ISSN : 2348-9502
American Journal of Ethnomedicine
Effects of Nigella sativa Seed Extract on Insulin Resistant Non-insulin-Dependent Diabetic Guinea Pigs
Department of Zoology, Ranchi University, Ranchi-834008, India
Abstract
In the present investigation, effects of ethanolic extract of Nigella sativa seed extract on insulin resistant non insulin dependent diabetic (NIDDM) guinea pigs were studied. Significant (p<0.05) decrease in blood glucose level and glycosylated haemoglobin were observed in animals treated with ethanolic extract of N. sativa. In addition, improvement in glucose tolerance, insulin level and insulin sensitivity was observed in treated diabetic animals. The results were more significant in animals treated with higher dose of N. sativa seed extract (500mg/kg BW) as compared to lower dose of extract (250 mg/kg BW). Protective effect of N. sativa seed extract was comparable to the standard drug (i.e., glimepride). Therefore it is concluded that N. sativa seed extract might prove beneficial in preventing hyperinsulinemia, impaired glucose tolerance, insulin resistance and eventually an effective means of improving hyperglycemia, without any toxic effect at the doses studied in the present study.
Keyword
Nigella sativa, Insulin resistance, Non-insulin dependent diabetes mellitus, STZ Guinea pigs.
INTRODUCTION
Type 2 diabetes mellitus (DM) is possibly the world’s fastest growing metabolic disorder which is characterized by hyperglycemia due to impaired insulin secretion with or without insulin resistance [1]. Diabetes mellitus is a heterogeneous group of disorders in which there is a specific genetic pattern, which includes environmental factors and pathophysiological mechanisms, leading to impairment of glucose tolerance [2]. Long-term damage, dysfunction and failure of various organs, especially the eyes, kidneys, nerves, heart and blood vessels are associated with chronic diabetic hyperglycemia. The resistance to insulin in diabetic patient is a result of autoimmune destruction of β-cells of the pancreas with consequent insulin deficiency to abnormalities [3]. Symptoms of marked hyperglycemia include polyurea, polydipsia, weight loss, sometimes with polyphagia, and blurred vision. Impairment of growth and susceptibility to certain infections may also accompany chronic hyperglycemia [4].
In recent years, plant derived medicines have received great deal of attention compared to synthetic ones for the cure and prophylaxis of various diseases. Management of type 2 DM without any adverse effect is still a challenge to researchers. A large number of herbal medicines are known which is found to possess antihyperglycemic effect and they are used because of minimal side effects and low cost of preparation. Nigella sativa is one of such plants used as an alternative medicine for diabetes. N. sativa has been used for many years for its diuretic, antihypertensive [5], anti- diabetic [6], anticancer and immunodulatory, analgesic antimicrobial [7], anti-helminths and anti-inflammatory [8], spasmolytic, bronchiodilator [9], gastroprotective [10], hepato-protective [11-13], renal protective and antioxidant properties. The seeds of N. sativa are widely used in the treatment of various diseases like bronchitis, asthama, diarrhea, rheumatism and skin disorders. Additionally, used as liver tonic, digestive, anti-diarrhoeal, appetite stimulant, and to support immune system [14]. Nearly 32 compounds have been identified, of which thymoquinone, thymohydro-quinone, dithymoquinone, p-cymene, arvacrol, 4- terpineol, t-anethol, sesquiterpene longifolene and á-pinene are some of the predominant compounds. Other derivatives found in trace amounts include carvone, limonene, citronellol. Many of these compounds are capable of inducing pharmacological effects in humans. Properties of whole seeds or their extracts are mainly attributed to quinine constituents, of which thymoquinone (TQ) is the most abundant as well as the potent pharmacologically active compound [15]. N. sativa oil as well as TQ have been shown to prevent oxidative injuries. It is reported that N. sativa and its derivative TQ inhibit eicosanoid generation in leucocytes and membrane lipid peroxidation [16].
Keeping in view, the severity of disease and potential of N. sativa, it was thought valuable to explore the antidiabetic properties of ethanolic extract of N. sativa seed by investigating the effect of N. sativa seed extract on hyperinsulinemia, glucose intolerance and insulin sensitivity in noninsulin dependent diabetes mellitus (NIDDM) model of guinea pigs.
MATERIALS AND METHODS
Animals
Male guinea pig (Cavia porcellus) of age 6-8 weeks old (weight approx. 150gm/rat) were used in this study and housed in stainless steel cages (5 animals/ cage). They were acclimatized under laboratory conditions viz. 24 ± 1°C of ambient temperature and relative humidity 55±10%, with a 12:12 h light-dark cycle. Animals were fed standard chow and water ad libitum for whole period of the experiment.
Grouping of animals
Sixty animals were distributed into 6 groups (ten animals in each group) as follows- Animals of Group-1: normal nondiabetic, Group-2: diabetic control, Group- 3: diabetic guinea pigs fed with ethanolic extract of N. sativa seed (250 mg/kg body wt.) in diet, Group-4: diabetic guinea pigs fed with ethanolic extract of N. sativa seed (500 mg/kg body wt.) in diet, Group-5: diabetic guinea pigs fed with Glimipiride (2 mg/kg body wt.) in diet and ethanolic extract of N. sativa seed was fed to guinea pigs of respective groups for eight weeks continuously. Group-6: Runner group (normal guinea pigs fed with ethanolic extract of N. sativa seed @ 500 mg/kg body wt.) for study of N. sativa seed toxicity. Guinea pigs were sacrificed at the 8th week of experimental period and assessed for various parameters.
Preparation of ethanolic extract of Nigella sativa seed
The seeds of Nigella sativa were purchased from local market. Seeds of N. sativa were later shade dried for 5 days and powdered using mixer grinder. One thousand gram of shade dried N. sativa seed powder was soaked for 48 hours in a flask containing 1.5L of petroleum ether. The flask was kept at room temperature for overnight. Following day the mixture was filtered with Whatman’s filter paper and supernatant powder was collected. Supernatant powder was dried for overnight. Next day extraction was done through soxhlet extractor by using ethanol. All the residue extract were collected and stored at room temperature.
Induction of type-2 diabetes (NIDDM) in rats
For induction of Type-2 diabetes (NIDDM), guinea pigs were fed with standard chow containing high carbohydrate for 15 days before STZ injection. Type-2 diabetes in guinea pigs was induced by a single dose of intra-peritoneal injection of STZ (freshly prepared in 0.1M citrate phosphate buffer) at a dose of 60mg/kg body weight [17] (Wang et.al., 2007). All rats were fasted for 12 hours before STZ injection.
Blood glucose test
Glucose levels were determined by using one drop of blood samples, blood was drawn from tail vain. Bayer Contour TS Glucometer (Bayer Healthcare Ltd., Hong Kong) was used for glucose level analysis following manufacturer’s protocol.
Oral glucose tolerance test (OGTT)
OGTT was performed between 0900-1400 h on 8th week as per the method described by Tran et al., (2003)18. The rats were deprived of food for 12-14 h before administration of oral glucose at concentration of 2 gm/kg BW. After glucose administration, blood samples were collected from the tail vein at 0 (before administration), 60 min and 120 min. Glucose levels were analysed by using one drop of blood samples in Bayer Contour TS Glucometer (Bayer Healthcare Ltd., Hong Kong).
Glycosylated hemoglobin (HbA1c) level
Bannon’s (1982) method was used to estimate glycosylated hemoglobin level [19], using a commercial diagnostic kit from Monozyme India Ltd., Secunderabad, India.
Insulin level
Plasma insulin level was estimated quantitatively by ELISA method of Morgan and Lazarow (1963) [20]. For this purpose Insulin ELISA kit was used.
Insulin sensitivity
Insulin tolerance test (ITT) is used to assess peripheral insulin resistance [21]. This test measures insulin sensitivity using KITT as an index of insulin mediated glucose metabolism. Rats were kept fasted overnight before administering insulin challenge. Insulin (0.2 U/100 g BW human regular insulin; Eli Lilly, Indianapolis, IN) was administered by slow i.v. injection through tail vein. Later, blood samples were collected at 0, 30, 60 and 120 min after administration of insulin injection. Glucose was then estimated by glucose oxidaseperoxidase method. KITT was determined from the slope of a linear portion of regression line of natural logarithm of glucose versus time using the formula:
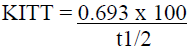
Where, t1/2 represents the half-life of plasma glucose decay. The half-life of plasma glucose was obtained by plotting plasma glucose concentrations versus time on semi-logarithmic graph paper.
HOMA-R
HOMA-R was calculated by using fasting blood glucose (FBG) and fasting insulin (FI) level. FBG and FI levels were used for the determination of hepatic insulin resistance [22]. The insulin sensitivity level was calculated using the following formula:
HOMA-R = FI (μU/mL) x FBG (mg/dL)/22.5.
Statistical analysis
The data were analyzed by one way ANOVA (Analysis of Variance). Values expressed in Mean ± S.E.M. Differences in mean were considered significant at P˂0.05.
RESULTS
Blood glucose test
Table 1 shows the effect of N. sativa seed extract on blood glucose level and glycosylated haemoglobin. After 8 weeks of treatment, diabetic guinea pigs fed with 500 mg/Kg BW N. sativa seed (group-IV) extract showed significantly (P<0.05) lower (103.66±3.17) blood glucose as compared to guinea pigs fed with 250 mg/Kg BW (group-III) N. sativa seed extract (189.33±2.60,). Comparable blood glucose concentration were recorded at 8th weeks of treatment in guinea pigs of 500 mg/Kg BW N. sativa (103.66±3.17), glimepiride (104±3), runner (79 ±2.5) and normal (78.33 ± 2.33) groups.
Table 1: Effect of ethanolic extract of N.Sativa seed on blood glucose and glycosylated haemoglobin (HbAlc) at 8th week of treatment indicating its anti-hyperglycemic effects
| Groups | Blood Glucose (mg/dl) | Glycosylated Haemoglobin in % |
|---|---|---|
| Normal | 78.33±2.33 | 4.79±0.81 |
| Diabetic control | 273.66±5.89* | 9.56±0.88* |
| 250mg/kg BW | 189.33±2.60* | 7.95±0.89 |
| 500mg/kg BW | 103.66±3.17 | 6.82±1.60 |
| Glimepride | 104±3 | 5.5±1.60 |
| Runner | 79 ± 2.5 | 4.89 ±0.81 |
Value are Mean ± S.E.M of three experiments, P<0.05.
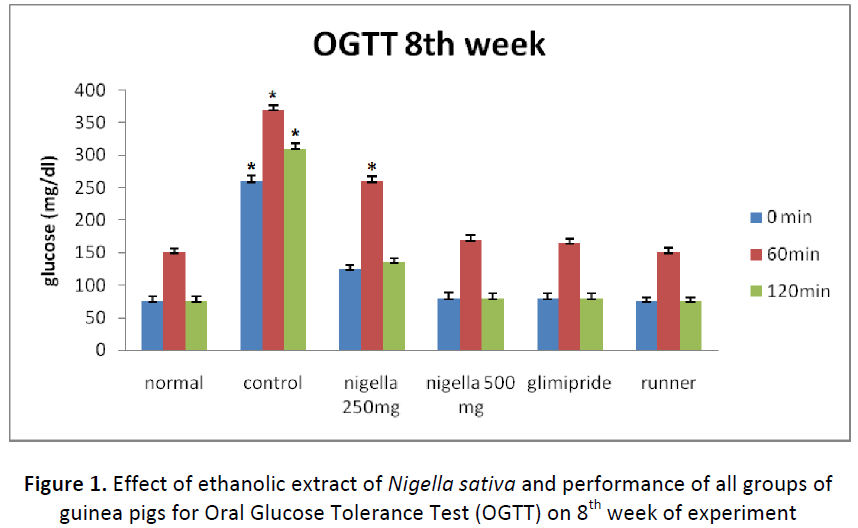
Oral glucose tolerance test (OGTT)
At the 8th weeks of treatment (Fig.- 1), blood glucose concentration was restored back to normal in guinea pigs fed with 500 mg/Kg BW N. sativa (group-IV) or glimepiride (group-V). Guinea pigs fed with 500 mg/Kg BW N. sativa seed extract showed significantly (P<0.05) better oral glucose tolerance as compared to guinea pigs fed with 250 mg/Kg BW N. sativa seed extract. Effects of N. sativa seed extract at the concentration of 500 mg/Kg BW was found to be comparable with glimepiride fed (group-V) and guinea pigs of normal (group- I) and runner group (group-VI).
Glycosylated haemoglobin
A significant (P<0.05) decrease in glycosylated hemoglobin was observed, in diabetic guinea pigs fed with ethanolic extract of N. sativa seed, at the end of 8th weeks of treatment (Table 1). At this stage 4.79±0.81 percent of hemoglobin was glycosylated in guinea pigs of normal group (group-I) where it was 9.56±0.88 percent in guinea pigs of diabetic control group (group- II). Diabetic guinea pigs fed with 250 mg/Kg BW (group-III) or 500 mg/Kg BW (group-IV) N. sativa seed extract showed 7.95±0.89 and 6.82±1.60 percent glycosylated hemoglobin respectively. Feeding N. sativa seed extract at the concentration of 500 mg/Kg BW to diabetic guinea pigs showed significantly (P<0.05) better results as compared to feeding 250 mg/Kg BW and found to be comparable with that of glimepiride (group-V) fed (5.5±1.60) and guinea pigs of runner group (group-VI, 4.89± 0.81).
Insulin level
Table 2 shows the effect of ethanolic extract of N. sativa seed on insulin level. At 8th week of treatment, feeding of 500 mg/Kg BW N. sativa seed extract to diabetic guinea pigs resulted in significant (P<0.05) increase in plasma insulin (17.55±1.60). This increased plasma insulin was found to be non significant (P<0.05) with guinea pigs of normal (18.5±0.81), glimepiride (17.95±1.60) and runner group (18.0±0.81).
Table 2: Effect of ethanolic extract of N.Sativa seed on Insulin resistance index (HOMA) at 8th week of treatment calculated by the formula, HOMA = insulin (µU/mL) X glucose (mg/dL)/22.5
| Groups | Plasma Insulin(µu/ml) | HOMA |
|---|---|---|
| Normal | 18.5±0.81 | 62.8±3.52 |
| Diabetic control | 7.8±0.88* | 90.2±4.07* |
| 250mg/kg BW | 14.2±0.89 | 68.41±3.67 |
| 500mg/kgBW | 17.55±1.60 | 63.05±4,42 |
| Glimepride | 17.95±1.60 | 62.8±3.51 |
| Runner | 18.0±0.81 | 62.6±3.44 |
Value are Mean ± S.E.M of three experiments, P<0.05.
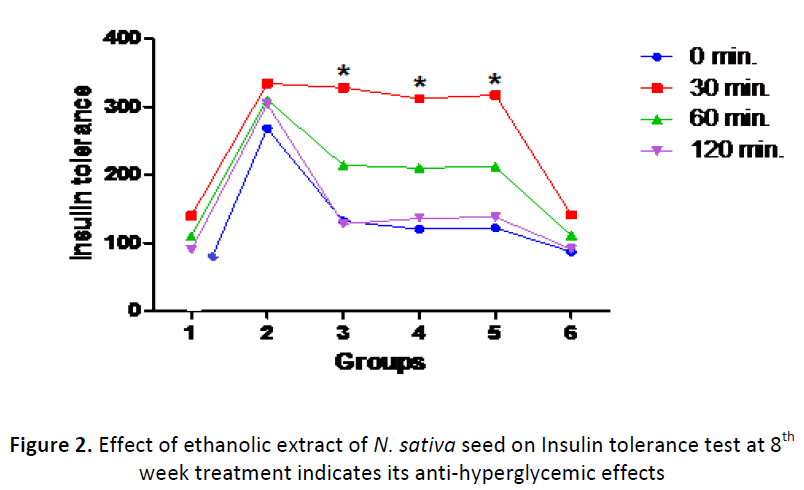
Insulin tolerance
Guinea pigs of diabetic control group (group-II) showed resistance towards insulin treatment. A non significant (P>0.05) decrease in blood glucose (mg/dl) was observed in guinea pigs of diabetic control group (group-II) at 30 min (334±6.5) and 90 min (310±5.7) of insulin administration. Guinea pigs of non diabetic normal (group-I, 140±8.11 Vs. 86±5.6) and runner (group-VI, 141±6.8 Vs. 87±7.9) groups showed statistically significant (P<0.05) decrease in blood glucose at 30 min and 60 min. Feeding N. sativa ethanolic extract to guinea pigs of diabetic groups for 8 weeks improved the insulin tolerance and showed blood glucose back to normal. N. sativa ethanolic seed extract at the concentration of 500 mg/Kg BW (312±5.7 Vs 210±8.5) and glimepiride (117±6.9. Vs 212±7.7) showed significantly better insulin tolerance at 30 min and 60 min as compared to guinea pigs fed with that of 250 mg/Kg BW (228±8.6 Vs 214±8.5).
HOMA
At the end of 8th week, feeding guinea pigs with 250 mg/Kg BW (68.41±3.67) or 500 mg/Kg BW (63.05±4.42) N. sativa seed extract resulted in significant (P<0.05) decrease in insulin resistance index. This came back to normal at 8th week (Table 2) and recorded to be statistically non significant (P>0.05) with guinea pigs of normal (group-I, 62.8±3.54), glimepiride (group-V, 62.8±3.51) and runner (group-VI, 62.6±3.44) group.
DISCUSSION
Type-2 diabetes is characterized by abnormalities in carbohydrate and lipid metabolism [23], resulting from defects in insulin secretion or action or both [24-26] which lead to postprandial and fasting hyperglycemia, dyslipidemia and hyperinsulinemia [27]. Insulin resistance is considered to be the significant pathogenic factor in type 2 diabetes [28]. Insulin resistance also leads to other disorders such as obesity, dyslipidemia, hypertension and cardiovascular disease, collectively termed as insulin resistance associated disorder (IRAD) [29]. One of the late complications of uncontrolled DM is the formation of advanced glycosylated end products (AGE) [30]. Some of these end products can react with other proteins and increased permeability and thickening of blood vessel walls with oxy radical damage [31]. Protein glycation and glucose auto oxidation may generate free radicals in the diabetic patient, which in turn catalyses lipid peroxidation, the antioxidant status of the diabetic is compromised and is unable to protect against harmful effect of free radicals [32].
There is an increased glycosylation of number of proteins, including haemoglobin and β-crystalline of lens in diabetic patients [33]. Glycosylated Haemoglobin (HbA1c) is an important tool of glycemic management [34]. Reason being that it provides an accurate measure to access the glycemic control and diagnosis of new diabetes mellitus. Samuel Rahbar et al (1969) [35] first described its relationship with diabetes. Individuals with inadequately controlled diabetes, the amount of these glycated haemoglobins are much higher than normal individual [36]. HbA1c level is directly proportional to the average blood glucose concentration. In the present study the HbA1c level was found to increase in NIDDM control animals. Treatment with two doses of N. sativa seed extract significantly decreased HbaA1c level in a dose dependent manner. Administration of N. sativa seed extract to NIDDM animals reduced the glycosylation of haemoglobin by virtue of its free radical scavenging property and thus decreased the level of HbA1c. A decrease in blood glucose level might also contribute to decreased level of HbA1c in N. sativa seed extract-treated NIDDM guinea pigs.
Feeding Nigella extract to diabetic guinea pigs at concentration of 250 mg/Kg BW or 500 mg/Kg BW or glimepiride, resulted in significant (P<0.05) increase in plasma insulin as compared to non fed guinea pigs of control group. This plant extract act in a similar fashion like that of Ocimum canum plant extract and sulphonylureas which also promote insulin release by β-cells of the pancreas [37]. Our result is in agreement with those of Ali BH (1997) [38] who showed increase in plasma insulin level after treating diabetic rats with Rhazya stricta extract. Tolan et al (2001) [39] showed increase in plasma insulin leading to decrease in insulin binding on the insulin receptor in diabetic dog following the treatment with capsaicin, the active principles present in Capsicum frutescens. The hypoglycaemic effect of N. sativa reported here is in agreement with the report of Abdel et al (1998) [40]. The hypoglycaemic effect may be mediated through enhancement of peripheral metabolism of glucose and an increase in insulin release or may be due to intestinal reduction of absorption of glucose or partially due to amelioration in the β cells of pancreatic islets causing an increase in insulin secretion.
An insulin-resistance state is a key phase of metabolic syndrome, constituting the most important risk factor for the development of glucose intolerance and diabetes mellitus [41]. Therefore, interventions to decrease insulin resistance may delay the progress of diabetic complications. In this study, when animals were subjected to oral glucose tolerance test (OGTT), glucose lowering effects were found in groups administered with N. sativa seed extract. Hypothetically, the extracts may have the properties to stimulate or regenerate the ßcell for the secretion of insulin and evidently effective in controlling diabetes [42]. Induction of diabetes with STZ can be associated with decrease in hepatic glycogen, which could be attributed to decrease in the availability of the active form of enzyme glycogen synthetase probably because of low levels of insulin [43]. Decreased activities of the enzymes involved in glucose homeostasis in liver and kidney such as hexokinase, which has been reported in diabetic animal, resulting in depletion of liver and muscle glycogen content [44]. Treatment with the N. sativa seed extract might increase the level of enzyme to the level which influence an over-all increase in glucose influx.
Evaluation of insulin sensitivity is an important mechanism for diagnosing the development and progression of diabetes and atherosclerotic disease, a common cause of mortality in diabetic individuals [29]. It is important to evaluate insulin resistance i.e., a decrease in the effect of insulin to stimulate glucose uptake at a given serum insulin concentration, for the prevention and treatment of NIDDM [45]. Thus Insulin tolerance test and HOMA levels were determined to check insulin sensitivity [46]. ITT was used to assess peripheral insulin resistance [21]. The results obtained here clearly showed that ITT was significantly improved by N. sativa seed extract treatment to STZ induced diabetic guinea pig. Additionally, N. sativa seed extract treatment significantly prevented the rise in HOMA in STZ treated animals. These findings suggest that N. sativa seed extract is pharmacologically effective in improving insulin sensitivity.
In the present study, significant decrease in blood glucose level, glycosylated haemoglobin, hyperinsulinemia and significant improvement in glucose tolerance and insulin sensitivity was observed in guinea pigs treated with N. sativa seed extract. Effects of higher doses were more significant than lower doses of extract. Protective effect of N. sativa seed extract was comparable to the standard drug i.e., glimepride. Therefore it is concluded that N. sativa seed extract might prove beneficial in preventing hyperinsulinemia, impaired glucose tolerance, insulin resistance and eventually an effective means of improving hyperglycemia, without any toxic effect at the dose used in the present study.
REFERENCES
- Hussain A, Claussen B, Ramachandran A, Williams R. Prevention of type 2 diabetes: A Review Diab Res Clin Prac. 2007; 76(3): 317-26.
- Klinegman RM, Behrman RE, Jenson HB, Stanton BF. (eds). Nelson Textbook of Pediatrics. 18th ed. Philadelphia Elsevier 2007: 2412-21.
- Paul SJ. Metabolic consequences of hyperglycemia and insulin resistance. Clin Cornerstone. 2007; (Suppl.7): 30-42.
- American Diabetic Association. Diagnosis and classification of diabetes mellitus Diabetes care. 2008; (31): 55-60.
- Zaoui A, Cherrah Y, Lacaille-Dubios MA, Settat A, Amarouch H, Hassar M. Diuretic and hypotensive effects of Nigella sativa in the hypertensive rat. Therapie. 2000; (55): 379-82.
- Kanter M, Meral I, Yener Z, Ozbek H, Demir H. Partial regeneration / proliferation of the β-cells in the islets of langerhans by Nigella sativa L in streptozotocin induced diabetic rats. Tohoku J Exp Med. 2003; (201): 213-19.
- Hanafy MS, Hatem ME. Studies on the antimicrobial activity of Nigella sativa seed (black cumin). J Ethmopharmacol. 1991; (34): 275-78.
- Al-Ghamdi MS. Anti-inflammatory analgesic and anti-pyretic activity of Nigella sativa. J Ethnopharmacol. 2001; (76): 45- 48.
- El-Tahir KEH, Ashour MMS, Al-Harbi MM. The cardiovascular actions of the volatile oil of the black seed (Nigella sativa) in rats. Elucidation of the mechanism(s) of action. Gen Pharmaco. 1993; (24): 1123-31.
- Khaled AAS. Gastroprotective effects of Nigella sativa oil on the formation of stress gastritis in hypothyroidal rats. Int J Physiol Pathophysiol Pharmacol. 2009; (1): 143-49.
- Nehar S, Kumari M. Protective effect of Nigella sativa seed oil on liver injury in rat. Proceedings of International Conference on Anthropogenic Impact on Environment & Conservation Strategy. The Ecoscan. 2012; (1): 409-12.
- Nehar S, Kumari M. Ameliorating Effect of Nigella sativa Oil in Thioacetamide-induced Liver Cirrhosis in Albino Rats. Indian J Pharm Edu Res. 2013; 47(2): 135-39.
- Nehar S. Hepatoprotective Effect of Nigella sativa Seed Oil on Rat Model of Alcoholic Liver Disease. International J Pharm Res Scho. 2014; 3(1): 464-72.
- Abel-salam BK. Immunomodulatory effect of black seed and garlic on alloxan induced diabetes in Albino rats. Allergol Immunopathol. (Madr) 2012; 40(6): 336-40.
- Nehar S, Rani P. HPTLC studies on ethanolic extract of Nigella sativa Linn. seeds and its phytochemical standardization. The Ecoscan. 2011; (1): 105-8.
- Houghton PJ, Zarka R, De Las Heras B, Hoult JRS. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995; (61): 33-36.
- Wang HJ, Jin YX, Shen W, Neng J, Wu T, Li YJ, Fu ZW. Low dose Streptozotocin (STZ) combined with high energy intake can effectively induce type-2 diabetes through altering the related gene expression. Asian Pac J Clin Nutr. 2007; 16(1): 412-217.
- Tran TT, Gupta N, Goh T, Naigamwalla D, Chia MC, Koohestani N, Mehrotra S McKeown-Eyssen G, Giacca A Bruce WR. Direct measure of insulin sensitivity with the hyperinsulinemic-euglycemic clamp and surrogate measures of insulin sensitivity with the oral glucose tolerance test: correlations with aberrant crypt foci promotion in rats. Cancer Epidemiol Biomarkers Prev. 2003; 12(1): 47-56.
- Bannon P. Effect of pH on the elimination of labile fraction of glycosylated Hemoglobin. Clin Chem. 1982; 28.
- Morgan CR, Lazarow A. Immunoassay of Insulin Two Antibody System. Diabetes. 1963; 12: 115.
- Murali B, Upadhyaya UM, Goyal RK. Effect of chronic treatment with Enicostemma littorale in non-insulin- dependent diabetic (NIDDM) rats. J Ethnopharmacol. 2002; 81: 199.
- Uno T, Ohsawa I, Tokudome M, Sato U. Effect of Goshajinkigan on insulin resistance in patients with type 2 diabetes. Diabetes Res Clin Pract. 2005; 69: 129.
- Cowie CC, Rust KF, Byrd-holt DD, Eberhardt MS, Flegal KM, Engelgau MM. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006; 29(6): 1263-68.
- Georg P, Ludvik KB. Lipid and Diabetes. J Clin Basic cardiol. 2000; (3):159-162.
- Chandra A, Singh RK, Tewari I. Anti- oxidative potential of herbal hypoglycaemic agents in diabetes an overview SFRR. Indian bullentin 2004; (3):24-26.
- Nyholm B, Porsen N, Juhi CB, Gravholt CH, Butler PC, Weeke J, Veldhuis J, Pincus S, Schmitz O. Secreation of insulin in relatives of patients with type 2 (Non– insulin dependent) Evidence of early b-cell dysfunction Metabolism. 2000; 49(7):896- 905.
- Defronzo RA. R.R.C.B and E.F. Pathogenesis of type 2 diabetes NIDDM: A Balanced overview. Dibetologia. 1992; 15(3)389-97.
- Olefsky JM. Insulin resistance in non– insulin dependent diabetes mellitus. Cur Opin Endocrin Diabet 1995; (2):290-299.
- Reaven GM. Role of insuli resistance in human diseases: Diabetes. 1988; (37):1595- 07.
- Vlassara H, Palace MR. Diabetes and advanced glycation end products. J Intern Med. 2002; 251(2): 87-180.
- Bonnefont – Rousselot D, Bastard JP, Joudon MC, Delattre J. Consequence of diabetic status on the Oxidant/Anti-oxidant balance: Diabetes Metab. 2000; (26):163- 176.
- Onorato JM, Jenkins AJ, Thorpes R, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation reactions alsoinhibits advanced lipoxidation reaction Mechanism of action of pyriodoxamine: J Biol Chem. 2000; (27)5: 21177-84.
- Al berti KGMM. The biochemistry and the complication of diabetes In Keen H JavveJ (eds) Complications of Diabetes: Edward Arnold Publishers London 1982; (2):31-70.
- Brownlee M, Hirsch IB. Glycemic variability a haemoglobin A1c independent risk factor for diabetic complications. JAMA. 2006; 295(14): 1707-08.
- Rahbar S, Blumenfeld O, Ramney HM. Studies on unusual haemoglobin in patients with diabetes mellitus: Bioche Biophys Res Commun. 1969; 36(5):838-43.
- Weykamp C, John WG, Mosca A. A review of the challenges in measuring haemoglobin A1c: Journal of Diabetes Science and Technology. 2009; 3(b): Symposium.
- Nyarkoa AK, Asare-Ananeb H ,Ofosuhenea M, Addyc M. Extract of Ocimum canum lowers blood glucose and facilitates insulin release by isolated pancreatic b-islet cell: Phytomedicine 2002; 9(4):346-51.
- Ali BH, Rasheed RA, Bashir AK, Padmanabhan R. Effect of Rhazya stricta on the rat fetus: Reprod Toxicol. 1997; 11(2- 3):191-9.
- Tolhan I, Ragoobirsingh D, Morrison EY. The effect of Capsaicin on blood glucose plasma insulin levels and insulin binding in dog models: Phytotheraphy Res. 2001; 15(5): 391-4.
- Abdel MA, El-Feki M, Saleh E. Effect of N sativa fish oil and localized on alloxan diabetic rats Biochemical and Histopath Studies: J Egypt Ger Soc Zool. 1998; (23):237-65.
- Groop L. Genetics of the metabolic syndrome: Br J Nutr 2000; (83):39-48.
- Ali MA, Sultana MC, Rahman BM, Khatune NA, Wahed MII. Antidiabetic activity of ethanolic extract of Semecarpus anacardium (linn.) stem barks in normal and alloxan induced diabetic rats: IJPSR. 2012; 3(8): 2680-85.
- Goel RK, Mahajan MP, Kulkarni, SK Evaluation of anti-hyperglycemic activity of some novel monocyclic beta lactams: J Pharm Sci. 2004; 7(1): 80-83.
- Brown JE Evans CAR. Luteolin rich artichoke extract protects low density lipoproti from oxidation in vitro: Free Radic Res. 1998; (29):242-55.
- Tenenbaum A, Fisman EZ, Motro M. Metabolic syndrome and type 2 diabetes mellitus focus on peroxisome proliferator activated receptors (PPAR): Cardiovasc Diabetol. 2003; 2-4.
- Bonara E, Francesco C, Simonetta L, Franco M, Luciano Z, Francesca S, Maurizio P, Sandro P, Andrea R, Vittorioe, Lorenza SGT, Ricardo B, Michele M. HOMA- estimated insulin-resistant is an independent predictor of cardiovascular diseases in type 2 diabetic subjects Prospective data for verolap diabetes complications study: Diacare. 2002; 5(7):1135-41.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences