EGFR - An Evolving Cancer Target
EL-Habib Dakir1, Mohamed El-Tanani1, Nadia-Maria Dakir2 Arnulfo Mendoza3 and Richard Morgan1
1Institute of Cancer Therapeutics, University of Bradford, UK
2Instituto de Biología Molecular y Celular del Cáncer, (CSIC)-Universidad de Salamanca, Campus Miguel de Unamuno, Salamanca, Spain
3Molecular Oncology Section, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute National Institutes of Health, USA
- *Corresponding Author:
- Dakir EL-H
Institute of Cancer Therapeutics
University of Bradford, UK
Tel: +44 (0) 1274 235367
E-Mail: dakir@usal.es; e.h.dakir@bradford.ac.uk
Received Date: January 21, 2017; Accepted Date: January 24, 2017; Published Date: January 31, 2017
Citation: Dakir EL-H, El-Tanani M, Dakir NM, et al. EGFR - An Evolving Cancer Target. Cancer Biol Ther Oncol. 2017, 1:1.
Abstract
The Epidermal Growth Factor Receptor (EGFR) plays an important role in initiating the signaling that directs the behaviour of epithelial cells and tumors of epithelial origin. EGFR is involved in cell signaling that controls cell proliferation, survival, and metastasis by regulating diverse cellular pathways, and is the most frequently mutated receptor in lung cancer (10% in the USA, 35% in Asia) [1,2], prompting an intensive effort to develop an effective drug to overcome EGFR-mutant-driven cancers. Resistance to EGFR tyrosine kinase inhibitors occurs in many cases of lung cancer. Here, we present a concise overview of the mechanisms involved in resistance and discusses a new approach to overcoming them.
Keywords
EGFR; Tyrosine kinase inhibitors; Monoclonal antibody; Tumors; Immunotherapy; Clinical trials
The Epidermal Growth Factor Receptor (EGFR) plays an important role in initiating the signaling that directs the behaviour of epithelial cells and tumors of epithelial origin. EGFR is involved in cell signaling that controls cell proliferation, survival, and metastasis by regulating diverse cellular pathways, and is the most frequently mutated receptor in lung cancer (10% in the USA, 35% in Asia) [1,2], prompting an intensive effort to develop an effective drug to overcome EGFR-mutant-driven cancers. Resistance to EGFR tyrosine kinase inhibitors occurs in many cases of lung cancer. Here, we present a concise overview of the mechanisms involved in resistance and discusses a new approach to overcoming them.
Since the 1980s, several reports described the overexpression of EGFR in a variety of epithelial tumors, supporting the hypothesis that dysregulation of EGFR gene expression and signaling could play a pivotal biological role in cancer. EGFR could be targeted via two fundamental approaches. The first approach was the development of antibodies against the EGFR extracellular domain and the second was to design and target EGFR with small-molecule tyrosine kinase inhibitors (TKIs). Both of these approaches have proven clinically useful but share the overarching limitation of the emergence of drug resistance, which prevents these therapies from eliciting lasting clinical benefit. However, it remains questionable whether these resistances (intrinsic and acquired) to both therapies are in fact acquired during the treatment or were already present in a small subpopulation of cancer cells that initially escaped detection.
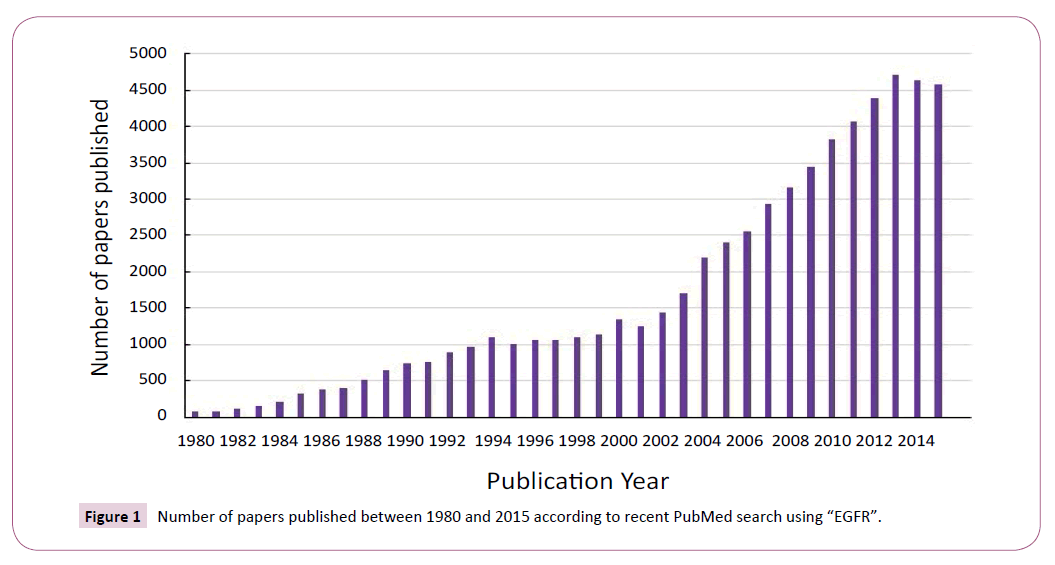
The important role of EGFR in lung oncogenesis and the prevalence of EGFR mutations in human cancers make it a promising therapeutic target. Over the past 35 years EGFR has received a resurgence of interest due to the outcomes of cancergenomic and proteomic studies (Figure 1). A number of indirect and direct strategies have been developed to target EGFR signaling by inhibiting tumor growth, survival, and metastasis.
EGFR is a transmembrane tyrosine kinase receptor belonging to the HER (ErbB) family of receptor tyrosine kinases [3]. Receptor activation upon ligand binding leads to downstream activation of the PI3K/AKT, RAS/RAF/MEK/ERK and PLCλ/PKC- pathways and therefore, exert an effect on cell proliferation, survival and the metastatic potential of tumor cells [3,4]. Many patients with Non- Small Cell Lung Cancer (NSCLC) have somatic mutations of EGFR, first identified in 2004 [1,2] which lead to aberrant constitutive signaling via the EGFR/HER family and their downstream protein markers. The EGFR mutations, including activating and resistant mutations, mostly occur in exons 18 to 21 of the EGFR gene encoding the ATP-binding pocket of the intracellular TK domain [5]. Gefitinib and erlotinib were the first-generation of EGFR-TKIs approved for use in the first-line setting for the treatment of advanced NSCLC patients [6], and thus targeting EGFR has been intensively investigated as a cancer treatment strategy over the last two decades.
Despite an initial response to EGFR-TKIs, the majority of patients will have disease progression within 1-2 years after treatment initiation due to acquired resistance [7-10]. The most common mechanism of TKI resistance is a second-site mutation (T790M) in the EGFR kinase domain in exon 20 [7,8], which can be detected in more than 50% of biopsies after resistance develops [11,12]. AZD9291 is an oral, irreversible mutant-selective EGFR-TKI that has a strong effect on tumors bearing the activating mutation L858R or an exon19 deletion in the presence of the T970M mutation [13-15]. This mutation leads to enhanced affinity for ATP, reducing the ability of ATP-competitive reversible EGFR-TKIs including gefitinib and erlotinib to bind to the TK domain of EGFR [4,5].
One approach to overcome this mechanism of resistance is the use of irreversible EGFR inhibitors [9]. Although the use of the irreversible TKIs afatinib and dacomitinib have been shown to be effective in preclinical models, they are associated with fewer response rates of less than 10% and a progression-free survival of less than 4 months in NSCLC patients who have received previous treatment with gefitinib or erlotinib, probably owing to an inability of afatinib or dacomitinib to inhibit EGFR T790M at clinically achievable doses [10]. EGFR T790M has been used as a prognostic biomarker, and in recent studies has been used also as a predictive biomarker for the efficacy of AZD929. The presence of the T790M mutation was observed in approximately 50% of the cases in which biopsy was obtained at the time of relapse following gefitinib or erlotinib treatment in patients with an exon 19 deletion or the L858R EGFR mutation.
Acquired resistance to EGFR-TKIs is mediated by non-T790M mechanisms in approximately 40% of cases, although the underlying mechanisms are not fully understood. However, known mechanisms include activation of non-EGFR bypass signaling pathways and a histologic change known as Epithelial to Mesenchymal Transition (EMT). Another frequent mechanism of resistance to EGFR-TKIs is activation of growth and survival pathways, the most studied of which is c-Met amplification. Like EGFR, c-Met is a transmembrane tyrosine kinase receptor that induces activation of PI3K/AKT/mTOR and MAPK pathways following binding to its ligand Hepatocyte Growth Factor (HGF). c-MET amplification accounts for about 20% of TKI acquiredresistance in tumors with a different molecular pathway [11]. Sustained amplification of c-MET phosphorylates HER3 and activates the PI3K/ AKT/mTOR and MAPK downstream signaling cascades [16-19]. It is considered one of the more common causes of acquired resistance (AR) in EGFR-mutant NSCLC, bypassing the inhibition of EGFR conferred by TKIs [19].
Current approaches to address lung cancer resistance to EGFRTKIs with non-T790M-depenedent resistance mechanisms include the combination of an EGFR inhibitor and a c-MET inhibitor. However, this approach has been limited both by toxicity and a lack of efficacy. The activity of AZD9291 coupled with its safety profile may provide the opportunity to evaluate combination treatments strategies with other inhibitors, and to further improve clinical outcomes in patients with resistance to EGFR tyrosine kinase inhibitors. Another newly emerged agent is heat shock protein 90 (HSP90) inhibitor [12]. HSP90 is a chaperone responsible for the conformational maturation and stabilization of its substrate proteins, such as the ErbB family (EGFR), MET and various downstream kinases, including AKT. Therefore, inhibiting the action of HSP90 is a potentially viable treatment strategy given that EGFR mutations associated with resistance to EGFR-TKIs do not compromise the capacity of HSP90 to regulate ErbB family members [13]. Moreover, HSP90 inhibitors have been described as suppressors of EGFR-mediated signaling in erlotinib-sensitive and erlotinib-resistant cell lines [13]. Recently, preclinical studies with a HSP90 inhibitor agent, CH5164840, showed potent anticancer activity and were highly effective in combination with erlotinib in NSCLC patients with EGFR mutations [14]. Other compounds targeting alternative pathways, such as HER2/HER3, PI3K/AKT/mTOR and MAPK inhibitors are in various stages of clinical development.
NSCLCs often express high levels of programed death-ligand 1 (PDL-1) that contributes to a poor prognosis and immune suppression [15,16]. Preclinical data showed that activation of the programed death (PD-1) pathway contributed to immune evasion in EGFR-driven lung cancers [17]. Clinical studies of PD-1/ PDL-1 targeted therapy using monoclonal antibodies in patients with advanced or metastatic NSCLC have been successfully conducted [18,19]. Considering such responses of NSCLC to PD-1/PDL-1 checkpoint therapy (nivolumab, pembrolizumab), the AKT/STAT3 pathway can be a potential target for regulating the surface expression of PD-1 on NSCLC with an EGFR mutation, therefore targeting either AKT or STAT3 could downregulate the expression of PD-1 even in gefitinib-resistant NSCLC and potential anti-tumor immune response. Therefore, targeting PD1-/PDL-1 is an attractive strategy to overcome immune escape in NSCLC and increase the efficacy of EGFR-TKIs. [17] Phase 1 trials combining EGFR-TKIs with immunotherapies are on-going, including the following: anti-PD-1 human monoclonal antibody, nivolumab (ClinicalTrials.gov, number NCT01454102); anti-PD-1 monoclonal antibody, pembrolizumab (ClinicalTrials. gov, number NCT02039674); and the anti-PDL-1 monoclonal antibody, MPDL3280A (ClinicalTrials.gov, number CT02013219).
TKIs are more appropriate clinical therapies for several human malignancies and represent a paradigm of treatment for EGFRmutated NSCLC. However, the development of resistance limits their efficiency and efficacy due to new EGFR mutations. Recent studies have provided new insights into the mechanism of resistance. For example, it was demonstrated that the T790M mutation in EGFR confers resistance through a change in ATP affinity. These advances have helped to drive the development of a new generation of inhibitors. A further understanding of the mechanisms of resistance will greatly aid in the development of the next generation of EGFR-TKIs.
As drug resistance appears to be pleomorphic, changes to standard treatment approaches, including different schedules and combination therapy, may also be an effective strategy in circumventing resistance. However, appropriate pharmacological evaluation should be accompanied with a deep understanding the genetic alterations of tumors cells and tumor heterogeneity. Plasma circulating cell-free tumor DNA (cfDNA) or circulating tumor cells (CTCs), have also helped facilitate the early detection of cancer and minimal residual disease to track tumor evolution and determine the mechanisms of resistance to any class of EGFR-TKIs. They are also non-invasive methods, and may help detect early cases of relapse.
The discovery of new biomarkers that will accurately predict the optimum response to TKIs, and the identification of other key signaling pathways that can be targeted to enhance TKI activity will increase the efficacy of clinical therapy to TKIs. Understanding the interaction between drug development, basic biology and translational studies will facilitate the development of new therapeutic approaches, such as first-line combinations of specific inhibitors targeting both EGFR activated mutations and oncoproteins involved in cell resistance and “pro-activation” of the immune system and thus further improve clinical outcomes of lung cancer patients.
Acknowledgement
The University of Bradford supported this work.
References
- Lynch TJ, Bell DW, Sordella R,Gurubhagavatula S, Okimoto RA,et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129-2139.
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, et al. (2004) EGFRmutations in lungcancer: Correlation with clinical response to gefitinib therapy. Science 304: 1497-1500.
- Yarden Y, Sliwkowski MX. (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127-137.
- Scaltriti M, Baselga J. (2006) The epidermal growth factor receptor pathway: A modelfor targeted therapy. Clin Cancer Res 12: 5268-5272.
- Rosell R, Moran T, Queralt C, Porta R, Cardenal F, et al. (2009) Screeningfor epidermal growth factor receptor mutations in lung cancer. N Engl J Med61: 958-967.
- Khozin S, Blumenthal GM, Jiang X, He K, Boyd K,et al. (2014) U.S. Food and Drug Administration approval summary: Erlotinib for the first-linetreatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist 19: 774-779.
- Zhou W, Ercan D, Chen L, Yun CH, Li D, et al. (2009) Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 462:1070-1074.
- Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA,et al.(2014) AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistanceto EGFR inhibitors in lung cancer. Cancer Discov4: 1046-1061.
- Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, et al. (2005) EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. NEngl J Med 352: 786-792.
- Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, et al. (2007) PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 67: 1924-11932.
- Ma C, Wei S, Song Y. (2011) T790M and acquired resistance of EGFR TKI: A literature review of clinical reports. J Thorac Dis 3: 10-18.
- Workman P, Burrows F, Neckers L, Rosen N. (2007) Drugging the cancer chaperoneHSP90: combinatorial therapeutic exploitation of oncogene addiction andtumorstress. Ann N Y AcadSci1113: 202-216.
- Shimamura T, Li D, Ji H, Haringsma HJ, Liniker E, et al. (2008) Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinaseinhibitor resistance. Cancer Res 68: 5827-5838.
- Ono N, Yamazaki T, Tsukaguchi T, Fujii T, Sakata K, et al. (2013) Enhanced antitumoractivityoferlotinibincombinationwiththeHsp90inhibitorCH5164840 against non-small-cell lung cancer. Cancer Sci. 104: 1346-1352.
- Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, et al. (2004)B7-H1 expression on non-small cell lung cancer cells and its relationship withtumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 10: 5094-5100.
- Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. (2011) High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escapethrough suppressing tumor infiltrating dendritic cells maturation. Med Oncol 28: 682-688.
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, et al. (2013) Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov3: 1355-1363.
- Soria JC,MarabelleA,BrahmerJR,GettingerS (2015) Immune checkpoint modulation for non-small cell lung cancer. Clin Cancer Res21: 2256-2262.
- Bustamante Alvarez JG, Gonzalez-Cao M, Karachaliou N, Santarpia M, Viteri S, et al. (2015) Advances in immunotherapy for treatment of lung cancer.Cancer Biol Med 12: 209-222.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences