ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Differential Desensitisation of Afferent Nerve Fibres by Iberis amara (STW 6) and Iberogast (STW 5) in the Murine Colon
Mueller MH1*, Seeliger H3*, Hering N3, Kasparek MS4, Abdel AH2 and Kreis ME3
1Vivantes Klinikum Neukoelln, Department of Visceral and Minimal Invasive Surgery, Berlin, Germany
2Steigerwald Arzneimittelwerk GmbH, Darmstadt, Germany
3Charité University Medicine, Campus Benjamin Franklin, Department of General Visceral and Vascular Surgery, Berlin, Germany
4Department of General Surgery, Josephinum Hospital, Munich, Germany
- *Corresponding Author:
- Mario M
Vivantes Klinikum Neukoelln, Department
of Visceral and Minimal Invasive Surgery
Berlin, Germany
Tel: +49030130142061
E-mail: mario.mueller@vivantes.de
- *Corresponding Author:
- Seeliger H
Department of General Visceral and Vascular Surgery
Charite University Medicine Campus, Benjamin Franklin
Berlin, Germany
Tel: +4930450522702
E-mail: hendrik.seeliger@charite.de
Received date: January 17, 2018; Accepted date: January 29, 2018; Published date: January 31, 2018
Citation: Mueller MH, Seeliger H, Hering N, Kasparek MS, Abdel AH , et al. (2018) Differential Desensitisation of Afferent Nerve Fibres by Iberis amara (STW 6) and Iberogast (STW 5) in the Murine Colon. Am J Phytomed Clin Ther Vol. 6 No. 1:4. doi: 10.21767/2321-2748.100340
Abstract
Spontaneous afferent discharge was not different among the subgroups. STW 6 reduced afferent nerve discharge following 5-HT administration to 0.2 ± 0.2 imp sec‑1 compared to 3.0 ± 1.0 imp sec‑1 following STW 5 and 5.0 ± 2.0 imp sec‑1 in vehicle controls (p<0.05 STW 6 vs. vehicle). There was no difference in the response to bradykinin after addition of STW6 (36.0 ± 5.0 imp sec-1) and vehicle (38.0 ± 6.0 imp sec‑1), respectively, while this was reduced to 9.0 ± 2.0 imp sec‑1 following STW 5 (p<0.05 compared to vehicle). Afferent discharge to luminal ramp distension up to 80 cmH2O was reduced following STW 5 and STW 6 pre-treatment compared to vehicle (p<0.05). At high-threshold distension pressures a more pronounced reduction was observed following STW 5.
Keywords
Afferent nerve discharge; Colon; Phytomedicine; Visceral hypersensitivity
Abbreviations: STW 5: Iberogast; STW 6: Bitter Candy Tuft; 5-HT: Serotonin; BK: Bradykinin; CED: Cambridge Electronic Design; EC cells: Enterochromaffin cells; ANOVA: Analysis of Variance; SEM: Standard Error of the Mean; Imp: Impulses; Sec: Seconds; Min: Minutes.
Introduction
Incidence of functional gastrointestinal disorders such as functional dyspepsia or irritable bowel syndrome is high [1] and, therefore, has tremendous epidemiologic significance [2]. These disorders are not life threatening; however, they may have severe impact on quality of life for affected patients [3]. Preparations containing herbal extracts such as STW 5 (Iberogast, Steigerwald Arzneimittelwerk GmbH, Darmstadt, Germany) were shown to be effective to treat these disorders in clinical trials [4]. STW 5 is a cocktail of different herbal plant extracts that has been used for the therapy of functional gastrointestinal disorders for decades [5]. Comprehensive research was performed to explore mechanisms of action of STW 5 in detail, since the drug was initially designed simply based on empirical physician experience. This research provided evidence that STW 5 exerts multiple effects on gastrointestinal physiology. Experimental data showed multi-target action of STW 5, spasmolytic and prokinetic effects depending on the part of the gastrointestinal tract investigated [6]. It was shown that STW 5 enhances antral motility [7]. Furthermore, STW 5 seems to have antiinflammatory [8] and pro-secretory effects [9]. Although experimental data provides evidence for STW 5 effects, it does not address visceral hypersensitivity that is considered to be a key mechanism in the development of functional gastrointestinal disorders [10]. The current concept of visceral hypersensitivity is well-established and suggests that physiological stimuli of the gastrointestinal tract, that are usually below threshold of consciousness, are not filtered as in healthy individuals but are rather perceived as pain or abdominal discomfort. To investigate a potential modulatory action of STW 5 on visceral sensitivity, we tested a panel of previously standardized stimuli in a rat model [11] and demonstrated that STW 5 reduced visceral afferent sensitivity to bradykinin (BK), serotonin (5-HT) and mechanical stimulation. These stimuli were chosen as they sensitize a wide range of intestinal afferents in small bowel and colon. STW 5 reduces afferent neuronal sensitivity to many stimuli. This may be attributed to the fact that the preparation contains different herbal extracts. These observations lead to the question whether a detailed investigation of single drug compounds may help to identify specific actions. This may potentially allow improving this herbal preparation by combining specific effects of single compounds. The aim of the present study was, therefore, to investigate the effect of the main compound bitter candytuft (Iberis amara, STW 6) on colonic afferent sensitivity. We hypothesized that bitter candytuft is capable to reduce afferent sensitivity only for specific stimuli in contrast to STW 5.
Methods
Herbal preparation
The herbal preparation STW 5 contains nine herbal extracts including an alcoholic fresh plant extract of bitter candytuft (Iberis amara) and alcoholic extracts of angelica root (Angelicae radix) milk thistle fruit (Silybi mariani fructus), celandine herb (Chelidonii herba), liquorice root (Liquiritiae radix, chamomile flower (Matricariae flos) balm leaf (Melissae folium) caraway fruit (Carvi fructus) and peppermint herb (Menthae piperitae folium). Solutions of STW 5 and bitter candytuft (STW 6) were prepared from lyophilised samples obtained from Steigerwald Arzneimittelwerk GmbH, Darmstadt, Germany by adding normal saline. The solutions were prepared as follows: STW 5 dissolved in normal saline 57.1 × 10-6 mg ml-1, lyophilised STW 6 21.2 × 10-6 mg ml-1 and vehicle (saline) (Table 1). Validated analytical methods were used to characterize the preparations and the single extracts which are classified as other herbal substances according to the guidelines of the European Medicine Agency. Quality control and extraction processes were performed according the Good Manufacturing Practice and Good Agricultural Practice of Medicinal and Aromatic Plants. Details were extensively described in previous studies [12-14].
| Code (Batch number) | Plant origin | Drug extract ratio | Concentration (micro l/ml) | Content in STW5 |
|---|---|---|---|---|
| STW 6 (13187) | Iberis amara totalis recens | 1:1.5-2.5 | 1.5 | 15% |
| STW 5KII (13182) | Menthae piperitae folium | 1:2.5-3.5 | 1 | 5% |
| STW KIII (13176) | Matricariae flos | 1:2-4 | 3 | 20% |
| STW KIV (13192) | Liquiritiae radix | 1:2.5-3.5 | 1 | 10% |
| STW KV (13175) | Angelicae radix | 1:2.5-3.5 | 1 | 10% |
| STW KVI (13188) | Carvi fructus | 1:2.5-3.5 | 2 | 10% |
| STW KVII (23033) | Silybi mariani fructus | 1:2.5-3.5 | 1 | 10% |
| STW KVIIII (13195) | Melissar folium | 1:.5-3.5 | 1.5 | 10% |
| STW KIX (23044) | Chelidonii herba | 1:2.5-3.5 | 1 | 10% |
Table 1: Specific plant extracts of STW5.
Animals
Male C57BL/6 mice (Charles River, Sulzfeld, Germany) weighing approximately 20 g were kept under a 12 h/12 h dark/light cycle with free access to food and water. The study protocol was approved by the local Institutional Review Board (Government of Bavaria) prior to experiments. Animals were sacrificed by an anesthetic overdose (Isoflurane, Abbott, Baar, Switzerland) before the colon was harvested with the adjacent mesentery. The distal colon was removed along with the lumbar colonic nerves, and the bundle containing the inferior mesenteric ganglion, intermesenteric nerve, inferior mesenteric artery was then transferred into pre-gazed Kreb’s solution described below.
Technique of afferent nerve recordings
Details of in vitro afferent nerve recording were described previously. The entire colon was placed in a culture dish containing ice-cold Kreb’s solution (mM: Na+ 143.5, K+ 5.9, Cl- 126, Ca2+ 2.5, Mg2+ 1.2, H2PO4 1.2, SO4 1.2, HCO3- 25, glucose 10 and sodium butyrate 1, pH 7). Then, the cecum was removed. The mesentery of the ascending colon was preserved and placed into a custommade two-chamber organ bath for afferent nerve recordings. The colonic segment was superfused with Kreb’s buffer gassed with an O2/CO2 mixture (95% / 5%) at a rate of 10 ml min-1, temperature 32°C. In order to avoid artefacts during afferent nerve recordings, spontaneous colonic motility was inhibited by the L-type calcium channel blocker Nifedipine (Sigma Chemicals, St. Louis, MO, USA). Nifedipine was added to the perfusion solution at a concentration of 1 μM as previously described to reduce smooth muscle activity and to inhibit degranulation of mast cells and enterochromaffin cells (EC cells) to avoid secondary effects [15,16]. The mesenteric neurovascular bundle was then pulled through a small gap into the recording chamber. The opening was closed with vaseline. Then the recording chamber was filled with colourless heavy liquid paraffin (Sigma Chemicals, St. Louis, MO, USA) for insulation and heated to 32°C. Cannulas were inserted into the lumen on both ends of the colonic segment. The lumen was continuously perfused with Kreb’s solution (10 ml per hour). The distal cannula remained open to air. Intraluminal pressure was recorded with a connected pressure transducer (Neurolog pressure amplifier NL 108, Digitimer Ltd., Welwyn Garden City, UK). In the recording chamber, the mesenteric nerve was dissected off the neurovascular bundle which is located in the mesentery. Then, the mesenteric nerve was placed on one arm of a bipolar platinum electrode, while connective tissue served as a reference on the other arm. Electrodes and pressure transducer were connected to an amplifier/filter system (1902 Cambridge Electronic Design (CED), Cambridge, UK) and transferred to a power Micro 1401 AD interface system (CED). Recordings were monitored online by running Spike 2 software (version 4.01; CED) and saved to the hard drive for offline analysis.
Chemical and mechanical stimulation
After establishing the afferent nerve recording, baseline discharge was monitored for 15 minutes to ensure a stable signal. Then, serosal perfusion of the organ bath was stopped and 10 μM 5-HT (Sigma Chemicals) was added to the perfusion chamber. After two minutes incubation time a washout period of 10 minutes was started to allow the signal to recover to baseline. For mechanical ramp distension up to 80 cmH2O, the cannula connected to the colonic outlet was closed until reaching an intraluminal pressure of 80 cmH2O. Then the outlet cannula was opened and pressure was released. After a recovery period of 10 minutes, the serosal perfusion was stopped again and BK (Sigma Chemicals) at a concentration of 0.5 μM was added to the organ bath for an incubation period of two minutes. 5-HT and BK dose response experiments were performed previously (data not shown). Each recording was started 30 s prior to 5-HT and BK application and maintained for 2 minutes.
Pretreatment
Colonic segments were pretreated in vitro to evaluate afferent nerve sensitivity as follows:
1. Superfusion of colonic segments in vitro with STW 6 10 minutes prior to chemical and mechanical stimulation (21.2 ×10-6 mg ml-1; lyophilized powder dissolved in 0.9% saline).
2. Superfusion of colonic segments in vitro with STW 5 prior to chemical and mechanical stimulation (57.1 ×10-6 mg ml-1; lyophilized powder, dissolved in 0.9% saline).
3. Superfusion with vehicle (0.9% saline) prior to chemical and mechanical stimulation.
STW 5 concentrations were chosen according to the previously published data.
Data analysis
Baseline afferent discharge frequency (imp sec-1) was determined as mean afferent nerve discharge per second over a 30 second period prior to administration of each test stimulus. Subsequent afferent nerve responses were quantified as peak increase in afferent discharge above baseline. Data was analysed by oneway ANOVA and post-hoc Dunnetts test. Data are presented as mean ± SEM. A probability of p<0.05 was considered statistically significant.
Results
Baseline afferent discharge
Colonic segments superfused with STW 6 showed an afferent baseline discharge of 16.3 ± 1.8 imp sec-1 that was not different compared to pretreatment with STW 5 (17.4 ± 2.0 imp sec-1) and vehicle controls (19.7 ± 1.8 imp sec-1 n=6 each).
Chemical stimulation
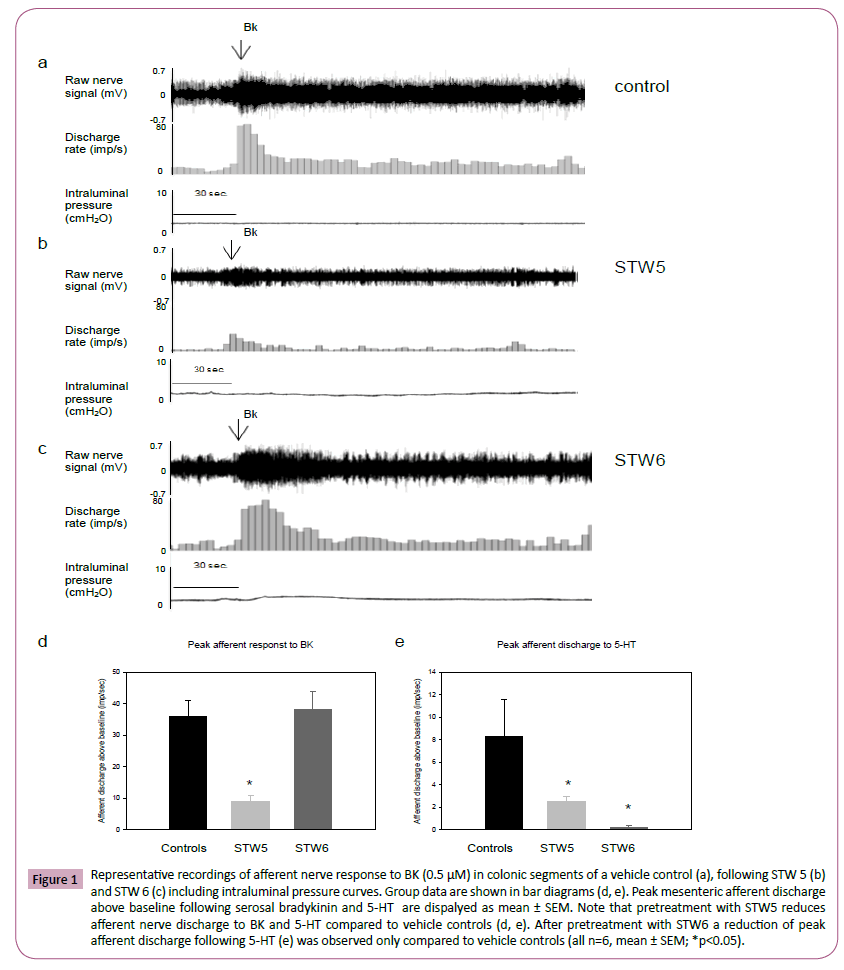
Following serosal 5-HT administration, an increase in afferent nerve discharge to 8.4 ± 3.3 imp sec-1 above baseline was observed in vehicle controls. This response was reduced to 0.2 ± 0.2 imp sec-1 following STW 6 pretreatment and to 2.5 ± 0.5 imp sec-1 after STW 5 pretreatment (each group n=6; p<0.05; Figure 1). Afferent nerve discharge in response to BK was 36.0 ± 5.0 imp sec-1 following STW 6 pre-treatment, which was unchanged compared to vehicle controls (38.0 ± 6.0 imp sec-1). STW 5 pretreatment significantly reduced afferent nerve discharge to 9.0 ± 2.0 imp sec-1(p<0.05, Figure 1).
Figure 1: Representative recordings of afferent nerve response to BK (0.5 μM) in colonic segments of a vehicle control (a), following STW 5 (b) and STW 6 (c) including intraluminal pressure curves. Group data are shown in bar diagrams (d, e). Peak mesenteric afferent discharge above baseline following serosal bradykinin and 5-HT are dispalyed as mean ± SEM. Note that pretreatment with STW5 reduces afferent nerve discharge to BK and 5-HT compared to vehicle controls (d, e). After pretreatment with STW6 a reduction of peak afferent discharge following 5-HT (e) was observed only compared to vehicle controls (all n=6, mean ± SEM; *p<0.05).
Mechanical stimulation
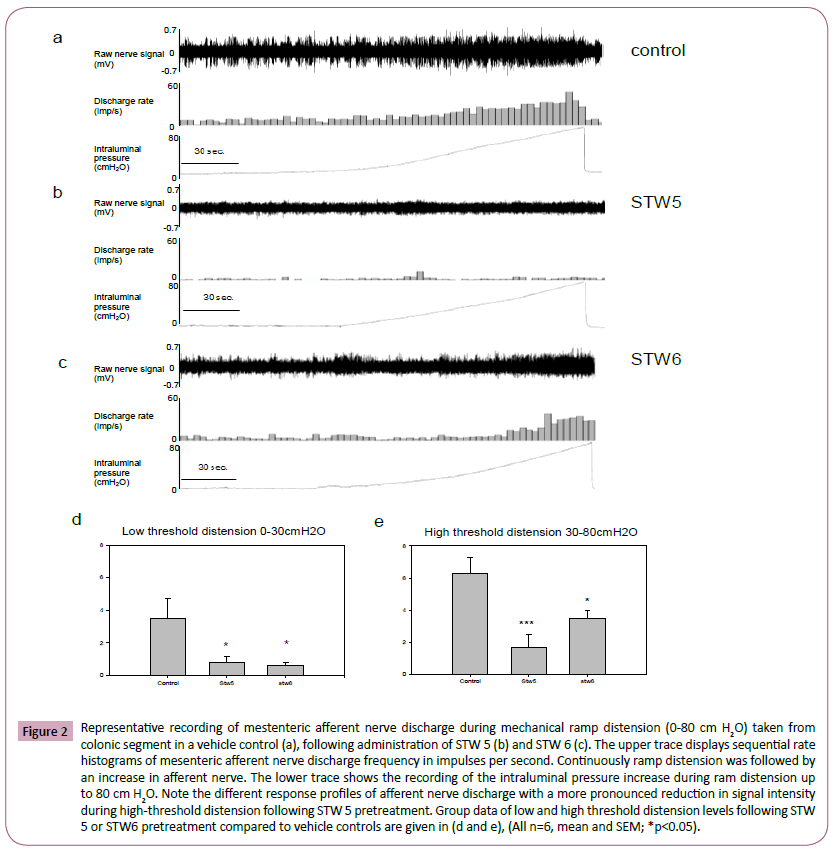
Luminal ramp distension up to 80 cm H2O led to a pressure dependent increase in afferent nerve discharge above baseline. The average peak firing frequency (imp sec-1) was similar after administration of STW 6, STW 5 and vehicle at low distension pressures from 0 to 10 cm H2O. At intraluminal pressures beyond that level, a decrease in peak afferent nerve discharge was observed following pre-treatment with STW 6 and STW 5 (Figure 2). At higher ramp distension pressures a significant reduction of peak afferent nerve discharge was observed following pretreatment with STW 5 (30-80 cm H2O; Figure 2), while reduced peak afferent nerve discharge was seen after pretreatment with STW 6 at intermediate pressure levels (10-30 cm H2O; Figure 2).
Figure 2: Representative recording of mestenteric afferent nerve discharge during mechanical ramp distension (0-80 cm H2O) taken from colonic segment in a vehicle control (a), following administration of STW 5 (b) and STW 6 (c). The upper trace displays sequential rate histograms of mesenteric afferent nerve discharge frequency in impulses per second. Continuously ramp distension was followed by an increase in afferent nerve. The lower trace shows the recording of the intraluminal pressure increase during ram distension up to 80 cm H2O. Note the different response profiles of afferent nerve discharge with a more pronounced reduction in signal intensity during high-threshold distension following STW 5 pretreatment. Group data of low and high threshold distension levels following STW 5 or STW6 pretreatment compared to vehicle controls are given in (d and e), (All n=6, mean and SEM; *p<0.05).
Discussion
The present study investigates the effect of STW 6 (Iberis amara), the main compound of STW 5, on colonic afferent sensitivity compared to STW 5. The present in vitro model allows to characterize colonic afferent nerve sensitivity to chemical and mechanical stimulation after administration of STW 5 and STW 6 without potential systemic interferences e.g., by changes in local tissue blood flow or secretion. However, baseline stimulation of afferent nerve fibers induced by intestinal ishemia might occur in this model. Those stimuli, are unlikely to provoke responses by alterations in blood pressure as described previously [17]. To avoid secondary nerve responses by intestinal motor events, we added the L-type calcium channel blocker, Nifedipine, to the organ bath. Nifedipine minimizes the nerve response to complex stimuli such as intestinal ischemia or spontaneous intestinal motor events. This L-type calcium channel blocker inhibits the degranulation of mast cells and enterochromaffin cells that may also give rise to indirect nerve responses. In our study, defined stimuli were administered with predominantly direct actions on afferent nerve terminals, so that they are unlikely to be altered by Nifedipine. Since the applied chemical test stimuli act on the serosal site of colon only, it is unlikely that additional subtypes of colonic afferent nerve fibers except lumbar splanchnic nerves, which predominantly contain spinal primary afferents, are involved [18]. Experimental studies describe that 5-HT has the potential to stimulate colonic lumbar splanchnic afferent nerve fibers via 5-HT3 receptors. In the colon, the 5-HT3 receptor mediated pathway is involved in signalling of non-physiological symptoms including pain since 5-HT3 receptor antagonists improved pain and discomfort in clinical settings. However, approximately 50% of colonic afferents do not respond to a 5-HT3 receptor selective agonists indicating that other 5-HT receptors such as 2-HT2, 5-HT4, 5-HT6 and 5-HT7 receptors are involved in the signal transduction in the colon. It was shown previously that STW 5 has the potential to alter afferent sensitivity to a panel of different stimuli such as 5-HT. In the present study colonic afferent nerve response to 5-HT was reduced by STW 6 and STW 5, with a more profound effect of STW 6. One can speculate that STW 6 may be a key component of STW 5 acting on 5-HT sensitivity on colonic lumbar spinal primary afferents. However, the exact mechanism warrants further examination. STW 5 but not STW 6 reduced intestinal afferent sensitivity to BK. BK stimulates visceral afferent neurons via B2 receptor [19,20]. In the colon BK is a selective stimuli of splanchnic serosal afferents and activates high and low threshold mechaosensitive nerve fibres. BK can sensitize mechanosensitive afferents in the colon [21]. In the current setting we applied BK after mechanical ramp distension to exclude a possible potentiation of mechnosensitive afferent nerve discharge induced by BK. Apparently the multicomponent drug STW 5 but not it’s single component STW 6 is capable to attenuate the afferent nerve reponse to BK. Thus, other compounds of STW 5 seem to mediate this action on BK sensitivity. Which components are involved in the desentization of colonic afferents process remains to be determined. Afferent nerve discharge to luminal ramp distension was reduced following pre-treatment with STW 6 and STW 5. Interestingly, STW 6 and STW 5 both desensitized low threshold afferent nerve fibers (10- 30 cm H2O) contrary to the reduction of high threshold mechanical afferent nerve response (30-80 cm H2O), which was only observed after STW 5 pre-treatment. It appears that STW 5 desensitizes low and high threshold mechanosensitive spinal colonic afferents. In contrast to the small bowel, where low threshold distension is mediated via vagal and high threshold distension via spinal afferent nerve fibers [22,23] colonic afferents have three distinct origins with different activation profiles. The mucosal colonic afferents closely resemble their counterparts in the small bowel (vagal afferents) since small pressure changes can activate these nerve fibres and the baseline discharge frequency was low. Serosal colonic afferents are non-responsive to circumferential stretch, however many of the colonic serosal afferent fibres are responsive to chemical stimulation such as BK indicating an important role in the transmission of signals related to potential noxious stimuli. Muscular colonic afferents convey small changes of intraluminal pressure. They are responsive to circumferential distortion, but not to graded increase of circumferential strain. Given these findings we assume that in the current study the single component STW 6 acts on mucosal and serosal colonic afferents whereas the multitarget drug STW 5 can differentially desensitze all three different origins of colonic afferent nerve fibres. In addition it may well be possible that mechanical ramp distension leads to an activation of intermesenteric fibers of nonspinal origin such as intestinofugal fibers which originate from enteric neurons within the gut wall such as mucosal or muscular afferents and project to prevertebral ganglia [24] and not to the spinal cord. It is important to consider this small number of fibers as a potential contributor to recorded afferent discharge following ramp distension. In conclusion, STW 6 and STW 5 are capable to attenuate colonic afferent intestinal neuronal sensitivity to chemical and mechanical stimulation and possibly reduce visceral hypersensitivity, which is generally accepted as the prime pathomechanism of functional bowel disorders [25-32]. In contrast to STW 5, which desensitizes afferents to a wide range of stimuli, STW 6 inhibits predominantly colonic serosal afferent nerve discharge. Clinical studies investigating the effect of the single compound STW 6 (Iberis amaris) on patients with functional dyspepsia or irritable bowel syndrome are required to elucidate a role for STW 6 in clinical medicine.
Acknowledgements
This study was funded by the Steigerwald Arzneimittel GmbH, Darmstadt, Germany.
Conflict of Interest
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work: Work was funded by the Steigerwald Arzneimittelwerk GmbH, Darmstadt, Germany.
References
- Fass R, Dickman R (2006) Non-cardiac chest pain: an update. Neurogastroent Motil 18: 408-417.
- Talley NJ (2006) Irritable bowel syndrome. Intern Med J 36: 724-728.
- Craig OF, Quigley EM (2011) Current and emerging therapies for the management of functional gastrointestinal disorders. Therapeutic Advances in Chronic Disease 2: 87-99.
- Melzer J (2005) Meta-analysis: phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast). Aliment Pharm Therap 20: 1279-1287.
- Saller R (2002) Iberogast (R): A modern phytotherapeutic combined herbal drug for the treatment of functional disorders of the gastrointestinal tract (dyspepsia, irritable bowel syndrome) from phytomedicine to 'evidence based phytotherapy'. A systematic review. Forsch Komp Klas Nat 9: 1-20.
- Ammon HPT (2006) Spasmolytic and tonic effect of Iberogast (R) (STW 5) in intestinal smooth muscle. Phytomedicine 13: 67-74.
- Hohenester B (2004) The herbal preparation STW5 (Iberogast (R)) has potent and region-specific effects on gastric motility. Neurogastroent Motil 16: 765-773.
- Michael S (2009) Inhibition of inflammation-induced alterations in rat small intestine by the herbal preparations STW 5 and STW 6. Phytomedicine 16: 161-171.
- Krueger D (2009) The multi-herbal drug STW 5 (Iberogast (R)) has prosecretory action in the human intestine. Neurogastroent Motil 21: 1203.
- Posserud I ( 2006) Functional findings in irritable bowel syndrome. World J Gastroentero 12: 2830-2838.
- Mueller MH (2009) A novel herbal preparation desensitizes mesenteric afferents to bradykinin in the rat small intestine. Neurogastroent Motil 21: 467-476.
- Kroll U, Cordes C (2006) Pharmaceutical perequisites for a multi-target therapy. Phytomedicine 13: 12-19.
- Allam S (2015) Extracts from peppermint leaves, lemon balm leaves and particular angelica roots mimic the pro-secretory action of the herbal preparation STW 5 in human intestine. Phytomedicine 22: 1063-1070.
- Schneider M (2016) Anti-inflammatory effects of herbal preparations STW 5 and STW5II in cytokine-challanged normal human colon cells. Frontiers of Phamacology 7: 1-12
- Hillsley K, Grundy D (1998) Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. J Physiol-London 509: 717-727.
- Hicks GA (2002) Excitation of rat colonic afferent fibres by 5-HT3 receptors. J Physiol London 544: 861-869.
- Jiang W (2011) Mast cells drive mesenteric afferent signalling during acute intestinal ischaemia. J Physiol-London 589: 3867-3872.
- Baron R (1988) Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical organization of the lumbar sympathetic nervous system of the rat. J Comp Neurol 275: 460-468.
- Booth CE (2008) Influence of the pattern of jejunal distension on mesenteric afferent sensitivity in the anaesthetized rat. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 20: 149-158.
- Brunsden AM, Grundy D (1999) Sensitization of visceral afferents to bradykinin in rat jejunum in vitro. J Physiol London 1521: 517-527.
- Brierley SM (2004) Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166-178.
- Ness TJ, Gebhart GF (1990) Visceral Pain - A Review of Experimental Studies. Pain 41: 167-234.
- Sengupta JN (1998) Stimulus-response function studies of esophageal mechanosensitive nociceptors in sympathetic afferents of opossum. Journal of Neurophysiology 64: 796-812.
- Luckensmeyer GB, Keast JR (1995) Distribution and morphological characterization of viscerofugal projections from the large intestine to the inferior mesenteric and pelvic ganglia of the male rat. Neuroscience 66: 663-671.
- Bergmann K (2003) A systematic review of tegaserod for the treatment of irritable bowel syndrome. Journal of Clinical Pharmacy and Therapeutics 28: 151-153.
- Brierley SM (2005) Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neuro gastro enterol Motil. 17: 854-862.
- Kreis ME (2002) Cosensitivity of vagal mucosal afferents to histamine and 5-HT in the rat jejunum. Am J Physiol Gastr L 283: 612-6177.
- Lacy BE (2004) Therapy for irritable bowel syndrome. The New England journal of medicine 350: 1261-1263.
- Liu CY (2004) The herbal preparation STW 5 (Iberogast (R)) desensitizes intestinal afferents in the rat small intestine. Neurogastroent Motil 16: 759-764.
- Regoli D, Barabe J (1980) Pharmacology of Bradykinin and Related Kinins. Pharmacol Rev 32: 1-46.
- Spiller R (2007) Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 19: 25-31.
- Wood JN, Docherty R (1997) Chemical activators of sensory neurons. Annual Review of Physiology 59: 457-482.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences