ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Dynamic kinetic Asymmetric Transformations with Hard Nucleophiles: Cyclopentens for Antituberculosis and Antibiotic
Hadia Almahli*
Department of Chemistry, University of Oxford, Oxford, United Kingdom
- *Corresponding Author:
- Hadia Almahli
Department of Chemistry
University of Oxford
Oxford, United Kingdom
Tel: +01865 276157
E-mail: hadia.almahli@yahoo.com
Received Date: September 19, 2018; Accepted Date: October 01, 2018; Published Date: October 11, 2018
Citation: Almahli H (2018) Dynamic kinetic Asymmetric Transformations with Hard Nucleophiles: Cyclopentens for Antituberculosis and Antibiotics. Synth Catal 3:4. DOI: 10.4172/2574-0431.100022
Introduction
Asymmetric additions of alkyl nucleophiles to racemic allylic chlorides will be used to access derivatives of important cyclopentene containing natural products. We describe in this paper the asymmetric additions of alkyl nucleophiles to racemic allylic chlorides, to access cyclopentenyl fatty acids. These natural products have timely biological activity and the eventual synthesis of derivatives will help develop structure-activity relationships. Cyclopentene natural products Alepric acid [1], aleprestic acid [1], and gorlic acid [1], have not previously had their synthesis reported. The asymmetric addition reaction is a dynamic kinetic asymmetric transformation (DYKAT) to a racemic allylic chloride to give cyclopentenes with high level of ee [2]. The bioassay results showed that gorlic acid has unusual selectivity against positive gram bacteria.
Copper-catalyzed asymmetric 1, 4-addition to α, β- unsaturated compounds is now well developed, but cyclopentenone remains a special case and addition of hard nucleophiles is notoriously difficult. Yields are generally limited by competitive reactions of the highly reactive enolate generated in the reaction, and much lower ee values than observed with other substrates are obtained. This commonly attributed to the “flatness” of the 5-memberd ring substrate.
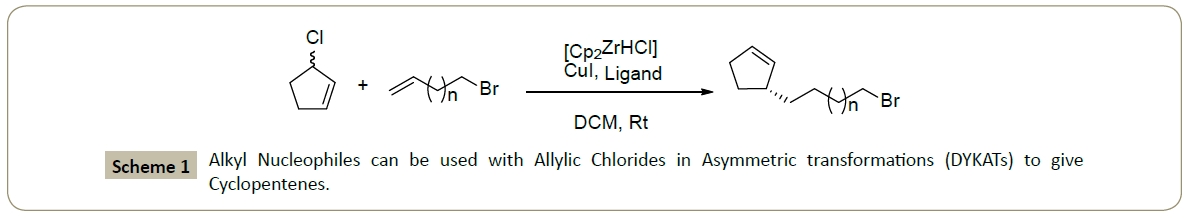
Fletcher group [2] recently demonstrated that alkyl nucleophiles can be used with racemic allylic chlorides in dynamic kinetic asymmetric transformations (DYKATs) to give cyclopentenes with high levels of ee (Scheme 1). These reactions work at room, rather than cryogenic-temperatures, which is unusual for asymmetric reactions with hard nucleophiles and practically significant.
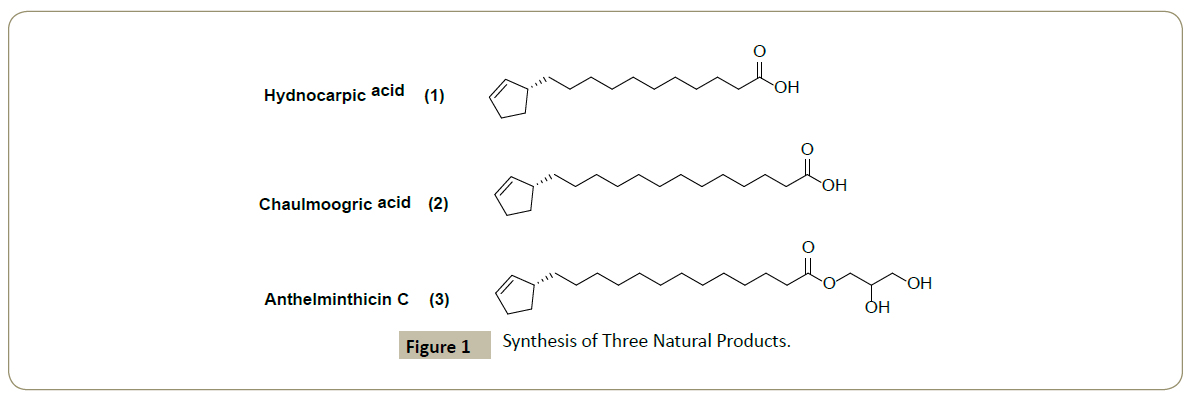
Fletcher group [2] applied the method to short, straightforward, enantioselective syntheses of three natural products (Figure 1) which compare favorably to previously reported syntheses, and will facilitate the preparation of analogues, these acids have been first isolated from Hydnocarpus wightiana oil (chaulmoogric oil) by Cole and Cardoso [1] in 1939, this oil had been used in the East against leprosy and various skin conditions for many hundreds of years, and was the standard remedy [3,4] for leprosy.
Hydnocarpic acid and chaulmoogric acid were used in ancient and traditional medicine, particularly as leprosy treatments; in both cases the cyclopentenyl ring is a requirement for biological activity. (1) is believed to act by blocking the activity of biotin or inhibiting microbial biotin synthesis [5], while the activity of (2) is likely due to incorporation into the cell wall lipids of M. leprae [6]. A recent report shows that (2) and anthelminthicin C (3) significantly inhibit M. tuberculosis growth with MIC values of 9.82 and 4.38 μM respectively [7]. (3) also inhibits para-aminobenzoic acid biosynthesis (PABA) [7], inhibitors of pABA are important leads for new antibiotics, as this pathway is not found in humans [8].
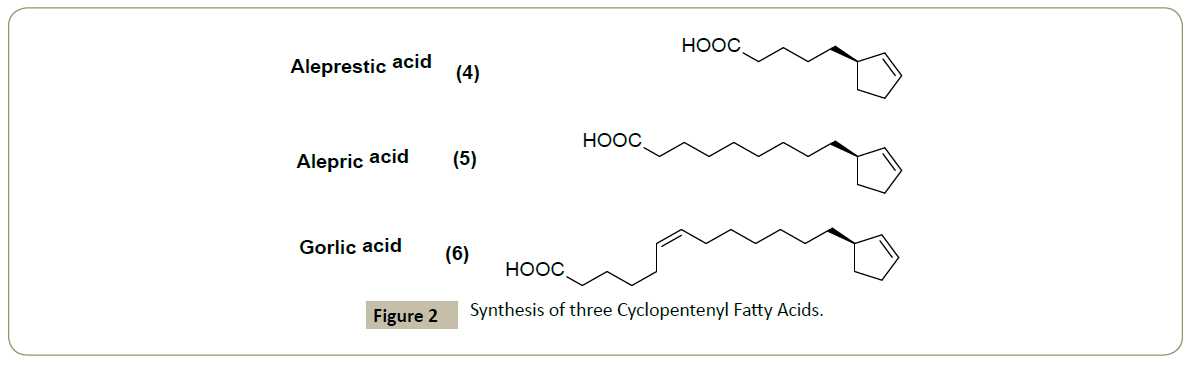
In our project, we prepared three cyclopentenyl fatty acids, which never been synthesized, Alepric acid (4), Aleprestic acid (5) and Gorlic acid (6) (Figure 2).
In the literature, it is believed that aleprestic acid has an inhibitory activity against glycolysis [9] and gorlic acid has an anti-bacterial activity [9,10]. These natural products are supposed to have an anti-bacterial, anti-leprosy and anti-tuberculosis activity.
Our bioassay results showed an interesting activity of gorlic acid 3.91 μg/mL towards the positive gram bacteria Staphylococcus aureus.
The goal of this study is to use DYKATs to rapidly access a variety of new natural products, it is hoped that these will be used in Structure activity relationship studies and as possible leads for new anti-tuberculosis and anti-biotic agents.
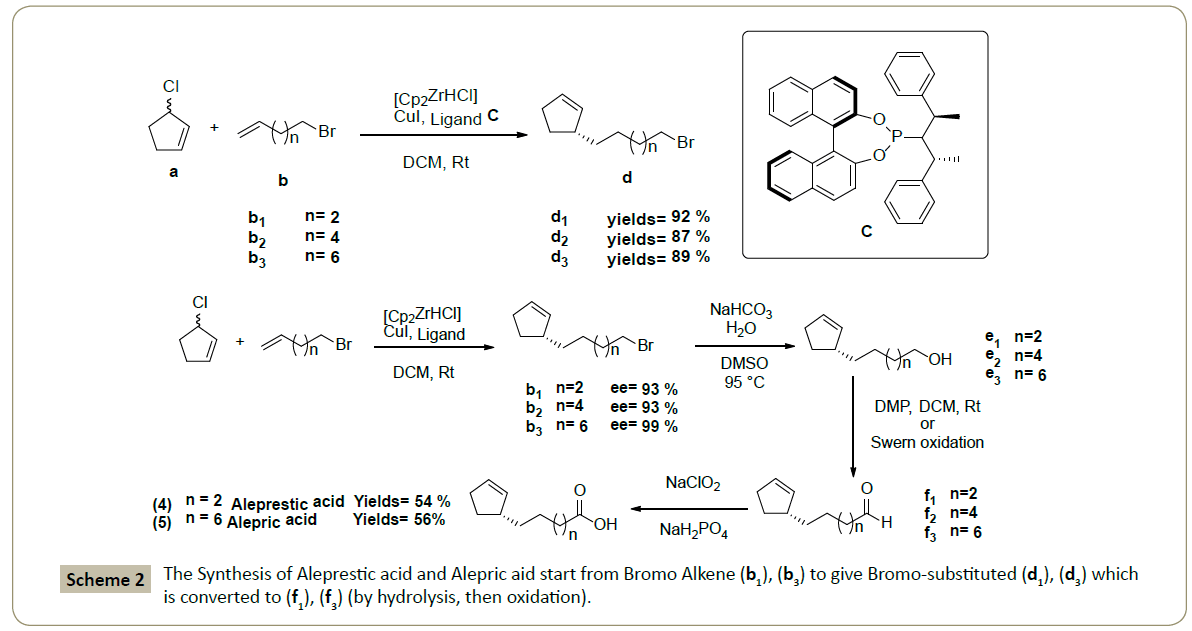
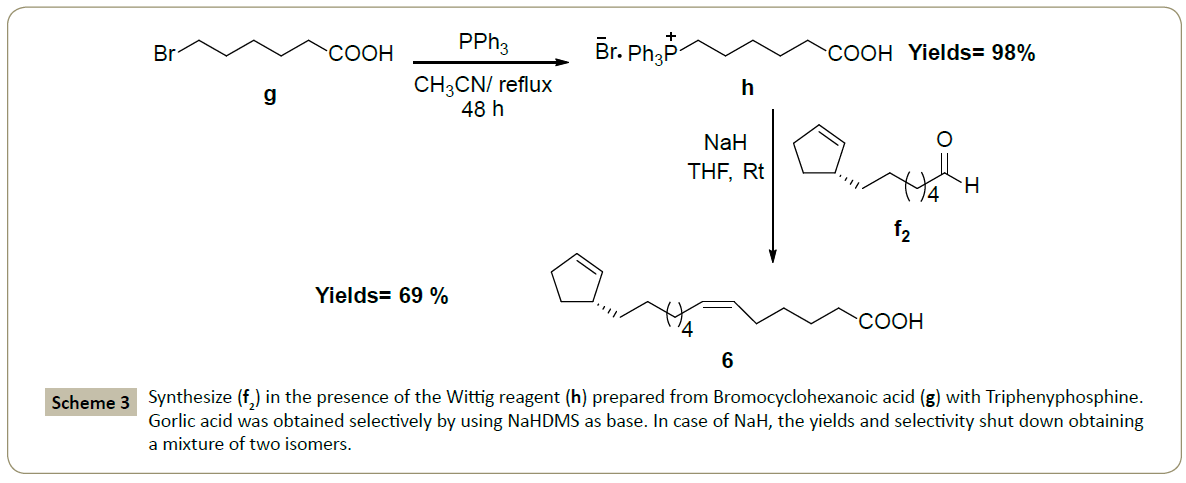
The syntheses of (4), (5) start from commercially available bromo alkene (b1), (b3) to give bromo-substituted (d1), (d3) which is converted to (f1), (f3) (by hydrolysis, then oxidation) (Scheme 2). We followed the same method to synthesize (f2) which was subjected to Wittig reaction [10,11] in the presence of the Wittig reagent (h) prepared [12,13] from bromocyclohexanoic acid (g) with triphenyphosphine (Scheme 3). Gorlic acid (6) was obtained selectively (one isomer: cis) with good yields 69 % by using NaHDMS as base (Scheme 3). In contrary, in case [14] of NaH, the yields and selectivity shut down (yields= 13%) obtaining a mixture of two isomers E/Z: 3/2 (Scheme 3), while the using of n-BuLi [14] didn’t led to the desired product.
Finally, we succeeded to prepare three natural products never been synthesized previously by selective methods (See supplementary information for full details).
Acknowledgement
We gratefully acknowledge financial support to HA from Christ Church College (Oxford), the CARA Fellowship Programme, and the Oxford Chemistry. We thank Prof. Stephen Fletcher for his support and guidance in this project. We also thank Alexander Pretsch and Dagmar Pretsch, Oxford Antibiotic Group, University and Research Centre, Konrad Lorenz Str for their assistance with bioassays.
References
- Cole HI, Cardodo HT (1939) Alepric, Aleprylic, Aleprestic and Aleprolic Acids, New Homologs of Chaulmoogric Acid. J Am Chem Soc 61: 2349-51.
- You H, Rideau E, Sidera M, Fletcher S (2015) Non-stabilized nucleophiles in Cu-catalysed dynamic kinetic asymmetric allylic alkylation. Nature 517: 351-355.
- Norton SA (1994) Useful plants of dermatology. I. Hydnocarpus and chaulmoogra. J Am Acad Dermatol 31: 683-86.
- Parascandola J (2003) Chaulmoogra oil and the treatment of leprosy. Pharm Hist 45: 47-57.
- Jacobsen PL, Levy L (1973) Mechanism by which hydnocarpic acid inhibits mycobacterial multiplication. Antimicrob Agents Chemother 3: 373-79.
- Cabot MC, Goucher CR (1981) Chaulmoogric acid: assimilation into the complex lipids of mycobacteria. Lipids 16: 146-48.
- Wang JF, Dai HQ, Wei YL, Zhu HJ, Yan YM, et al. (2010) Antituberculosis agents and an inhibitor of the para-aminobenzoic acid biosynthetic pathway from Hydnocarpus anthelminthica seeds. Chem Biodivers 7: 2046-53.
- O'Connell KM, Hodgkinson JT, Sore HF, Welch M, Salmond GP, et al. (2013) Combating multidrug-resistant bacteria: current strategies for the discovery of novel antibacterials. Angew Chem Int Ed: 52: 10706-33.
- Manly RS, Hargreaves G, Pillard R (1959) Glycolysis inhibitors among compounds containing aldehydes, ketones, and organic acids. Arch Oral Biol 1: 145-60.
- Almahli H (2017) Cyclopentenyl Fatty Acids: History, Biological Activity and Synthesis. Curr Top Med Chem 17: 2903-12.
- Peng S, McGinley CM, van der Donk WA (2004) Synthesis of sitespecifically labeled arachidonic acids as mechanistic probes for prostaglandin H synthase. Org Lett 6: 349-52.
- Marinzi C, Offer J, Longhi R, Dawson PE (2004) An o-nitrobenzyl scaffold for peptide ligation: synthesis and applications. Bioorg Med Chem 12: 2749-57.
- Ramon-Azcon J, Galve R, Sánchez-Baeza F, Marco MP (2006) Development of an enzyme-linked immunosorbent assay for the determination of the linear alkylbenzene sulfonates and longchain sulfophenyl carboxylates using antibodies generated by pseudoheterologous immunization. Anal Chem 78: 71-81.
- Sankaranarayanan S, Chattopadhyay S (1998) Asymmetric synthesis of a cytotoxic amide of Telesto riisei. Tetrahedron: Asymmetry 9: 1345-50.
- Huleatt PB, Lau J, Chua S, Tan YL, Duong HA, et al. Concise, efficient and practical assembly of bromo-5,6-dimethoxyindole building blocks. Tetrahedron Lett 52: 1339-1342.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences