Effects of Adansonia Digitata Pulp Extract on Ovarian Damage Induced by Carbon Tetrachloride (CCl4) in Adult Female Wistar Rats (Rattus Novergicus)
Ogunbiyi OE1, Oyewopo AO2, Johnson Olawumi F2*, Adunmo GO3 and Oyabambi AO4
1Department of Anatomy, Babcock University, Colleges of Health Sciences, IIishan-Remo, Nigeria
2Department of Anatomy, University of Ilorin, Ilorin, Nigeria
3Department of Medical Laboratory Science, University of Ilorin, Ilorin, Nigeria
4Department of Physiology, University of Ilorin, Ilorin, Nigeria
- *Corresponding Author:
- Johnson Olawumi F
Department of Anatomy
Faculty of Basic Medical Sciences
University of Ilorin
Ilorin
Nigeria
Tel: +2348035466102
E-mail: olawumi_2005@yahoo.co.uk
Received Date: April 06, 2021; Accepted Date: April 21, 2021; Published Date: April 28, 2021
Citation: Ogunbiyi OE, Oyewopo AO, Johnson Olawumi F, Adunmo GO, Oyabambi AO (2021) Effects of Adansonia digitata Pulp Extract on Ovarian Damage Induced by Carbon Tetrachloride (CCl4) in Adult Female Wistar Rats (Rattus Novergicus). J Women’s Health Reprod Med Vol. 5 No. 3: 13.
Abstract
Infertility, a couple’s inability to conceive after one year of unprotected regular intercourse, is an important issue in the world. The use of natural products in the treatment of infertility has been considered as a possible alternative to conventional therapies. This study was carried out in order to determine the curative effect of Adansonia digitata pulp extract on ovarian damage induced by carbon tetrachloride on the histology, level of hormone and the level of oxidative stress in the ovary of adult female Wister rats. Twenty adult female Wister rats were used as subjects for the experiment. The rats were then grouped into four (A, B, C, and D groups) containing five rats each. Group A (control) were given animal feed and distilled water, Group B rats were treated with 500 mg/kg of Adansonia digitata pulp extract, Group C rats received 2.5 ml/kg of carbon tetrachloride (CCl4 ), and Group D rats were treated with both Adansonia and CCl4 . Their ovaries were then removed and processed for histological examination, while their sera were taken for biochemical analysis and the supernatant from the homogenized tissues were used to analyse the level of oxidative stress. There were observable improvement in the structural appearance of the ovary of rats treated with Adansonia digitata pulp extract (Group B) while the ovary of rats treated with CCl4 (Group C) showed extreme damage to the structural outlook of the tissue. The ovaries of rats treated with both CCl4 and Adansonia digitata pulp extract showed an improvement in the damaged areas of the tissue. There was also an increase in the level of progesterone in rats treated with CCl4 compared to the control (Group A) and also an increase was observed in the level of eostrogen in the rats treated with CCl4 compared to control (Group A). The level of oxidative stress in the rats treated with CCl4 also increased when compared with the control group which indicates that damage has occurred to the tissue.
Keywords
Adansonia digitata; Carbon chloride; Female infertility; Ovarian damage; Oxidative stress
Introduction
Accumulating evidence indicates that the prevalence of human infertility has increased over the past decades [1]. Female infertility can be caused by failures at various steps, including ovulation, fertilization, embryo development, embryo transport, and implantation [2]. The different responses of environment toxicity include reduced fertility, spontaneous abortions, low birth weight, impaired folliculogenesis, and even damage to the ovaries [3]. Oxidative stress induces infertility in woman through a variety of mechanisms [4], having a direct effect on the oocyte, embryo, and implantation by causing cell membrane lipid peroxidation, cellular protein oxidation, and DNA damage [5]. Excess ROS in the follicle may overwhelm follicular fluid antioxidant defense and hinder the endometrium which normally functions to support the embryo and its development [4]. Appropriate development of embryo and receptive endometrium are crucial factors for successful implantation [6].
The human race is blessed with vast amount of plants which are consumed for their nutrients or for their medicinal purposes and in recent years have been shown to possess valuable antioxidants of great nutritional and therapeutic values. Antioxidants are substances which when present at low concentration compared to those of an oxidizable substrate [7], significantly delay or prevent the oxidation of that substrate. They are capable of preventing or attenuating damages such as lipid peroxidation, oxidative damage to membranes, glycation of proteins and inactivation of enzymes caused by free radicals. Some areas of human well-being and some forms of life depend on plants directly or indirectly [8]. The medicinal properties of some plants used locally have been scientifically proven over the years to be potent in managing different prevailing diseases and ailments [9].
Adansonia digitata (Baobab) is a tropical plant of African origin belonging to the Malvaceae family and is important to the livelihood of the people in arid zones of Africa [10]. Baobab is the common name of a genus (Adansonia) containing eight species of trees, Adansonia digitata is the most widespread of the Adansonia species on the African continent, found in the hot, dry savannahs of sub-Saharan Africa. It also grows, having spread secondary to cultivation, in populated areas and in neighbourhood villages. Its English common names include baobab, dead-rat tree (from the appearance of the fruits), monkey-bread tree (the soft, dry fruit is edible), upside-down tree (the sparse branches resemble roots) and cream of tartar tree it usually grows as solitary individuals, and are large and distinctive trees in the scrub on the savannah, and near settled areas, with some large individuals living to well over a thousand years of age [11]. Adansonia digitata fruit pulp has a very high vitamin C content which is ten times that of orange, and can be used in seasoning, as an appetizer and to make juices. Seeds contain appreciable quantities of crude protein, digestible carbohydrates and oil, whereas they have high levels of lysine, thiamine, calcium and iron. They can be eaten fresh or dried, ground into flour and thus added to soups and stews. Processing eliminates a number of anti-nutritional factors present in the seed [12].
Free radicals react with polyunsaturated fatty acids to propagate a chain reaction leading to lipid peroxidation or bind covalently to lipids and proteins, resulting in the destruction of membranes [13]. Peroxidation of the polyunsaturated fatty acids in biological membranes is believed to be the mechanism of toxicity for some chemicals such as carbon tetrachloride, ethanol, etc. Carbon tetrachloride was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent. It is a colourless, clear non-flammable liquid with a characteristics odour [14] that can be detected at low levels. It is a non-polar solvent suited for dissolving other non-polar compounds, fats, and oil and iodine. It is volatile giving off vapours with a characteristics smell of other chlorinated solvents. Exposure to high concentration of carbon tetrachloride can affect the central nervous system, degenerate liver, reproductive system and kidneys and it may also result into coma after a prolonged exposure to it cancer and even death in human and experimental animals [15]. Carbon tetrachloride is frequently used as a chemical inducer of experimental tissue damages and as a result of its molecular characteristics CCl4 does induce oxidative stress with the production of free peroxy radicals and lipoperoxides thereby damaging protein, DNA, and lipids [16]. There are several evidences that show that oxidative stress resulting from reactive oxygen species including free radicals such as Hydroxyl (OH), Superoxide (O-2), Nitric Oxide (NO), Nitrogen Dioxide (NO2), Peroxyl (ROO) and non-free radical like hydrogen peroxide and singlet oxygen play an important role in the development of several pathological conditions such as lipid peroxidation, protein oxidation, DNA damage and cellular degeneration. These have been implicated in the aetiology of these pathological conditions related to cardiovascular diseases, diabetes, cancer, Alzheimer and Parkinson disease, inflammatory diseases and infertility [17-19]. The present study was carried out to access the effect the pulp extract of Adansonia digitata will have on CCl4 induced ovarian damage in adult Wistar rats.
Materials and Methods
Experimental animals
Twenty (20) adult female Wistar rats weighing between 120-160 g were obtained from Ladoke Akintola University of Technology, Ogbomosho. The rats were housed at the animal house of the College of Health Sciences, University of Ilorin, Nigeria, kept in wooden cages at room temperature of 24 ± 3°C under a 12 h dark/light cycle. They were fed with standard rat pellets and allowed access to water. Study was performed in accordance with the ethical guidelines stipulated by the ethical committee of the College of Health Sciences, University of Ilorin, Nigeria. These guidelines were in accordance with the internationally accepted principles for laboratory animal use and care. The rats were randomly divided into four (4) groups with five (5) rats in each group. The groups are as follows: A (Control, distilled water), B (Adansonia digitata), C (CCl4) and D (CCl4 and Adansonia digitata).
Plant collection and the extraction
Adansonia digitata pod was plucked from College of Health Science, University of Ilorin premises. Identification and authentication was done at the Department of Plant biology, University of Ilorin, Nigeria. A voucher with specimen number was deposited at the herbarium in the department of plant biology, University of Ilorin, Nigeria. The outer white fruit pulp coating the seeds was separated from the seeds by soaking the seeds in mild water for 5 minutes. The suspension of pulp and seeds was subsequently filtered using filter paper and beaker the substrate was handpicked to remove the shaft and seed from the fruit pulp. The fruit pulp is now air dried for 72 hours after which it was grinded with mortar and pestle and dissolved in distilled water.
Standard dose of Adansonia digitata fruit pulp=500 mg/kg.
Each rat in group B and D were given extract solution according to their weights.
Experimental design
The rats were fed twice a day and Adansonia digitata extract was administered to the rats in groups B and D through oral administration using 2 ml calibrated syringe and cannula every day for 14 days. Carbon tetrachloride (2.5 ml/kg) was administered orally to the rats in group C and D for 3 days.
Animal sacrifice and collection of blood
The rats in each group were sacrificed by anaesthetizing them with chloroform after which the limbs were pinned to board and medial incision was done using a surgical blade, scalpel and forceps and the organ of study (ovary) was removed and it was immediately fixed in a labelled bottle containing fixative (10% formal saline) for 24 hours before tissue processing.
Blood samples were collected from the rats through the apex of the heart for biochemical analysis. Serum levels of progesterone, oestrogen was done using Enzyme Linked Immunosorbent Assay (ELISA).
Assessment of Malonaldehyde (MDA) level
To analyse lipid peroxidation, ovarian tissues were transferred into 5 ml ice-cold sucrose solution (0.25 M) and homogenized. The homogenates were further centrifuged at 3000 rpm for 15 min to obtain the supernatant, which was then aspirated with Pasteur pipette into sample bottle, stored overnight at 4°C before being used for assays. Tissue activities of MDA were determined by the method of [20].
Statistical analysis
Data were analyzed by Graph pad prism software, 8.0.25. Results were expressed as mean ± Standard Deviation (SD) and subjected to statistical analysis using one-way Analysis of Variance (ANOVA) followed by Duncan’s post-hoc test. The significant level considered was p<0.05 [21].
Results and Discussion
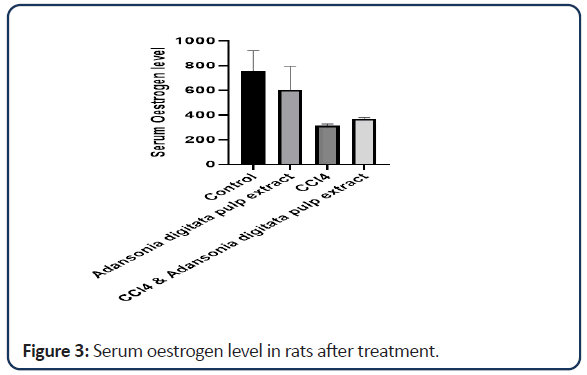
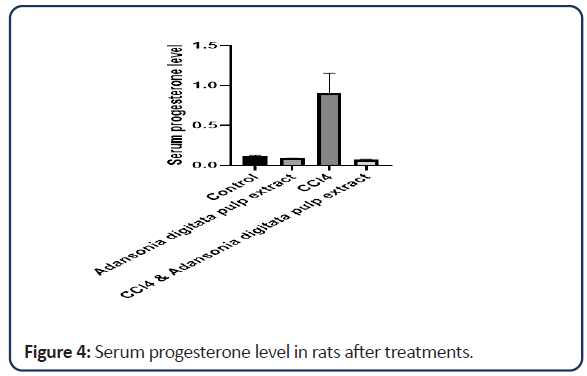
According to the data obtained (Tables 1-3 and Figures 1-6) it was discovered that the rats treated with Adansonia digitata pulp extract showed no significant effect on oestrogen level but there is moderate decrease in progesterone level when compared with the rats in the control group. Treatment with CCl4 was discovered to extremely increase the level of progesterone when compared with control group. This could be as a result of damage to the corpus luteum which is responsible for the production of progesterone in the ovary which may be as a result of haemorrhage due to the fragility of the blood vessels [22].
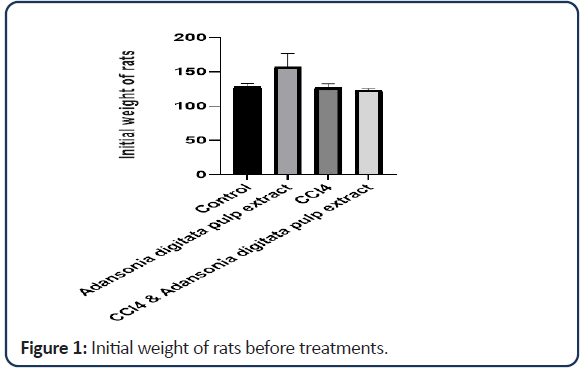
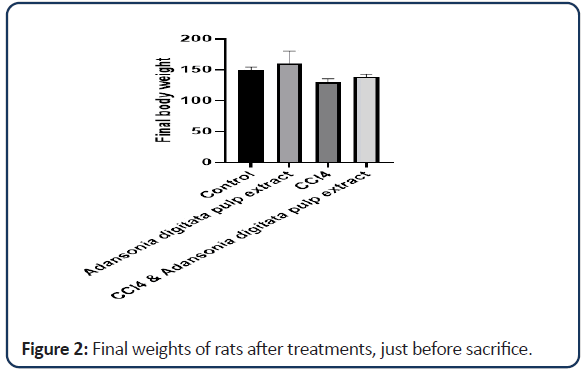
| Groups | Initial mean weight | Final mean weight | Weight differences |
|---|---|---|---|
| Control | 128.42 ± 4.73 | 150.00 ± 4.07 | 21.58 |
| Adansonia digitata pulp extract only | 157.26 ± 19.80 | 160.94 ± 19.72 | 3.68 |
| CCl4 only | 127.10 ± 5.56 | 129.84 ± 5.39 | 2.74 |
| CCl4 and Adansonia digitata pulp extract | 123.12 ± 2.46 | 138.60 ± 4.74 | 15.48 |
Note: Value expressed as mean ± SD; *P<0.05
Table 1: Effect of Adansonia digitata pulp extract and CCl4 on mean weight of rats.
| Groups | Oestrogen | Progesterone |
|---|---|---|
| Control | 759.15 ± 160.25 | 0.118 ± 0.008 |
| Adansonia digitata pulp extract only | 603.90 ± 191.20 | 0.087 ± 0.004 |
| CCl4 only | 316.05 ± 12.55 | 0.901 ± 0.2498* |
| CCl4 and Adansonia digitata pulp extract | 369.55 ± 10.95 | 0.070 ± 0.010 |
Note: Value expressed as mean ± SD; *P<0.05
Table 2: Effect of Adansonia digitata pulp extract and CCl4 level of oestrogen and progesterone in the rats.
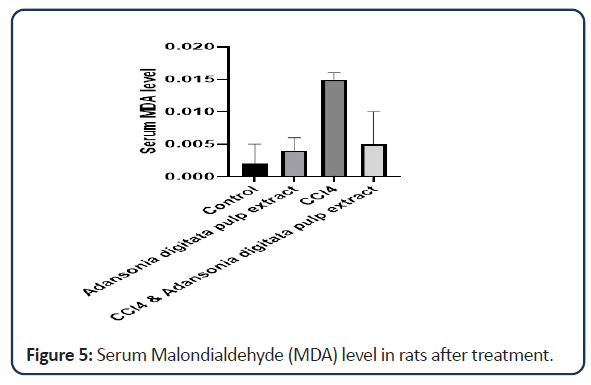
| Groups | Malondialdehyde (MDA) |
|---|---|
| Control | 0.002 ± 0.001 |
| Adansonia digitata pulp extract only | 0.004 ± 0.002 |
| CCl4 only | 0.015 ± 0.003 |
| CCl4 and Adansonia digitata pulp extract | 0.005 ± 0.005 |
Note: Value expressed as mean ± SD; P<0.05 (compared with control group)
Table 3: Effect of Adansonia digitata pulp extract and CCl4 level of Malondialdehyde (MDA).
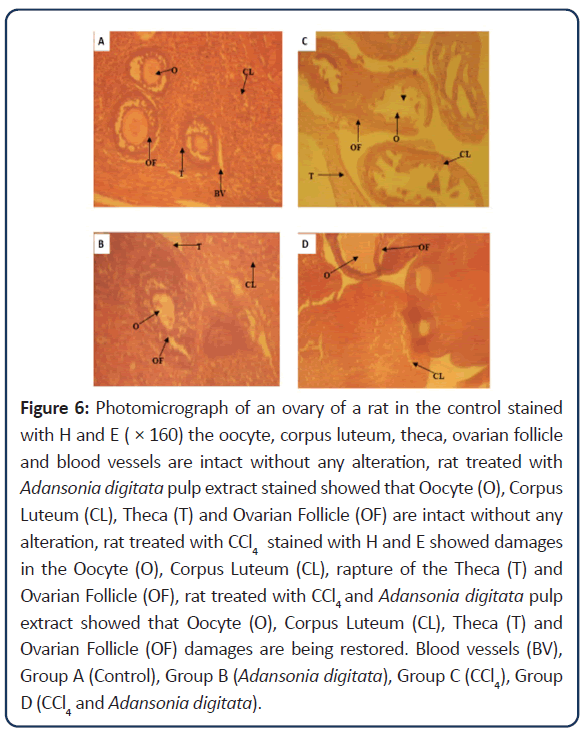
Figure 6: Photomicrograph of an ovary of a rat in the control stained with H and E ( × 160) the oocyte, corpus luteum, theca, ovarian follicle and blood vessels are intact without any alteration, rat treated with Adansonia digitata pulp extract stained showed that Oocyte (O), Corpus Luteum (CL), Theca (T) and Ovarian Follicle (OF) are intact without any alteration, rat treated with CCl4 stained with H and E showed damages in the Oocyte (O), Corpus Luteum (CL), rapture of the Theca (T) and Ovarian Follicle (OF), rat treated with CCl4 and Adansonia digitata pulp extract showed that Oocyte (O), Corpus Luteum (CL), Theca (T) and Ovarian Follicle (OF) damages are being restored. Blood vessels (BV), Group A (Control), Group B (Adansonia digitata), Group C (CCl4), Group D (CCl4 and Adansonia digitata).
Female infertility can be caused by failures at various steps, including ovulation, fertilization, embryo development, embryo transport, and implantation [2]. Oxidative Stress induces infertility in woman through a variety of mechanisms [4], having a direct effect on the oocyte, embryo, and implantation by causing cell membrane lipid peroxidation, cellular protein oxidation, and DNA damage [5]. Excess ROS in the follicle may overwhelm follicular fluid antioxidant defense and hinder the endometrium which normally functions to support the embryo and its development [4]. Oxidative stress has been implicated in a number of different reproductive scenarios such as endometriosis, folliculogenesis, oocyte maturation, hydrosalpingeal fluid, necrozoospermia, asthenozoospermia and sperm DNA damage [23]. In the context of female infertility, oxidative stress has been poorly characterized [24]. Nevertheless, oxidative stress in the female reproductive system has been demonstrated to correlate with fertility. Markers of oxidative stress in follicular fluid such as lipid peroxidation, total antioxidant capacity and superoxide dismutase activity are strongly correlated with oocyte fertilization and pregnancy rates following IVF [25]. A decrease in their total antioxidant capacity may lead to oxidative stress. The rats that were exposed to carbon tetrachloride showed a significant increase in the level of Malonialdehyde (MDA) which is an oxidative stress marker. The increase in the level of oxidative stress could be as a result of injury caused by exposing the ovary to high concentration of carbon tetrachloride. The rats treated with both CCl4 and Adansonia digitata pulp extract decreases the stress that has been caused by exposure to CCl4. But there is no significant difference between the rats treated with Adansonia digitata pulp extract and the rats in the control group.
Numerous studies have shown that CCl4 exposure causes morphological and functional reproductive disorders through oxidative toxicity [26]. Many antioxidants have been used to overcome these reproductive disorders [27-29]. Oxidative stress results from the free oxygen radicals, which include superoxide anion (O⁻₂), H2O2 and the Hydroxyl Ion (OH), in excess of the enzymatic and non-enzymatic antioxidants of the stressed tissue. Free radicals have high affinity to cell membrane lipids, especially PUFAs, leading to tissue damage due to the LPO [30]. According to Manibusan, Manjrekar and others [31,32,28] shown that CCl4 causes structural and functional damages in other organs of body such as kidney, lung, reproductive organs and brain. The free radicals initiate Lipid Peroxidation (LPO) by attacking Polyunsaturated Fatty Acids (PUFAs) in membranes, setting off a free radical chain reaction sequence. LPO is known to cause membrane disruption, resulting in the loss of membrane integrity and the leakage of microsomal enzymes. By products of LPO can form protein and DNA adducts and may contribute to toxicity and carcinogenicity [28,31,32]. The Histological findings in this study observed a collapse of the ovarian follicles which comprises of granulosa cells in the animals treated with carbon tetrachloride, and resulted in a degree of cytoarchitectural distortion when compared with the Control group. The cytoarchitecture of the ovary of rats treated with Adansonia digitata pulp extract remained unchanged when compared with the rats in the control group, but there is improvement in the cytoarchitecture of the ovary of rats treated with both carbon tetrachloride and Adansonia digitata pulp extract when compared with the rats treated with carbon tetrachloride.
Conclusion
In conclusion, the observations from this study showed that Adansonia digitata pulp extract attenuated the effect of the damage caused by carbon chloride on the ovary which can lead to infertility through causing a decline in lipid peroxidation and ameliorated the damage on the histology of the testes.
Conflict of Interest
The authors declare no actual or potential conflict of interest.
References
- Kokubu D, Ooba R, Abe Y, Ishizaki H, Yoshida S, et al. (2019) Angelica keiskei (ashitaba) powder and its functional compound xanthoangelol prevent heat stress-induced impairment in sperm density and quality in mouse testes. Dev Reprod 2018-2141.
- Choi HJ, Chung TW, Park MJ, Jung YS, Lee SO, et al. (2017) Water-extracted tubers of cyperus rotundus l. Enhance endometrial receptivity through leukemia inhibitory factor-mediated expression of integrin avß3 and avß5. J Ethnopharmacol 208:16-23.
- Uchewa OO, Ezugworie OJ (2019) Countering the effects of lead as an environmental toxicant on the microanatomy of female reproductive system of adult wistar rats using aqueous extract of Ficus Vogelii. J Trace Elem Med Biol 52:192-198.
- Adeoye O, Olawumi J, Opeyemi A, Christiania O (2018) Review on the role of glutathione on oxidative stress and infertility. JBRA Assist Reprod 22(1):61.
- Smits RM, Mackenzie-Proctor R, Fleischer K, Showell MG (2018) Antioxidants in fertility: Impact on male and female reproductive outcomes. Fertil Steril 110(4):578-580.
- Kim EY, Choi HJ, Chung TW, Choi JY, Kim HS, et al. (2016) Water-extracted perilla frutescens increases endometrial receptivity though leukemia inhibitory factor-dependent expression of integrins. J Pharmacol Sci 131(4):259-266.
- Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine.
- Oyetunji OA, Babatunde IR, Chia SL, Abraham OA, Adewale FB, et al. (2015) Ameliorative effects of Adansonia digitata leaf extract on Carbon Tetrachloride (CCl 4) induced testicular toxicity in adult male wistar rats. AJOA 4(1):481-487.
- Sofowora A, Ogunbodede E, Onayade A (2013) The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med 10(5):210-229.
- Sidibe M (2002) Baobab, Adansonia Digitata L. Crops for the Future.
- Al-Qarawi AA, Al-Damegh MA, El-Mougy SA (2003) Hepatoprotective influence of Adansonia digitata pulp. J Herbs Spices Med Plants 10(3):1-6.
- de Caluwé E, Halamouá K, Van Damme P (2010) Adansonia digitata L: A review of traditional uses, phytochemistry and pharmacology. Afrika Focus 23(1):11-51.
- Aneja R, Upadhyaya G, Prakash S, Dass SK, Chandra R (2005) Ameliorating effect of phytoestrogens on CCl4-induced oxidative stress in the livers of male Wistar rats. Artificial cells, blood substitutes, and biotechnology 33(2):201-213.
- Saleh TA, Alhooshani KR, Abdelbassit MS (2015) Evaluation of AC/ZnO composite for sorption of dichloromethane, trichloromethane and carbon tetrachloride: Kinetics and isotherms. J Taiwan Inst Chem E 55:159-169.
- Rood AS, McGavran PD, Aanenson JW, Till JE (2001) Stochastic estimates of exposure and cancer risk from carbon tetrachloride released to the air from the rocky flats plant. Risk Analysis 21(4):675-696.
- Khan MR, Younus T (2011) Prevention of CCl4-induced oxidative damage in adrenal gland by digera muricata extract in rat. Pak J Pharm Sci 24(4):469-473.
- Jenardhanan P, Panneerselvam M, Mathur PP (2016) Effect of environmental contaminants on spermatogenesis. Semin Cell Develop Biol 59:126-140.
- Kabir ER, Rahman MS, Rahman I (2015) A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol 40(1):241-258.
- Krieg SA, Shahine LK, Lathi RB (2016) Environmental exposure to endocrine-disrupting chemicals and miscarriage. Fertil Steril 106(4):941-947.
- Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE (2005) Biomarkers of oxidative stress study: III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radic Biol Med 38(6):711-718.
- Duncan RC, Knapp RG, Miller III CM (1977) Introductory biostatistics for the health sciences. Biometrics 40(4):1212.
- Campbell J, Conklin F, Chang V, Singh K, Hurteau G (1974) Ovarian apoplexy, ovarian pregnancy and the IUCD. Obstet Gynecol Surv 29(4):287.
- Kamarzaman S, Shaban M, Abdul Rahman S (2014) The prophylactic effect of Nigella Sativa against cyclophosphamide in the ovarian follicles of matured adult mice: A preliminary study. J Anim Plant Sci 24:81-88.
- Agarwal A, Allamaneni SS (2004) Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online 9(3):338-347.
- Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, et al. (2004) Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril 81(4):973-976.
- Abdou HS, SH S, Booles HF, EA AR (2012) Effect of pomegranate pretreatment on genotoxicity and hepatotoxicity induced by Carbon Tetrachloride (CCl4) in male rats. JMPR 6(17):3370-3380.
- Fadhel ZA, Amran S (2002) Effects of black tea extract on carbon tetrachloride-induced lipid peroxidation in liver, kidneys, and testes of rats. Phytother Res 16(S1):28-32.
- Manjrekar AP, Jisha V, Bag PP, Adhikary B, Pai MM, et al. (2008) Effect of Phyllanthus niruri Linn. treatment on liver, kidney and testes in CCl4 induced hepatotoxic rats.
- Soliman AM, Fahmy SR (2011) Protective and curative effects of the 15 KD isolated protein from the Peganum harmala L. Seeds against carbon tetrachloride induced oxidative stress in brain, tests and erythrocytes of rats. Eur Rev Med Pharmacol Sci 15(8):888-899.
- Sonmez M, Turk G, Ceribasi S, Ciftci M, Yuce A, et al. (2014) Quercetin attenuates carbon tetrachloride-induced testicular damage in rats. Andrologia 46(8):848-858.
- Manibusan MK, Odin M, Eastmond DA (2007) Postulated carbon tetrachloride mode of action: A review. J Environ Sci Health C 25(3):185-209.
- Khan MR, Ahmed D (2009) Protective effects of Digera muricata (L.) Mart on testis against oxidative stress of carbon tetrachloride in rat. Food Chem Toxicol 47(6):1393-1399.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences