study Site
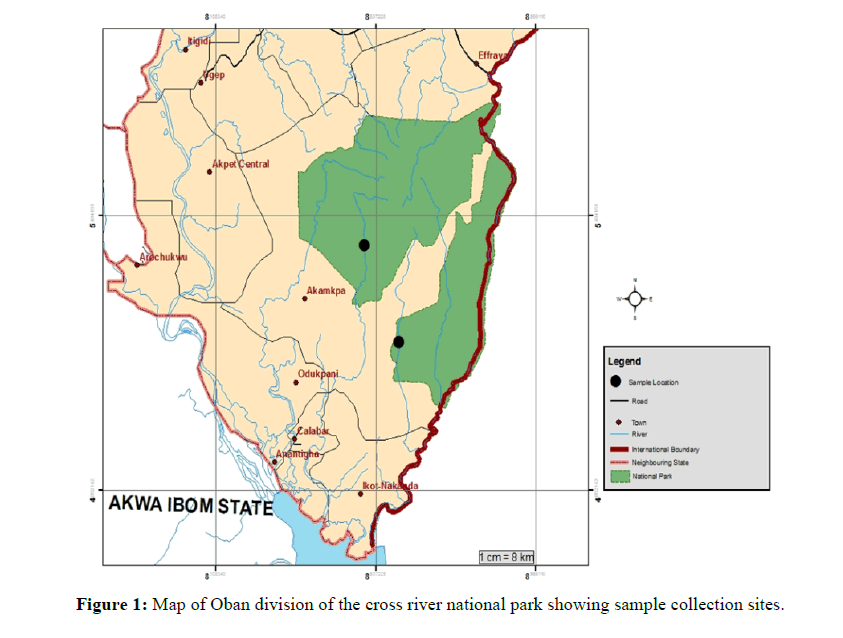
The study involved collecting healthy leaves and barks of I. gabonensis from Erokut (Latitude 5⁰ 21' 13.74264ʺ E and Longitude 8⁰ 22' 9.737764ʺ N) in Akamkpa (Oban West) and Aking (Latitude 5⁰ 26' 26ʺN and Longitude 8⁰ 38'.96456"E) in Oban (Oban East) in the Oban Division of the Cross River National Park, Nigeria. The leaves and barks were collected by convenience sampling from four I. gabonensis trees each from Erokut in Akamkpa and Aking in Oban.
Sample Collection
Using ethanol disinfected knives, 10 each of bark tissues and asymptomatic leaves were collected in the months of February - March and June - September, 2019 for the dry and wet seasons sampling respectively, from each of the four selected trees. Sampling was done twice in the dry season and twice in the wet season (Figure 1).
The barks and leaves were collected and put in well labeled sterile polyethylene bags and taken in an ice box to the laboratory for isolation of endophytic fungi within forty eight hours. Four 1 cm2 pieces were made from each of ten leaves, yielding a composite of forty (40) pieces. The same process was done with the barks to yield 40 pieces of barks. To eliminate epiphytic microorganisms, all the samples were surface sterilized by dipping in ethanol 70% for 3 min, followed by a solution of sodium hypochlorite (4% available chlorine) for 3 min and then rinsed in ethanol 70% for 2-5 sec, before a final rinse in sterilized double distilled water. Samples were then allowed to surface dry under sterile conditions [19]. Six segments each of leaves and barks from Akamkpa and Oban were plated on each Petri dish. Four Petri dishes were cultured for each plant part, during each round of sampling. This yielded 384 surface sterilized segments that were plated on Petri-dishes containing Potato Dextrose Agar (PDA) amended with Chloramphenicol 150 mg/l.
The Petri dishes were then incubated at 27 ± 2⁰C. The plates were screened on a routine basis and hyphal tips that grew out from the tissues were subsequently transferred onto fresh PDA plates for pure cultures and onto slants. For characterization of the morphology of fungal isolates, slides prepared from cultures were stained with lacto phenol in cotton blue and examined with a compound light microscope. The endophytic fungi were identified according to their macroscopic and microscopic characteristics such as the morphology of fruiting structures and spore, surface texture, margin character, aerial mycelium, mechanism of spore production and characteristics of the spore [20]. Colonies were counted and grouped by their morphological characteristics, and representative isolates of fungal diversity were collected, purified and preserved for future analysis.
Statistical Analysis
The endophytic fungal isolates from I. gabonensis explants were analyzed based on the percentage of density of colonization (colonization frequency) 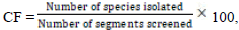
Relative percentage occurrence of different groups of fungi,  and
and
Percentage of endophytic infection rate (EIR), EIR 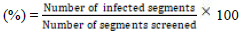 [21-23].
[21-23].
The diversity indices; Simpson’s Diversity index D, Shannon-Wiener Diversity index, H; Shannon’s Equitability Index, EH; Species richness, D and Jaccard’s Similarity Index were calculated Shannon-Wiener Diversity Index, H= - Σ (Pi × ln Pi),
Simpson’s Diversity index = 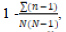
Shannon’s Equitability Index, 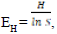
Species richness, 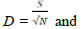
Jaccard’s Similarity Index = 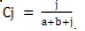
The experiment was laid out in a Randomised Complete Block Design (RCBD). Data generated was subjected to analysis of variance (ANOVA) by SPSS [24-26].
Results
A total of 228 isolates were recovered from 384 plant parts (leaves and barks) of I. gabonensis from Akamkpa and Oban in Oban Division of the Cross River National Park, Nigeria during the dry (February-March) and wet (June- September) seasons of 2019, as can be seen from Table 1. 11 species of endophytic fungi were isolated; Hyphomycetes 10 and Zygomycetes 1.
| S/N | Endophytic Fungi Species | Akamkpa | Oban | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaves (96) | Barks (96) | Leaves (96) | Barks (96) | |||||||
| NOI | CF% | NOI | CF% | NOI | CF% | NOI | CF% | |||
| 1 | Acremonium sp | 19 | 19.79 | 19 | 19.79 | 13 | 13.54 | 16 | 16.67 | |
| 2 | Altenaria sp. | 2 | 2.08 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | Aspergillius niger | 5 | 5.21 | 2 | 2.08 | 10 | 10.42 | 3 | 3.13 | |
| 4 | Aspergillius parasiticus | 0 | 0 | 4 | 4.17 | 0 | 0 | 7 | 7.29 | |
| 5 | Epicoccum sp. | 5 | 5.21 | 0 | 0 | 5 | 5.21 | 0 | 0 | |
| 6 | Fusarium sp. | 2 | 2.08 | 2 | 2.08 | 0 | 0 | 0 | 0 | |
| 7 | Geotrichum candidum | 2 | 2.08 | 0 | 0 | 3 | 3.13 | 11 | 11.46 | |
| 8 | Penicillium sp.1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | |
| 9 | Penicillium sp. 2 | 1 | 1 | 5 | 5.21 | 0 | 0 | 4 | 4.17 | |
| 10 | Rhizopus stolonifer | 4 | 4.17 | 12 | 12.5 | 11 | 11.46 | 10 | 10.42 | |
| 11 | Verticillium sp. | 22 | 22.92 | 3 | 3.13 | 19 | 19.79 | 5 | 5.21 | |
| 62 | 64.58% | 47 | 48.96% | 62 | 64.58% | 57 | 59.38% | |||
*NOI= Number of Isolates; +CF% = Colonization Frequency
Table 1: Colonisation frequency of endophytic fungi isolated from irvingia gabonensis growing at Akamkpa and Oban.
Acremonium sp., colonies typically grow gradually, often compacted and humid at first, becoming powdery, suedelike or floccose as they age. They may be white, grey, pink, rose or orange in colour. The hyphae are filamentous, septate and hyaline and produce mostly simple awl-shaped erect phialides bearing one-celled conidia on slender conidiophores.
Alternaria alternate,, Colonies are black greyish in colour, fast growing, and filamentous soma. The hyphae are septate, with long often branched ellipsoidal conidia in chains with septate conidiophores. Microscopically, branched acropetal chains (blastocatenate) of multicellular conidia (dictyoconidia) are produced from simple, branched, conidiophores. Conidia are ellipsoidal (Figure 2).
Aspergillus niger, this is one of the commonest and easily recognizable species of the genus Aspergillus. Colonies are dense white to black when they begin to produce spores, and grow moderately. The soma are filamentous, hyphae are septate with cylindrical asexual spores. The conidiophores are smooth walled and erect, with globose conidial heads, the vesicle are equally globose. Conidiophores dark, mostly simple; determinate or sympodial, rather short or elongate; conidia (porospores) dark, typically with both cross and longitudinal septa; variously shaped, obclavate to elliptical or ovoid, frequently borne acropetally in apical simple or branched appendage.
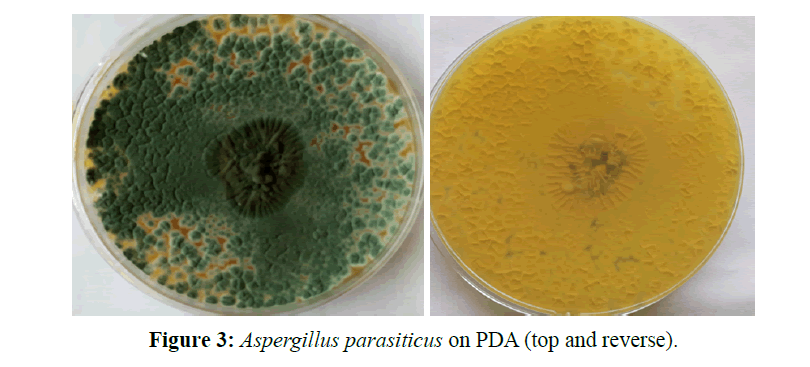
Aspergillus parasiticus, this one is a member of the Aspergillus complex. The conidiophores are upright, simple, terminating in a globose or clavate swelling, bearing phialides at the apex or radiating from the apex or the entire surface; conidia (phialospores) 1-celled, globose, often variously colored in mass, in dry basipetal chains. The conidia are rough and thick walled and spherical in shape, with short conidiophores and small vesicles to which the phialides are directly attached. The colonies are dark green in colour (Figure 3).
Epicoccum sp., the sporodachia are dark, more or less club-shaped, with short, dark and compact conodiophores. The conidia are short, dark and dictysporous (with several cells).
Fusarium sp., the mycelia are extensive and white in colour and yellow coloration on the reverse side of the plate. The conidiophores are inconstant and simple having a spiral of philiades. The conidia are hyaline (Figure 4).
Geotrichum candidum, has white smooth colonies with dichotomously branched hyphae bearing artrosporous cylindrical hyaline spores without conidiophores.
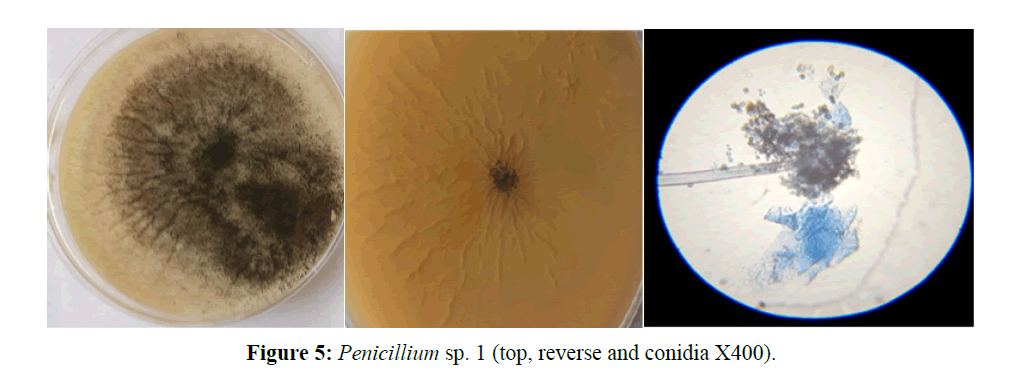
Penicillium sp.1, colony growth is moderate, dense white to green in colour and the reverse side was off white. Hyphae are septate. Has long erecting conidiophores arising singly from the mycelium terminating in a whorl of phialides, bearing hyaline, globose conidia (Figure 5).
Penicillium sp. 2, colonies are fast growing, green in colour with the reverse side off white. Has filamentous, septate, brown like hyphae produced in columns. Conidiophores are dense and bear single celled, globose conidia which are produced basipetally.
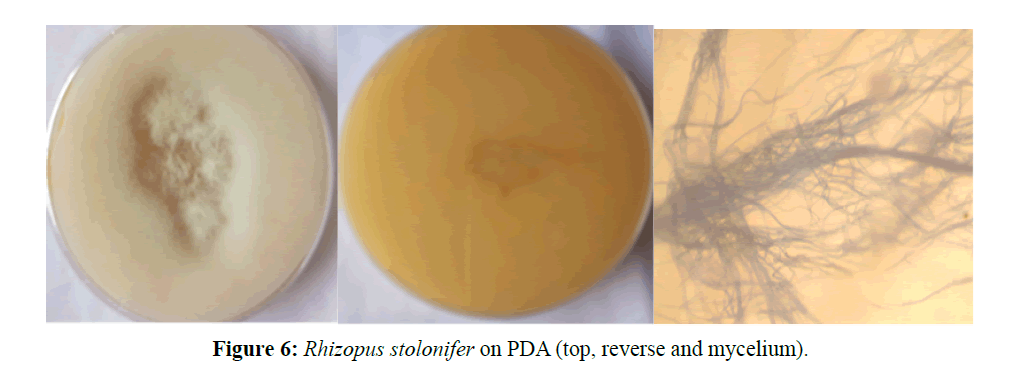
Rhozipus stolonifer, colony is white in colour with filamentous soma and coenocytic hyphae bearing stolons and rhizoids. Have tall sporangiophores in groups, each bearing black brownish, ovoid sporangiopores (Figure 6).
Verticillium sp., colony is cottony white, soma filamentous with septate hyphae. Conidia are one-celled and cylindrical. Philides are solitary without chlamydospores.
Table 1 presents the Colonization Frequency (CF) of endophytic fungi isolated from the leaves and barks of I. gabonensis collected from Akamkpa and Oban. For Akamkpa there were 62 isolates from the leaves giving a colonisation frequency of 64.58%, while the barks yielded 47 isolates giving a colonization frequency of 48.96%. I. gabonensis leaves from Oban yield 62 isolates of endophytic fungi too giving a colonization frequency of 64.58% too, while the barks gave 57 isolates with a colonization frequency of 59.38%. Acremonium sp was the most abundant with 67 isolates approximately.
The Endophytic Infection Rates (EIR) of endophytic fungi isolated from the leaves and barks of I. gabonensis are presented in Table 2. The following endophytic fungi had the highest infection rates in decreasing order; Verticillium sp 28.13% for leaves from Oban, 26.04% for leaves from Akamkpa, Acremonium sp 26.04% for leaves from Akamkpa, 19.79 for barks from Akamkpa and 16.67% for barks from Oban. Others are Aspergillus niger 16.67% for leaves from Oban; Rhizopus stolonifer 12.5% for leaves from Akamkpa and Oban each; Epicoccum sp 11.46% for leaves from Akamkpa.
| S/No | Endophytic Fungi Species |
Akamkpa | Oban | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Barks | Leaves | Barks | ||||||||||
| S1 | I2 | EIR3% | S1 | I2 | EIR3% | S1 | I2 | EIR3% | S1 | I2 | EIR3% | ||
| 1 | Acremonium sp | 96 | 25 | 26.04 | 96 | 19 | 19.79 | 96 | 10 | 10.4 | 96 | 16 | 16.67 |
| 2 | Alternaria sp | 96 | 2 | 2.08 | 96 | 0 | 0 | 96 | 0 | 0 | 96 | 0 | 0 |
| 3 | Aspergillus niger | 96 | 7 | 7.29 | 96 | 2 | 2.08 | 96 | 16. | 16.67 | 96 | 3 | 3.13 |
| 4 | Aspergillus parasiticus | 96 | 2 | 2.08 | 96 | 4 | 4.17 | 96 | 0 | 0 | 96 | 7 | 7.29 |
| 5 | Epicoccum sp. | 96 | 11 | 11.46 | 96 | 0 | 0 | 96 | 9 | 9.38 | 96 | 0 | 0 |
| 6 | Fusarium sp. | 96 | 2 | 2.08 | 96 | 2 | 2.08 | 96 | 0 | 0 | 96 | 0 | 0 |
| 7 | Geotrichum candidum | 96 | 2 | 2.08 | 96 | 0 | 0 | 96 | 9 | 9.38 | 96 | 11 | 11.46 |
| 8 | Penicillium sp. 1 | 96 | 0 | 0 | 96 | 0 | 0 | 96 | 1 | 1.04 | 96 | 1 | 1.04 |
| 9 | Penicillium sp.2 | 96 | 0 | 0 | 5 | 5.21 | 0 | 96 | 0 | 0 | 96 | 4 | 4.17 |
| 10 | Rhizopus stolonjifer | 96 | 8 | 8.33 | 96 | 12 | 12.5 | 96 | 12 | 12.5 | 96 | 10 | 10.42 |
| 11 | Verticillium sp. | 96 | 25 | 26.04 | 96 | 3 | 3.13 | 96 | 27 | 28.13 | 96 | 5 | 5.21 |
| - | 84 | - | - | 57 | - | - | 86 | - | - | 64 | - | ||
S1 = No of segments screened; I2 = No of isolates; EIR3 = Endophytic Infection Rates
Table 2: Endophytic infection rates of endophytic fungi in I. gabonensis from Akamkpa and Oban.
The relative percentage of occurrence of the different groups of fungi is given in Figure 7. The Hypomycetes dominated in leaves from Akamkpa 93.55% to 74.47% in the barks from Akamkpa. Zygomycetes ranged from 25.53% in the barks from Akamkpa to 6.45% in the leaves. Saccaromycets were not isolated.
The ecological indices are presented in Table 3. Simpson’s diversity index D for barks from Oban was 0.8371, followed by 0.8086 for leaves from still Oban; the values for leaves and barks from Akamkpa were slightly lower at 0.7721 and 0.7567 respectively. Shannon Wiener diversity index was highest, 1.8628 in the barks from Oban followed by 1.7119 for the leaves from Akamkpa. The index was 1.7072 and 1.6071 for the barks got from Akamkpa and leaves from Oban repectively. The Jaccard similarity index Cj for the barks from Akamkpa and Oban 0.27 while that for leaves from the two locations was also 0.27. The Shannon-Wiener equitability index EH ranged from 0.8958 for barks from Oban to 0.7788 for leaves from Akamkpa. Species richness D was higher in Akamkpa than Oban. The values for the leaves and barks were 1.14 and 1.02 respectively, while for Oban 0.8958 and 0.8259 for the barks and leaves respectively.
| Activity | Akamkpa | Oban | ||||
|---|---|---|---|---|---|---|
| Leaves | Barks | Total | Leaves | Barks | Total | |
| Number of segments screened | 96 | 96 | 192 | 96 | 96 | 192 |
| Number of segments colonized by fungi | 60 | 47 | 107 | 62 | 57 | 119 |
| Total number of fungal species | 9 | 7 | - | 7 | 8 | - |
| Total number of fungal isolates | 62 | 47 | - | 62 | 57 | - |
| Colonisation frequency (CF) % | 64.58 | 48.96 | - | 64.58 | 59.38 | - |
| Simpson’s diversity index D | 0.7721 | 0.7567 | - | 0.8086 | 0.8371 | - |
| Jaccard Similarity Index Cj | 0.27 | 0.29 | - | 0.27 | 0.29 | - |
| Shannon-Wiener Equitability index EH | 0.7788 | 0.8259 | - | 0.8210 | 0.8958 | - |
| Species Richness D | 1.14 | 1.02 | - | 0.96 | 0.93 | - |
Table 3: Ecological Indices of Endophytic Fungi from I. gabonensis from Akamkpa and Oban.
Discussion
Endophytic fungi have been found to exist in every plant species examined. While some have been shown to been beneficial to their hosts, some tend to be latent parasites. 11 species of endophytic fungi were recovered during this study, 10 Hyphomycetes and 1 Zygomycete. This is in agreement with Arnold et al. that endophytic fungi assemblages found in tropical tree species are hyperdiverse [5].
The result derived from this study showed no significant differences (P>0.05) between endophytic fungi frequencies in the wet and dry seasons, though endophytic fungal species were consistently higher in the wet season than the dry season. During the wet season the large surface area of leaves tends to provide a better chance for the fungal propagules in the air to shelter hence a higher chance of infecting the internal tissues, as indicated below.
Acremonium sp. with 67 isolates from both the leaves and barks from both locations had the highest colonization frequency. Acremonium sp. colonized both barks and leaves more in the wet seasons in both locations. The presence of Acremonium sp. on bark was lower when compared with their level in the leaves especially in Akamkpa which was similar (P<0.05) to the level obtained in the wet season.
Verticillium sp. was the next in abundance with 49 isolates mostly from the leaves. It colonized mainly leaves, 41 isolates compared with 8 from the barks from both locations; thereby being significantly (P<0.05) more present in the leaves than the barks.
There were 37 isolates of Rhizopus stolonifer, with 22 coming from the barks and 15 from the leaves. Barks from Akamkpa had significantly (P<0.05) more isolates than the leaves and in the wet season than the dry.
Aspergillus niger was next in abundance with 20 isolates, mostly from the leaves 15. Of the fifteen, 10 were obtained during the wet season in both locations, hence a significant (P<0.05) presence in the leaves.
There were 10 isolates of Epicoccum sp. from the leaves alone throughout the study, evenly distributed between the two locations. The species was not isolated from the bark.
Geotrichum candidum could only colonize the bark of I. gabonensis significantly (P<0.05) in the wet season only in Oban. Its presence during dry season was lower but not significantly (P>0.05) below the values obtained during the wet season in the same location. This organism was completely absent in leaves of the plant in both Oban and Akamkpa, and in barks of in Akamkpa.
A. parsiticus was isolated from the barks of I. gabonensis in both Akamkpa and Oban, it did not record any presence in leaves of the plat in both locations across the two seasons under consideration.
Penicillium sp. 2 was next in abundance with 10 isolates which mostly occurred in the barks from both locations.
Fusarium sp. was only present at very minimal and insignificant level on both barks and leaves of of the plant only in Akamkpa. There was no presence of this organism in Oban.
Alternaria sp. was barely present 2 throughout the study. Penicillium sp. 1 had the least presence of 1 isolate each from the leaves and barks from Oban alone. The result from this study is in tandem with the report of Arnold et al. about endophytic fungi being hyperdiversive [5]. The colonization frequency of endophytes reported here were well within that reported by [27]. The colonization frequencies were consistently higher in the leaves than the barks. It was 62% each for Akamkpa and Oban while for the barks Oban had a value of 57% and Akamkpa 47%. Few studies have quantified endophyte colonisation patterns in tropical plants. However, among tropical host species studied, proportions of leaf segments colonised in individual leaves appear to differ widely. In this study, the colonization frequency recorded ranged from 48.96% in I. gabonensis barks from Akamkpa to 64.58% in leaves from Akamkpa and Oban. This seems to agree with Rodrigues and Petrini, that leaves are the sites for photosynthesis, hence a higher probability of the presence of more of the needed carbon and other essential nutrients [28].
The Endophytic Infection Rates (EIR) ranged between 8.13% and 8.33% for some species and as low as 2.08% to zero. The Shannon-Wiener diversity index H commonly runs from 1.5 to 3.5 and it takes into accounts both abundance and evenness of species. The equally-common species required to give a particular value of an index is called the "effective number of species" [25]. The Shannon-Wiener values for I. gabonensis ranged from 1.8628 for endophytes isolated from barks from Oban, through 1.7119 for those got from leaves collected from Akamkpa to the least 1.601 for endophytes isolated from barks got from Akamkpa. Species richness gives a total count of the species in the site. If the species richness is high, it means there are varieties of species in the area under study. A high species richness value points to a more stable environment and is good for the biodiversity of the area in question. The highest value 1.14 was recorded for the leaves from Akamkpa which had 9 species; the next was 1.02 for barks from Akamkpa. The least value recorded was 0.93 recorded for endophytes got from barks of I. gabonensis obtained from Akamkpa. A close look at the data shows that species richness varies as the colonization frequency.
The Shannon-Wiener equitability index EH takes into account both species abundance and species richness into account. The value increases as both the richness and the evenness of the community increases. The relatively low values recorded during study (0.7788-0.8958) suggest dominance by a few species as supported by the data.
Simpson’s diversity index ranged from the least 0.7567 for Akamkpa barks, to the highest 0.8371 for the endophytic fungi isolated from the Oban barks. These values which are quite high indicate that the biodiversity among the endophytic assemblage within the plant under study in both sites is on the high side. The Jaccard similarity index for the two locations ranged between 0.27 and 0.29. These are low values and can be interpreted to mean that the two sites are not very similar.
Conclusion
The result presented seems to show that I. gabonensis from Akamkpa and Oban habour a diverse assemblage of endophytic fungi with the leaves having a higher infection rate. The Jaccard similarity index seems to suggest a not very dissimilar assemblage between the two locations. With the use of this species in the anti-obesity supplement, more survey is needed to possibly enumerate more endophytic fungi that may lead to the isolation of bioactive molecules of great importance.
References
- De Bary. Morphologie und Physiologie der Pilze, Flechten und Myxomyceten. Hofmeister’s Hand Book of Physiological Botany. 1866.
- Petrini, O. Fungal endophytes in tree leaves In: Andrews JH, Harino SS (eds), Microbial Ecology of tree leaves. Springer, New York. 1991, 179-197.
- Petrini O. Taxonomy of endophytic fungi of aerial plant tissues. In: Fokkenna NJ, Van Den HJ (eds), Microbiology of the phylospere. Cambridge University Press, Cambridge. 1986, 175-187.
- Arnold AE. Understanding the diversity of foliar endophytic fungi: progress, challenges and frontiers. Fungal Biol Rev. 2007, 21:51-66.
- Arnold A, Maynard Z, Gilbert G, Coley P, Kursar T. Are tropical endophytes hyperdiverse? Ecol Lett. 2000,3(4):267-274.
- Mohali S, Burgess TI, Wingfield MN. Diversity and host association of the tropical tree endophytic Lasiodiplodia threobrome revealed using simple sequence repeat markers. Forest Pathol. 2005, 35(6):385-396.
- Ganley RJ, Brunsfeld SJ, Newcombe G. A community of unknown, endophytic fungi in western white pine. Proceedings of the National Academy of Sciences, USA. 2004,101:10107-10112.
- Suryanarayanan TS, Wittlinger SKJ, Faeth SH. Endophytic fungi associated with cacti in Arizona. Mycol Res. 2005, 109(5):635-639.
- Sraj-Krzic N, Pongrac P, Klemenc M, Kladnik A, Regvar M, et al.. Mycorrizal colonization in plants from intermittent aquatic habitats. Aquat Bot. 2006, 85(4):340-346.
- Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM. Thermotolerance generated by plant/fungal symbiosis. Science. 2002, 298:158.
- Dreyfuss M, Chapela I. Potential of fungi in the discovery of novel, low-molecular weight pharmaceuticals. In: Gullo VP (edtr), The Discovery of Natural Products with Therapeutic Potential. 1994, 26(5):49-80.
- Schulz B, Boyle C, Draeger S, Rommert AK. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol Res. 2002, 99:996-1004.
- Strobel G, Daisy B. Bioprosecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003, 67:491-502.
- Verma VC, Kharwara RN, Strobel GA. Chemical and functional diversity of natural products from plant associated endophytic fungi. Nat Prod Commun. 2009,4(11):1511-1532.
- Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S. Agroforestree Database: a tree reference and selection guide version 4.0. 2009.
- Oben JE, Ngondi JL, Momo CN, Agbor GA, Sobgui CSM. The use of a Cissus quadrangularis/Irvingia gabonensis combination in the management of weight loss: a double-blind placebo-controlled study. Lipids Health Dis. 2008, 7(12).
- Vuksan V, Jenkins D, Spadafora JP, Sievenpiper JL, Owen R, et al. Konjac-mannan (glucomannan) improves glycemia and other associated risk factors for coronary heart disease in type 2 diabetes. A randomized controlled metabolic trial. Diabetes Care. 1999, 14:913-919.
- Ezuruike UF, Prieto JM. The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J Ethnopharmacol. 2014, 155(2):857-924.
- Naik S, Shashikala J, Krishnamurthy Y. Diversity of fungal endophytes in shrubby medicinal plants of Malnad region, Western Ghats, Southern India. Fungal Ecol. 2008, 1:89-93.
- Barnett HL, Hunter BB. Illustrated Genera of Imperfect Fungi. 4th edition, MacMillan Publ. Co. ISBN: 0-89054-192-2, New York. 1998.
- Hata K, Futai K. Endophytic fungi associated with healthy pine needles and needles infested by the pine needle gall midge, Thecodiplosis japonensis. Canadian J Bot. 1995,73:384-390.
- Suryanarayanan TS, Senthilarasu G, Muruganandam V. Endophytic fungi from Cuscuta reflexa and its host plant. Fungal Divers. 2000, 4:117-123.
- Suryanarayanan, T. S., & Thennarasan, S. ‘Temporal variation in endophyte assemblages of Plumeria rubra leaves’. Fungal Diversity. 2004, 15:197- 204.
- Simpson EH. Measurement of diversity. Nature. 1949, 163:688.
- Shannon CE, Wiener W. The Mathematical Theory of Communication. University of Illinois Press, Urbana. 1963, 117.
- Jaccard P. "Étude comparative de la distribution florale dans une portion des Alpes et des Jura", Bulletin de la Société vaudoise des sciences naturelles, 1901, 37: 547-579.
- Fröhlich J, Hyde KD, Petrini LE. Endophytic fungi associated with palms. Mycol Res. 2000, 104:1202-1212.
- Rodrigues KF, Petrini O. Biodiversity of endophytic fungi in tropical regions. In: Hyde KD (edtr), Biodiversity of Tropical Micro Fungi. Hong Kong University Press, Hong Kong. 1997, 57-69.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences