ISSN : 0976-8505
Der Chemica Sinica
Electron Ionization (EI) Induced Mass Spectral Analysis of Substituted Phenylacetamides
Priyanka Gupta1, Sanjay Kumar Srivastava2 and Kumaran Ganesan1*
1Synthetic Chemistry Division, Defence Research and Development Establishment (DRDE), Jhansi Road, Gwalior (MP), India
2School of Studies in Chemistry, Jiwaji University, Gwalior (MP), India
Abstract
A variety of substituted Phenylacetamides of piperidine and morpholine as potent mosquito repellents have been synthesized and their mass spectral fragmentation pattern under electron impact have been studied. All these amides follow a unique pattern in their mass spectra fragmentation and formation of the fragment ions depends on the substitution on both the phenyl ring and amines. These fragmentation patterns are useful for unambiguous identification of these amides during their GC MS analysis.
Keywords
Phenylacetamides, Piperidines, Morpholines, Electron impact, Mass spectra
Introduction
Amides and their derivatives constitute an important class of organic compounds and exhibit a wide range of biological properties such as anti-inflammatory [1], anticonvulsant [2], antifungal [3], insecticidal [4] anti-tumor [5], anti-tuberculosis [6] and mosquito repellent activity [7]. In fact, about 25% of known pharmaceuticals contained at least one amide bond [8]. Amides like N, N-Diethyl-m-toluamide (DEET) [9], Diethylbenzamide (DEB) [10] and N, N-Diethyl-2- phenylacetamide (DEPA) [11] are the most commonly used mosquito repellents all over the world. Among them, DEPA has been extensively evaluated as multi-insect repellent in the laboratory and field and found effective against different insect vectors such as mosquitoes, simuliidaes, sand flies, ticks, rat flea and blood sucking flies [12-14]. It has been granted approval by the Drug Controller of India for use on humans [15] and is now available in market as lotion, cream and in spray formulations. Two DEPA analogues, viz., DM 156 and DM 34, show promising repellency and low toxicity [16] against Aedes aegypti. As an extension of our previous work on the development of more potent DEPA analogues [17], our present research program involves the synthesis and characterization of substituted phenylacetamides followed by their screening as potent mosquito repellents. Mass spectral analysis of these amides is an interesting research area. Numerous reports have been published in the literature for their spectral analysis. However, to the best of our knowledge, no systematic study could be found on the mass spectral analysis of phenylacetamides of piperidine and morpholine and we, herein, describe the electron induced mass spectral fragmentation pattern of these amides differing in the nature of substituents both on the phenyl ring of the carboxylic acid as well as in the nature of amine counterparts.
Materials and Methods
General method for the synthesis of amides
In a three neck round bottom flask, 1, 1’-carbonyldiimidazole (1.2 eq) was added to a solution of carboxylic acid (1 eq) in freshly distilled THF at RT. After complete consumption of carboxylic acid (TLC), amine (1.1 eq) was added and the reaction continued at room temperature. After completion of the reaction, reaction mixture was diluted with ethyl acetate and treated with 10% aqueous. NaOH solution (100 ml). The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined organic phase was washed successively with 2 N HCl (100 ml) and brine (100 ml), dried over anhydrous Na2SO4 and concentrated under reduced pressure. Pure amide was then obtained after flash chromatography (hexane-ethyl acetate).
Mass spectral analysis
The electron impact-mass spectrometric analysis (EI-MS) analysis was carried out on Agilent 6890 N GC-5973 Quadrupole Mass Selective Detector equipped with BP-5 capillary column (30 m × 0.32 mm id, 0.25 μm film thickness). Ionization was done by electron impact at 70 eV and 230°C. Helium was used as the carrier gas at the flow rate of 1.0 ml/min.
Results and Discussion
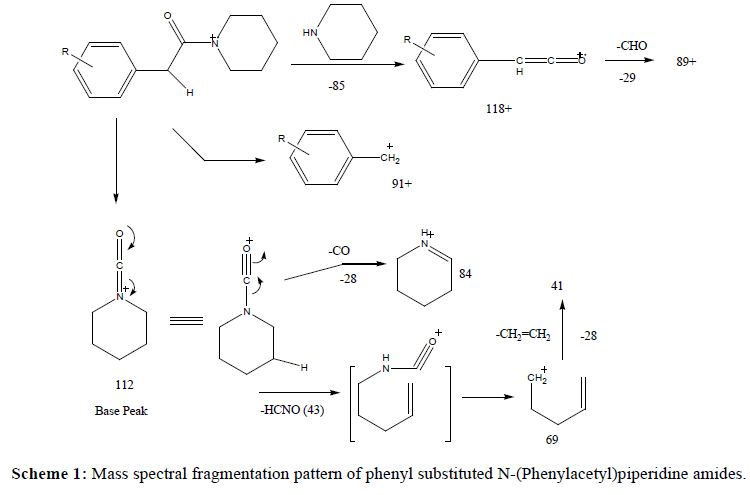
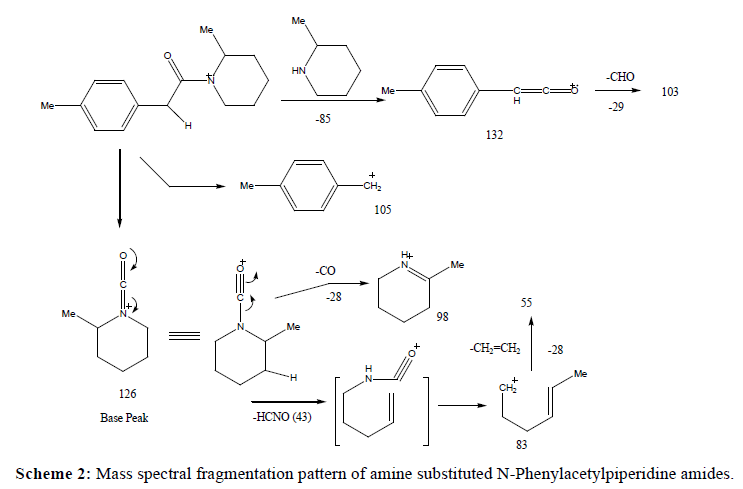
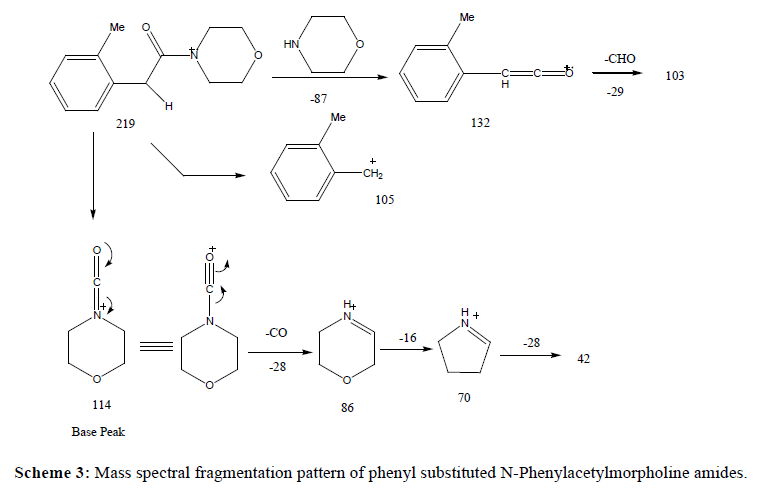
A series of substituted phenylacetamides were synthesized by the reaction of substituted phenylacetic acids with piperidines/morpholines and their EI-MS spectra was recorded on Agilent GC MS. The peculiar fragmentation pattern in their mass spectra was found to be dependent on the substitutions on both the phenyl ring and on the amine counterpart. Both piperidine and morpholine derived amides exhibited prominent molecular ion peak and the characteristic fragmentation pattern was observed in the mass spectrum. The main fragmentation pathway can be described by two major events including α-cleavages at the carbonyl functions at C-C and C-N bonds which, depending upon the retention of charge, produce fragment ions characteristics of the acid and amine components of the amide respectively (Table 1).
| Amide | M Wt | Fragment Ions m/z (Intensity) |
|---|---|---|
| 1-(2-Phenylacetyl)piperidine | 203 | 203 (48), 118 (3), 112 (100), 91 (20), 89 (3), 84 (10), 69 (42), 41 (10), 56 (6) |
| 1-(2-(2-Methylphenyl)acetyl) piperidine | 217 | 217 (68), 216 (2), 202 (9), 132 (2), 112 (100), 105 (22), 91 (2), 103 (7), 84 (9), 69 (36), 41 (11), 77 (11), 56 (4) |
| 1-(2-(3-Methylphenyl)acetyl) piperidine | 217 | 217 (63), 216 (8), 202 (2), 132 (3), 112 (100), 105 (22), 91 (7), 103 (9), 84 (12), 69 (48), 41 (14), 77 (12), 56 (7) |
| 1-(2-(4-Methylphenyl)acetyl) piperidine | 217 | 217 (55), 216 (7), 132 (4), 112 (100), 105 (22), 91 (2), 103 (7), 84 (9), 69 (44), 41 (11), 77 (11), 56 (7) |
| 1-(2-(2-Methoxyphenyl)acetyl) piperidine | 233 | 233 (98), 232 (1), 202 (38), 148 (7), 132 (5), 112 (100), 121 (24), 91 (55), 119 (3), 84 (12), 69 (67), 41 (15), 77 (8), 56 (6) |
| 1-(2-(3-Methoxyphenyl)acetyl) piperidine | 233 | 233 (77), 232 (12), 218 (2), 148 (4), 112 (100), 121 (17), 91 (12), 119 (2), 84 (10), 69 (39), 41 (9), 78 (8), 56 (6) |
| 1-(2-(4-Methoxyphenyl)acetyl) piperidine | 233 | 233 (98), 232 (4), 148 (6), 132 (1), 112 (100), 121 (94), 91 (9), 119 (4), 84 (7), 69 (64), 41 (14), 78 (14), 56 (7) |
| 1-(2-(2-Fluorophenyl)acetyl) piperidine | 221 | 221 (42), 220 (3), 202 (3), 136 (3), 112 (100), 109 (39), 107 (5), 84 (10), 69 (42), 41 (11), 83 (11), 56 (6) |
| 1-(2-(3-Fluorophenyl)acetyl) piperidine | 221 | 221 (29), 220 (7), 136 (3), 112 (100), 109 (37), 107 (3), 84 (12), 69 (43), 41 (12), 83 (10), 56 (7) |
| 1-(2-(4-Fluorophenyl)acetyl) piperidine | 221 | 221 (29), 220 (4), 136 (3), 112 (100), 109 (37), 107 (4), 84 (8), 69 (43), 41 (12), 83 (10), 56 (6) |
| 1-(2-(2-Methylphenyl)acetyl) morpholine | 219 | 219 (97), 204 (9), 132 (13), 114 (100), 105 (55), 103 (14), 86 (8), 70 (54), 42 (7), 77 (14), 56 (8) |
| 1-(2-(3-Methylphenyl)acetyl) morpholine | 219 | 219 (97), 132 (20), 176 (7), 114 (100), 105 (62), 103 (13), 86 (12), 70 (55), 42 (8), 77 (14), 56 (12) |
| 1-(2-(4-Methylphenyl)acetyl) morpholine | 219 | 219 (58), 132 (10), 176 (2), 114 (100), 105 (42), 103 (8), 86 (6), 70 (38), 42 (6), 77 (6), 56 (7) |
| 1-(2-(2-Methoxyphenyl)acetyl) morpholine | 235 | 235 (100), 204 (18), 148 (20), 114 (93), 121 (65), 91 (57), 119 (3), 86 (7), 70 (39), 42 (6), 77 (6), 56 (7) |
| 1-(2-(3-Methoxyphenyl)acetyl) morpholine | 235 | 235 (73), 204 (2), 148 (25), 114 (100), 121 (40), 91 (15), 86 (11), 70 (44), 42 (5), 77 (6), 56 (9) |
| 1-(2-(4-Methoxyphenyl)acetyl) morpholine | 235 | 235 (33), 148 (4), 114 (30), 121 (100), 91 (4), 86 (2), 70 (19), 42 (3), 77 (5), 56 (3) |
| 1-(2-(4-Methylphenyl)acetyl)-2-methylpiperidine | 231 | 231 (50), 216 (1), 132 (4), 126 (100), 112 (2), 105 (28), 103 (6), 98 (12), 83 (12), 55 (12), 91 (3), 70 (4) |
| 1-(2-(4-Methylphenyl)acetyl)-3-methylpiperidine | 231 | 231 (69), 216 (3), 132 (3), 126 (100), 105 (24), 103 (5), 98 (9), 83 (12), 55 (20), 91 (3), 70 (3) |
| 1-(2-(4-Methylphenyl)acetyl)-4-methylpiperidine | 231 | 231 (46), 132 (4), 126 (100), 105 (23), 103 (4), 98 (4), 83 (17), 55 (25), 91 (3), 70 (4) |
Table 1: Mass spectral fragment ions appearing in the mass spectra of Piperidine and Morpholine derived substituted phenylacetamides
In case of piperidine derived amides, the most important pathway involves α-cleavage leading to carboxamide ion [CONR2]+ leading to base peak at m/z 112. The major fragmentation route followed by carboxamide ion involves the ring opening followed by expulsion of HCNO molecule which leads to a prominent peak at m/z 69. The later also fragments with the loss of ethylene molecule to give peak at m/z 41. An alternate fragmentation involves CO-N cleavage with hydrogen transfer to nitrogen leading to less abundant peak at m/z 84 corresponding to [HNR2]+ ion. It can be considered as a result of methylene hydrogen transfer to carboxamide nitrogen, subsequent homolytic cleavage with charge retention on nitrogen and expulsion of a CO molecule. These fragment ions were observed in all the mass spectra and are characteristic of the piperidine nucleus. On the other hand, rest of the ions is characteristics of the substituents present on the phenyl ring of the acetic acid moiety. In all the cases, the molecular ion peak was quite strong and prominent. A peak, although very weak, at M-1 was also observed in mass spectra of all the molecules. However, the most abundant ion resulting from the cleavage of bond alpha to the CO group (CO-N bond) leads to the fragment ion at m/z 118 in case of unsubstituted phenyl acetic acid while in substituted amides, the corresponding ion was observed at its respective position. On the other hand, cleavage of another α-CO bond leads to the formation of ion at m/s 91 and corresponding ions in the mass spectra of other substituted amides. Loss of substituent from the phenyl ring of the amides was also observed with some of the amides but not with all the amides. For example, a very faint peak at M-1 was observed in the mass spectra of 2- and 3-methyl and methoxy substituted amides and that of 4-fluoro amide only (Scheme 1).
In case of amides differing in the nature of amine only, similar trend of fragmentation was followed. The fragment ions characteristics of the acetic acid moiety, i.e., ions at 132, 105 and 103 were observed consistently in mass spectra of all these amides. On the other hand, other ions appeared in the mass spectra contained the amine component and observed at different m/z values in amides of different amines (Scheme 2).
Replacement of piperidine with morpholine as amine part in the amides led to the changes as expected from the proposed fragmentation scheme. This replacement resulted in the stronger molecular ion peak in morpholides in comparison to the piperidine amides. Such molecular ion peak enhancement was more prominent in case of 2- and 4-substituted carboxylic acids. However, like piperidine amides, in the mass spectra of morpholides, the base peak results from the cleavage of C-N bond α to the CO group and appears at m/z 114 (m/z 112 in piperidine amides). However, an exception to it was observed in the mass spectrum of 4-methoxyphenylacetic acid morpholide where the base peak appeared at m/z 121 due to the cleavage of C-C bond cleavage α to the CO group which may resulted from the formation of more stable resonance stabilized 4-methoxybenzyl carbocation. Surprisingly, such behaviour was not observed in the corresponding piperidine amide. Other fragment ions containing the morpholine residue was confirmed by comparing with mass spectra of piperidine amides.
Another noticeable difference between the mass spectra of piperidine amide and morpholides was observed in the subsequent fragmentation of the carboxamide ion. In case of piperidine amides, it eliminates HCNO (43) molecule to give rise to a peak at m/z 69 which is followed by loss of ethylene molecule to give peak at m/z 41. On the other hand, in case of morpholides, the carboxamide preferentially eliminates CO molecule to give a peak at m/z 86 which readily loses oxygen atom to give rise to peak at m/z 70. Subsequent loss of another ethylene molecule leads to the appearance of a peak at m/z 42 (Scheme 3).
Conclusion
In summary, the mass spectral fragmentation patterns of these amies were found to be unique and depends on the substitution on both the phenyl ring and amines. These fragmentation patterns are useful for unambiguous identification of these amides after their GC MS analysis.
Acknowledgements
The authors thank Head, Syntheic Chemistry Division and Director, Defence Reserach and Development Establishment (DRDE), Gwalior for his keen support and encouragement.
References
- Galewicz-Walesa K, Pachuta-Stec A (2003) The synthesis and properties of n-substituted amides of 1- (5-methylthio-1, 2, 4-triazol-3-yl) -cyclohexane-2-carboxylic acid. Medical Academy in Lublin9: 118-125.
- Siddiqui N, Alam MS, Ahsan W (2008) Synthesis, anticonvulsant and toxicity evaluation of 2-(1H-indol-3-yl)acetyl-N-(substituted phenyl)hydrazine carbothioamides and their related heterocyclic derivatives. Acta Pharm 58: 445-454.
- Moise M, Sunel V, Profire L, Popa M, Lionte C (2008) Synthesis and antimicrobial activity of some new (sulfon-aminophenyl)-amide derivatives of N-(4-nitrobenzoyl)-phenylglycine and N-(4-nitrobenzoyl)-phenylalanine. Farmacia 41: 283-290.
- Graybill TL, Ross MJ, Gauvin BR, Gregory JS, Harris AL, et al. (1992) Synthesis and evaluation of azapeptide-derived inhibitors of serine and cysteine proteases. Bioorg Med Chem Lett. pp.1375-1380.
- Warnecke A, Fichtner I, Sab G, Kratz F (2007) Synthesis, cleavage profile and antitumor efficacy of an Albumin-binding prodrug of methotrexate that is cleaved by Plasmin and Cathepsine B. Arch Pharm Pharm Med Chem 340: 389-395.
- Hegab MI, Abdel-Fattah AM, Yousef NM (2007) Synthesis, X-ray structure and Pharmacological activity of some 6, 6-disubstituted chromeno[4,3-b] and chromeno-[3,4-c]-quinolines. Archiv der Pharmazie, Chemistry in Life Sciences: 340: 396-399.
- Kalyansundram M (1982) A preliminary report on the synthesis and testing of mosquito repellents. Indian J Med Res 76: 190-194.
- Ghose AK, Viswanadhan VN, Wendoloski JJ (1999) A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery.1. A qualitative and quantitative characterization of known drug databases. J Comb Chem 1: 55-68.
- Roberts JR, Reigart JR (2004) Does anything beat DEET. Pediatr Ann 33: 443-453.
- Mittal PK, Sreehari U, Razdan RK, Dash AP, Ansari MA (2011) Efficacy of Advanced Odomos repellent cream (N, N-diethyl-benzamide) against mosquito vectors. Indian J Med Res 133: 426-430.
- McCabe ET, Barthel WF, Gertler SI, Hall SA (1954) Insect repellents. III. N, N-Diethylamides. J Org Chem 19: 493-498.
- Vijayaraghavan R, Rao SS, Swamy RV, Suryanarayana MVS (1991) Acute and subacute inhalation toxicity studies of a new broad spectrum insect repellent, N,N-diethylphenylacetamide. Toxicology 67: 85-96.
- Kumar S, Prakash S, Sharma RK, Jain SK, Kalyanasundaram M, et al. (1984) Field evaluation of three repellents against mosquitoes, black flies and land Leeches. Indian J Med Res 80: 541-545.
- Rao SS, Prakash S, Kumar S, Kaveeshwar U, Bhattacharya BK, et al. (1987) Toxicologic studies of an insect repellent N, N-diethylphenylacetamide. Indian J Med Res 85: 626-633.
- DRDO (2004) Multi-insect Repellent Diethylphenylacetamide Spray. Technology focus.
- Debboun M, Wagman J (2004) In vitro repellency of N, N-diethyl-3-methylbenzamide and N, N-diethylphenylacetamide analogs against Aedes aegypti and Anopheles stephensi (Diptera: Culicidae). J Med Entomol 41: 430-434.
- Garud A, Ganesan K, Prakash S, Vijayaragavan R, Shinde CK (2011) Behavioral responses and bio efficacy of some aromatic amides against Aedes aegypti. J Econ Entomol 104: 1369-1378.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences