Development of Multigenic Sugarcane to Decrease the Production Cost in Pakistan
Zahida Qamar1*, Idrees Ahmad Nasir1, Mounir G Abouhaidar2, Kathleen L Hefferon3, Muhammad Ahmed1 and Ahmad Ali Shahid1
1Department of Molecular Biology, University of the Punjab, Lahore, Pakistan
2Department of Cell and Systems Biology, University of Toronto, Toronto, Canada
3Department of Microbiology Cornell University, Ithaca, New York, USA
- *Corresponding Author:
- Zahida Qamar
Department of Molecular Biology, University of the Punjab, Lahore, Pakistan
E-mail:Zahida.Qamar@cemb.edu.pk
Received date: January 31, 2022, Manuscript No. IPJPSAR-22-12504; Editor assigned date: February 02, 2022, PreQC No. IPJPSAR-22-12504(PQ); Reviewed date: February 16, 2022, QC No. IPJPSAR-22-12504;Revised date:23, 2022, Manuscript No. IPJPSAR-22-12504 (R); Published date: March 02, 2022, DOI: 10.36648/plant-sciences.6.1.66
Citation: Qamar Z, Nasir IA, Abouhaidar MG, Hefferon KL, Ahmed M, et al. (2022) Development of Multigenic Sugarcane to Decrease the Production Cost in Pakistan. J Plant Sci Agri Res Vol.6 No.1: 66.
Abstract
Due to higher amount of sucrose, sugarcane is grown commercially. In order to save sucrose yields, various studies have been designed to develop resistance in sugarcane against weeds and stemborers. In this study, two problems had been addressed by genetic manipulation of sugarcane to make them resistant against both herbicides and insects by expressing glyphosate resistant gene (CEMB-GTGene) and borer resistant genes (CEMB-Cry1Ac and CEMB-Cry2A) under control of Nos terminator and maize ubiquitin promoter. Mortality percentage of shoot borers Chilo infuscatellus was determined by assessing the cry proteins through insect bio-toxicity assays. Results showed that in 80 days old transgenic plants, 100% mortality rates of Chilo infuscatellus have been found showing that there was high resistance in transgenic sugarcanes against shoot borers and sufficient gene expression to fully resist target pests. Weed management was done by glyphosate spray assays. 70%-76% of the transgenic plants were identified to be glyphosate resistant (3000 ml/Ha) in V1 generation while 100% tolerant in V2 generation. Thus, this transgenic sugarcane will help to boost sugarcane yield in the country as it now successfully provides resistance against both stemborers and glyphosate herbicides.
Keywords
Sugarcane; Cash crop; Development of pest resistance; Herbicide tolerance; Commercialization
Introduction
Sugarcane is considered to be the world’s significant cash crop as it is being cultivated around the globe in 58 countries, 26.9 million hectares of area is used for sugarcane cultivation worldwide [1-4]. 80% of world’s sugar need is fulfilled by sugarcane via chemically synthesized sweetener known as sucrose [5]. A wide range of products are obtained from sugarcane like chemicals, biofuels, fibers, paper, beverages, detergents, insecticides, industrial enzymes, plastics, paints, pharmaceutical products, synthetics, chipboard and industrial chemicals like dextran, furfural and alcohol [6]. Sugarcane contributes to 0.7% GDP and 3.4% of agriculture sector and is cultivated on ~1.3 million hectare area in Pakistan. 37% of the agriculture production in Pakistan is lost out of which 13% is because of insects. Sugarcane crop is destroyed by ~1300 insect pests all over the world and by 61 insect species in Pakistan. In Pakistan, 15%-36% of sugarcane yield is lost due to stemborers, 10%-20% by root-borers and 10%-15% by top-borers. Main objective of this study to prevent yield loss by making sugarcane resistant against stemborers and herbicides.
Materials and Methods
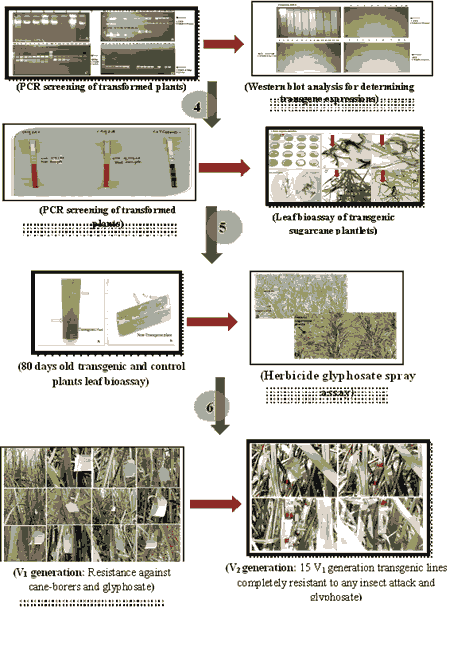
A gene cassette was designed that contains herbicides and stemborers resistant genes i.e. CEMB-GTGene, CEMB-Cry1Ac and CEMB-Cry2A under control of No terminator and maize ubiquitin promoter. These constructs pCEMB-SGTG and pCEMB-SC12 were introduced via electroporation into the agrobacterium cells. Colony PCR was performed to confirmed gene transformation via gene specific primers. 8 weeks-10 weeks old leaves of tobacco plants were co-cultured with agrobacterium to induce agrobacterium mediated transformation [7]. Expression of transgenes was indicated by histochemical detection of the GUS activity that was used as a reporter using agroinfiltrated leaves. Biolistic transformation method was used for transformation of transgene in 4 sugarcane varieties i.e. CPF-246, HSF-240, SPF-234 and SPF-213 [8]. During early transgenesis, transgene expression was determined by performing GUS assays on young shoots. Presence of transgenes was confirmed through PCR screening using CEMB-GTGene, CEMB-Cry1Ac and CEMB-Cry2A genes specific primers. Stable transgene integration was determined by performing southern blotting on PCR positive transformants. CEMB-GTGene, CEMB-Cry1Ac and CEMB-Cry2A genes expression were determined through dipstick assays that were coated with the IgG monoclonal antibodies for each gene [9]. These sticks were dipped in total proteins that were isolated from the fresh transgenic plants leaves. ELISA was performed to quantify the transgene expressions. Toxicity effects of CEMB-Cry1Ac and CEMB-Cry2A endotoxins were determined by performing leaf biotoxicity assay on the leaves. CEMB-GTGene expression and activity was confirmed by spraying glyphosate on the transgenic plants. Comparison between different lines (control and transgenic) was done through statistical analysis (Dunnett’s tests, LSD and ANOVA).
Results
Restriction of the CEMB-GTGene, CEMB-Cry1Ac and CEMB-Cry2A genes generated 1.4 kb, 1.8 kb and 1.9 kb fragments respectively which were then integrated into expression vector (pCAMBIA-1301). These constructs were introduced via electroporation into agrobacterium. PCR analysis confirmed presence of transgenes. PCR positive transformants were subjected to agro-filtration using tobacco leaves in presence of GUS receptor. The expression of GUS was confirmed by bluish green color under fluorescent microscope. For sugarcane transformation and tissue culturing, CPF-246, HSF-240, SPF-234 and SPF-213 varieties of sugarcane were selected. To obtain maximum embryogenic calli from the selected varieties, 4 different combinations were used for callus induction media. Maximum embryogenic calli was observed in CPF-246 (100%) followed by SPF-213 (90%), SPF-234 (90%) and HSF-240 (81%). Plasmid constructs were then transferred to these varieties via biolistic methods. Total of 400 explants were used for transformation. On single selection media (kanamycin), 91% of CPF-246, 74% of SPF-234, 70% of SPF-213 and 45% of HSF-240 survived while on double selection media (glyphosate and kanamycin), 81% of CPF-246, 40% of SPF-234, 34% of SPF-213 and 29% of HSF-240 transformed calli survived. Then after it, GUS assay was performed to screen the transgenic putative plants. PCR, southern blotting, dipstick assay and ELISA was performed for transgenic plants at Vo, V1 and V2 generation. Leaf bio-assay was performed to determine the efficiency of CEMB-GTGene, CEMB-Cry1Ac and CEMB-Cry2A genes. 60%-100% mortality rate Chilo infuscattellus was determined in transgenic leaves. Weed management was done by glyphosate spray assays. 70%-76% of the transgenic plants were identified to be glyphosate resistant (3000 ml/Ha) in V1 generation while 100% tolerant in V2 generation.
Discussion
Main objective of crop production is to obtain high yields even for sugarcane. Different viruses, drought stress, weeds and insects are the major constrains for sugarcane. Present study aimed to control insects and weeds through genetic manipulation of sugarcanes. In this study, for maximum callus regeneration, an efficient procedure was developed to instill tolerance against glyphosate and cane borers. For embryogenic callus formation, immature leaves were found to be excellent explants [10]. It basically strengthens the procedure for gene transformation in sugarcane. From a callus inducing media with 2,4-D embryogenic calli were obtained for all 4 varieties. To enhance potential of embryogenic calli of sugarcane, it was supplemented with casein [11]. Tissue culture response was observed to critically screen all 4 varieties. For genetic modification, varieties were selected on basis of regeneration response [12]. Studies have also disclosed that resistant against lepidopteran insects were best provided by cry proteins [13]. Most commonly used herbicide for weed control is glyphosate which is a broad spectrum herbicide. One of the main drawbacks of using glyphosate is that along with weeds and herbs, it also stunts the plant growth thus affecting its yield [14]. It inhibits formation of EPSPS enzyme in shikimate pathway which leads to shikimate pathway being shut down. This inhibits the formation of 3 essential amino acids i.e. phenylalanine, tyrosine and tryptophan that humans can’t synthesize and is required from plant source [15]. In this study, resistant against glyphosate and stemborers are provided by introducing CEMB-GTGene, CEMB-Cry1Ac and CEMB-Cry2A genes into the sugarcane varieties.
Conclusion
In this study, 100% mortality rates of Chilo infuscatellus have been found in CPF-246 variety showing that there was high resistant in transgenic sugarcanes against shoot borers and sufficient gene expression to fully resist target pests. Weed management was done by glyphosate spray assays. 70%-76% of the transgenic plants were identified to be glyphosate resistant (3000 ml/Ha) in V1-generation while 100% tolerant in V2-generation. This study reported that after approval from biosafety committee, farmers can use this sugarcane variety as starting material for cost effective weeds and insect’s control. More studies should be done to enhance stable Bt. toxin expression. Glyphosate resistant crops against 5000 mL/ha were recommended to be successful in controlling all sugarcane weeds.
References
- Feng M, Yu Q, Chen Y, Fu Z, Xu L, et al. (2022) ScMT10, a metallothionein-like gene from sugarcane, enhances freezing tolerance in Nicotiana tabacum transgenic plants. Environ Exp Bot 194: 104750.
[Cross Ref], [Google Scholar]
- Qamar Z, Nasir IA, Abouhaidar MG, Hefferon KL, Rao AQ, et al. (2021) Novel approaches to circumvent the devastating effects of pests on sugarcane. Sci Rep 11: 12428.
[Crossref], [Google Scholar]
- Yao W, Ruan M, Qin L, Yang C, Chen R, et al. (2017) Field performance of transgenic sugarcane lines resistant to sugarcane mosaic virus. Front Plant Sci 8: 104.
[Crossref], [Google Scholar], [Indexed]
- Mayavan S, Subramanyam K, Jaganath B, Sathish D, Manickavasagam M, et al. (2015) Agrobacterium-mediated in planta genetic transformation of sugarcane setts. Plant Cell Rep 34: 1835-1848.
[Crossref], [Google Scholar]
- Gao S, Yang Y, Wang C, Guo J, Zho D, et al. (2016) Transgenic sugarcane with a cry1Ac gene exhibited better phenotypic traits and enhanced resistance against sugarcane borer. PLoS ONE 11: e0153929.
[Crossref], [Google Scholar]
- Raghavi S, Sindhu R, Binod P, Gnansounou E, Pandey A (2016) Development of a novel sequential pretreatment strategy for the production of bioethanol from sugarcane trash. Bioresour Technol 199: 202-210.
[Crossref], [Google Scholar], [Indexed]
- Bhaskar PB, Venkateshwaran M, Wu L, Ané JM, Jiang J (2009) Agrobacterium-mediated transient gene expression and silencing: A rapid tool for functional gene assay in potato. PLoS ONE 4: e5812.
[Crossref], [Google Scholar], [Indexed]
- Nasir IA, Tabassum B, Qamar Z, Javed MA, Tariq M, et al. (2014) Herbicide-tolerant sugarcane (Saccharum officinarum L.) plants: An unconventional method of weed removal. Turk J Biol 38: 439-449.
[Crossref], [Google Scholar]
- Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19: 1349.
[Crossref], [Google Scholar], [Indexed]
- Snyman S, Meyer G, Richards J, Haricharan N, Ramgareeb S, et al. (2006) Refining the application of direct embryogenesis in sugarcane: Effect of the developmental phase of leaf disc explants and the timing of DNA transfer on transformation efficiency. Plant Cell Rep 25: 1016-1023.
[Crossref], [Google Scholar]
- Joyce P, Hermann S, O'Connell A, Dinh Q, Shumbe L, et al. (2014) Field performance of transgenic sugarcane produced using Agrobacterium and biolistics methods. Plant Biotechnol J 12: 411-424.
[Crossref], [Google Scholar], [Indexed]
- Bakhsh A, Rao AQ, Shahid AA, Husnain T (2012) Spatio temporal expression pattern of an insecticidal gene (cry2A) in transgenic cotton lines. Notulae Scientia Biologicae 4: 115-119.
[Crossref], [Google Scholar], [Indexed]
- Riaz N, Husnain T, Fatima T, Makhdoom R, Bashir K, et al. (2006) Development of Indica Basmati rice harboring two insecticidal genes for sustainable resistance against lepidopteran insects. S Afr J Bot 72: 217-223.
[Crossref], [Google Scholar], [Indexed]
- Nawaz A, Haseeb A, Malik HA, Ali Q, Malik A (2020) Genetic association among aorphological araits of zea mays seedlings under salt stress. Biol Clin Sci Res J.
[Crossref], [Google Scholar]
- Castle LA, Siehl DL, Gorton R, Patten PA, Chen YH, et al. (2004) Discovery and directed evolution of a glyphosate tolerance gene. Science 304: 1151-1154.
[Cross Ref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences