ISSN : 0976-8505
Der Chemica Sinica
Design, Synthesis and Pharmacological Evaluation of New Series of 2-Pyrazoline Containing s-Triazine and their Derivatives
Ramkumar P Dongre and Shantilal D Rathod*
Milind College of Science, Aurangabad-431002, Maharashtra, India
Abstract
In the present investigation, a series of some novel 4,6-diethoxy-N-(4-(4,5-dihydro-5-phenyl-1H-pyrazol-3-yl) Substituted phenyl)-1,3,5-triazin-2-amine 7(a-h) have been synthesized by the treatment of 1-(4-(4,6-diethoxy-1,3,5- triazin-2-ylamino)phenyl)-3-substituted phenyl prop-2-en-1-one (CHAlcone) (6a-6h) with hydrazine hydrate in DMF. The structure of newly synthesized compounds was confirmed by the IR, 1H NMR and Mass spectral analysis. All the synthesized compounds were evaluated for anti-fungal and anti-bacterial activity. Most of the compound showed potent activity.
Keywords
Cyanuric chloride, 2-chloro-4,6-diethoxy-1,3,5-triazine, Triazine CHAlcone, Triazine pyrazoline
Introduction
Heterocyclic compounds play important roles in the drug discovery process, substituted heterocyclic compounds offer a high degree of structural diversity and have proven to be broadly useful as therapeutic agents. Of these heterocycles 1,3,5-triazine core have been reported to possess a wide range of biological activities. These include antiviral and anticancer [1-3], anti-tuberculosis [4], antibacterial [5,6], antifungal [7,8], antimalarial [9,10], antiviral [11], herbicidal [12], anesthetic [13] and anti-inflammatory [14] activities. Moreover, 1,3,5-triazine are useful intermediates in the construction of several other heterocycles.
Pyrazoline are prominent nitrogen containing five membered heterocyclic bioorganic molecules, which occupy unique position in medicinal chemistry, due to the broad range of pharmacological activities. They are known to possess antibacterial [15], anticancer [16], antioxidant [17] and anti-inflammatory [18] activities.
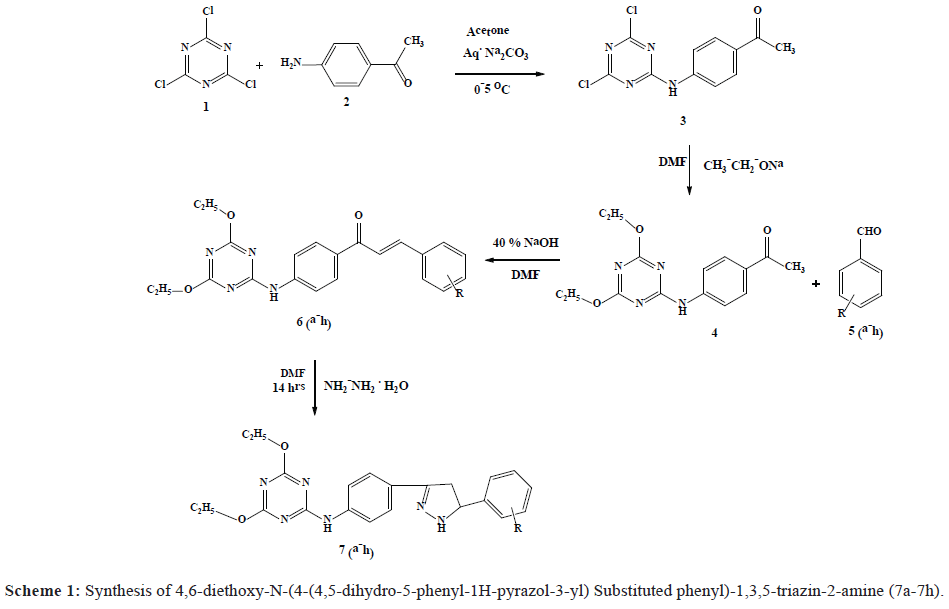
In view of these inspections and in persistence of the research work on 1,3,5-triazine and 2-Pyrazoline and in continuation of our research program [19,20]. It was thought of interest to merge both 1,3,5-triazine and 2-Pyrazoline moieties which may enhance the drug activity and viewing them for antimicrobial activities. In the present work, 4,6-diethoxy-N-(4-(4,5-dihydro-5-phenyl-1H-pyrazol-3-yl) Substituted phenyl)-1,3,5-triazin-2-amine (7a-7h) have been synthesized by the treatment of 1-(4-(4,6-diethoxy-1,3,5 triazin-2-ylamino)phenyl)-3-substituted phenylprop-2- en-1-one (CHAlcone) (6a-6h) with hydrazine hydrate in DMF (Scheme 1). The structures of all synthesized compounds were assigned on the basis of IR, Mass, 1H NMR spectral data and elemental analysis. Further these compounds were subjected for antifungal and antibacterial activity.
Materials and Methods
Experimental
Melting points were determined in open capillaries and are uncorrected.IR spectra were recorded using Perkin Elmer spectrometer. 1H NMR spectra were recorded on Brucker Advance II 400 spectrometer in DMSO-d6 by using TMS as internal standard. Thin layer chromatography was performed with E. Merk precoated TLC plates, silica gel 60F254 with thickness of 0.25 mm and spots were visualized by irradiation with ultraviolet light (254 nm).
General procedure for the synthesis of 1-(4-(4,6-dichloro-1,3,5-triazin-2-yl amino) phenyl)ethanone (3) [21]
4-amine acetophenone (0.01 M) was added slowly to cyanuric chloride (0.01 M) in acetone (30 ml) with constant stirring over a period of 4 h at 0°C to 50°C. Then, sodium carbonate (0.005 M) dissolved in water (10 ml) and added drop wise to neutralize HCl evolved during the reaction. Finally, the contents were poured into crushed ice. The solid was separated out by filtration and washed with water. The product is dried, recrystallized from alcohol to give the product (3).
General procedure for the synthesis of 1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino) phenyl)ethanone (4)
1-(4-(4,6-dichloro-1,3,5-triazin-2-yl amino)phenyl)ethanone (3) (0.01 M) was added slowly to sodium ethoxide (0.02 M) with constant stirring in DMF: H2O (9:1 ml) over a period of 4 h at room temperature and refluxed for 4 h at 80°C. The contents were poured onto ice cold water and filtered. The product 4 was obtained and recrystallized from DMF.
General procedure for the synthesis of substituted 1-(4-(4,6-diethoxy-1,3,5-triazin-2-yl amino)phenyl)-3- phenylprop-2-en-1-one (CHAlcone) (6a-6h)
Compound 4 (0.01 M) was dissolved in DMF (25 ml) and substituted benzaldehyde (5a-h) (0.01 M) was added with constant stirring at room temperature for 30 min, then sodium hydroxide (40% w/v) was added to the reaction mixture which was again stirred at RT for 24 h. The progress of reaction was monitored by TLC. After completion of the reaction, crushed ice was added in the reaction mixture and neutralized with HCl. The product separated was filtered, washed with water, dried and recrystallized from DMF to get pure product (CHAlcone) (6a-6h).
General procedure for the synthesis of substituted 4,6-diethoxy-N-(4-(4,5-dihydro-5-phenyl -1H-pyrazol-3-yl) phenyl)-1,3,5-triazin-2-amine (7a-7h)
A mixture of substituted 1-(4-(4,6-diethoxy-1,3,5-triazin-2-yl-amino)phenyl)-3-phenylprop-2-en-1-one (CHAlcone) (6a-6h) and hydrazine hydrate (0.002 M) in 30 mL DMF was refluxed for 14 h. After completion of reaction (checked by TLC), the reaction mixture was cooled and poured into ice cold water. The separated solid product was filtered, washed with cold water, dried and then recrystallized from DMF.
Results and Discussion
The synthesis of compounds substituted 1-(4-(4,6-diethoxy-1,3,5-triazin-2-yl-amino)phenyl)-3-phenylprop-2-en-1- one (CHAlcone) (6a-6h) was accomplished by reacting 1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino)phenyl)ethanone (4) with substituted benzaldehyde (5a-5h) in DMF. The CHAlcones (6a-6h) underwent ring closure via condensation with hydrazine hydrate to give substituted 4,6-diethoxy-N-(4-(4,5-dihydro-5-phenyl-1H-pyrazol-3-yl)phenyl)-1,3,5- triazin-2-amine (7a-7h). The synthetic pathway followed for the synthesis of the title compounds is described in Scheme 1.
The structure of the synthesized compounds was confirmed by elemental analysis and spectral data (IR, 1H NMR and MS spectroscopy). The IR spectra of compounds (7a-7h) showed absorption peaks at 3262 (N-H), 3081 (Ar-H), 2981 Ali(C-H), 1502 (C=N), 1329 (C-N) absence of >C=O at 1606 cm-1 but it is presence in CHAlcones (6a-6h) it confirmed the formation of (7a-7h).
Further, in their 1H NMR (DMSO-d6) spectrum the appearance of a signal at δ 4.83-4.81 (dd, 1H, Hx pyrazoline), 3.22-3.20 (dd, 1H, HB pyrazoline) and 2.94-2.87 (dd, 1H, HA pyrazoline) and singlet at 8.02 due to (N-H) confirms the presence of the pyrazoline ring. The synthetic pathway followed for the synthesis of the title compounds is described in Scheme 1.
Spectral data of synthesized compounds (6a-6h) and (7a-6h)
(6a): 1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino)phenyl)-3-(4-methylphenyl)prop-2-en-1-one
Yield 75%; M.P. 225°C: Elemental analysis Calcd for (C23H24N4O3); C, 68.30; H, 5.98; N, 13.85; found: C, 68.25; H, 5.80; N, 13.80%; IR (KBr pellets cmâ€ÂÂ1): 3310 (N-H), 1655 (>C=O), 1606 (CH=CH), 840 (Câ€ÂÂCl): 1H NMR (DMSO-d6, 400 MHz), δ 9.42 (s, 1H, N-H), 8.10-7.62 (m, 8H, Ar-H), 7.50-7.45 (dd, 1H, >C=CHB), 7.42-7.40 (dd, 1H, CHA=C<), 3.43-3.19 (q, 6H, CH3-CH2-), 2.70-2.50 (t, 4H, -CH2-CH3), 2.35 (s, 3H, Arâ€ÂÂCH3), MS: m/z 405 (M+1).
(6b): 1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino)phenyl)-3-(4-methoxyphenyl)prop-2-en-1-one
Yield 78%; M.P. 234°C: Elemental analysis Calcd for (C23H24N4O4); C, 65.70; H, 5.75; N, 13.33; found: C, 65.60; H, 5.60; N, 13.24%; IR (KBr pellets cmâ€ÂÂ1): 3312 (N-H), 1652 (>C=O), 1600 (CH=CH), 843 (Câ€ÂÂCl): 1H NMR (DMSO-d6, 400 MHz), δ 9.40 (s, 1H, N-H), 8.14-7.65 (m, 8H, Ar-H), 7.48-7.46 (dd, 1H, >C=CHB), 7.44-7.43 (dd, 1H, CHA=C<), 3.85 (s, 3H, -OCH3), 3.40-3.17 (q, 6H, CH3-CH2-), 2.70-2.48 (t, 4H, -CH2-CH3), MS: m/z 421 (M+1).
(6c): 1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino)phenyl)-3-(2,3,4-trimethoxyphenyl)prop-2-en-1-one
Yield 74%; M.P. 232°C: Elemental analysis Calcd for (C25H28N4O6); C, 62.49; H, 5.85; N, 11.66; found: C, 62.45; H, 5.80; N, 11.60%; IR (KBr pellets cmâ€ÂÂ1): 3325 (N-H), 1650 (>C=O), 1620 (CH=CH): 1H NMR (DMSO-d6, 400 MHz), δ 9.43 (s, 1H, N-H), 8.9-7.25 (m, 6H, Ar-H), 7.52-7.50 (dd, 1H, >C=CHB), 7.47-7.46 (dd, 1H, CHA=C<), 3.90 (s, 3H, 3X-OCH3), 3.38-3.10 (q, 6H, CH3-CH2-), 2.62-2.45 (t, 4H, -CH2-CH3), MS: m/z 481 (M+1).
(6d):1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino)phenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one
Yield 80%; M.P. 210°C: Elemental analysis Calcd for (C25H28N4O6); C, 62.49; H, 5.85; N, 11.66; found: C, 62.44; H, 5.80; N, 11.14%; IR (KBr pellets cmâ€ÂÂ1): 3325 (N-H), 1648 (>C=O), 1622 (CH=CH): 1H NMR (DMSO-d6, 400 MHz), δ 9.45 (s, 1H, N-H), 8.10-7.27 (m, 6H, Ar-H), 7.51-7.48 (dd, 1H, >C=CHB), 7.44-7.43 (dd, 1H, CHA=C<), 3.92 (s, 3H, 3X-OCH3), 3.43-3.19 (q, 6H, CH3-CH2-), 2.63-2.50 (t, 4H, -CH2-CH3), MS: m/z 481 (M+1).
(6e): 1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino)phenyl)-3-(4-fluorophenyl)prop-2-en-1-one
Yield 76%; M.P. 241°C: Elemental analysis Calcd for (C22H21FN4O3); C, 64.70; H, 5.18; N, 13.72; found: C, 64.65; H, 5.15; N, 13.65%; IR (KBr pellets cmâ€ÂÂ1): 3316 (N-H), 1650 (>C=O), 1610 (CH=CH), 743 (Câ€ÂÂF): 1H NMR (DMSO-d6, 400 MHz), δ 9.50 (s, 1H, N-H), 8.13-7.75 (m, 8H, Ar-H), 7.52-7.50 (dd, 1H, >C=CHB), 7.47-7.46 (dd, 1H, CHA=C<), 3.43-3.19 (q, 6H, CH3-CH2-), 2.71-2.46 (t, 4H, -CH2-CH3), MS: m/z 409 (M+1).
(6f): 1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino)phenyl)-3-(2-chlorophenyl)prop-2-en-1-one
Yield 79%; M.P. 236°C: Elemental analysis Calcd for (C22H21ClN4O3); C, 62.19; H, 4.98; N, 13.19; found: C, 62.15; H, 4.80; N, 13.14%; IR (KBr pellets cmâ€ÂÂ1): 3316 (N-H), 1650 (>C=O), 1608 (CH=CH), 842 (Câ€ÂÂCl): 1H NMR (DMSO-d6, 400 MHz), δ 9.48 (s, 1H, N-H), 8.14-7.65 (m, 8H, Ar-H), 7.54-7.52 (dd, 1H, >C=CHB), 7.44-7.43 (dd, 1H, CHA=C<), 3.40-3.17 (q, 6H, CH3-CH2-), 2.68-2.48 (t, 4H, -CH2-CH3), MS: m/z 425 (M+1).
(6g): 1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino)phenyl)-3-(4-chlorophenyl)prop-2-en-1-one
Yield 75%; M.P. 237°C: Elemental analysis Calcd for (C22H21ClN4O3); C, 62.19; H, 4.98; N, 13.19; found: C, 62.15; H, 4.80; N, 13.14%; IR (KBr pellets cmâ€ÂÂ1): 3316 (N-H), 1650 (>C=O), 1606 (CH=CH), 843 (Câ€ÂÂCl): 1H NMR (DMSO-d6, 400 MHz), δ 9.45(s, 1H, N-H), 8.14-7.65 (m, 8H, Ar-H), 7.52-7.50 (dd, 1H, >C=CHB), 7.44-7.43 (dd, 1H, CHA=C<), 3.43-3.19 (q, 6H, CH3-CH2-), 2.72-2.50 (t, 4H, -CH2-CH3), MS: m/z 425 (M+1).
(6h): 1-(4-(4,6-diethoxy-1,3,5-triazin-2-ylamino)phenyl)-3-(2,4-dichlorophenyl)prop-2-en-1-one
Yield 78%; M.P. 240°C: Elemental analysis Calcd for (C22H2°C12N4O3); C, 57.53; H, 4.39; N, 12.20; found: C, 57.50; H, 4.30; N, 12.16%; IR (KBr pellets cmâ€ÂÂ1): 3322 (N-H), 1650 (>C=O), 1615 (CH=CH), 840 (Câ€ÂÂCl): 1H NMR (DMSO-d6, 400 MHz), δ 9.45 (s, 1H, N-H), 8.14-7.65 (m, 7H, Ar-H), 7.52-7.50 (dd, 1H, >C=CHB), 7.44-7.43 (dd, 1H, CHA=C<), 3.43-3.19 (q, 6H, CH3-CH2-), 2.72-2.50 (t, 4H, -CH2-CH3), MS: m/z 460 (M+1).
(7a): 4,6-diethoxy-N-(4-(4,5-dihydro-5-(4-methylphenyl)-1H-pyrazol-3-yl)phenyl)-1,3,5-triazin-2-amine
Yield 80%; M.P. 150°C: Elemental analysis Calcd for (C23H26N6O2); C, 60.48; H, 4.84; N, 19.24; found: C, 60.45; H, 4.80; N, 19.22%; IR (KBr pellets cmâ€ÂÂ1): 3255 (N-H), 3088 (Ar-H), 2972 Ali(C-H), 1508 (C=N), 1325 (C-N): 1H NMR (DMSO-d6, 400 MHz), δ 8.08 (s, 2H, N-H), 7.90â€ÂÂ6.75 (m, 8H, Arâ€ÂÂH), 4.85-4.80 (dd, 1H, Hx pyrazoline), 3.38-3.42 (dd, 1H, HB pyrazoline), 3.22-3.20 (dd, 1H, HA pyrazoline), 2.80-2.16 (q, 6H, CH3-CH2-), 2.40-2.35 (t, 4H, -CH2- CH3), 2.30 (s, 3H, Arâ€ÂÂCH3), MS: m/z 419 (M+1).
(7b): 4,6-diethoxy-N-(4-(4,5-dihydro-5-(4-methoxyphenyl)-1H-pyrazol-3-yl)phenyl)- 1,3,5-triazin-2-amine
Yield 78%; M.P. 152°C: Elemental analysis Calcd for (C23H26N6O3); C, 63.58; H, 6.03; N, 19.34; found: C, 63.50; H, 6.00; N, 19.22%; IR (KBr pellets cmâ€ÂÂ1): 3260 (N-H), 3012 (Ar-H), 2972 Ali(C-H), 1510 (C=N), 1320 (C-N): 1H NMR (DMSO-d6, 400 MHz), δ 8.12 (s, 2H, N-H), 7.96â€ÂÂ6.75 (m, 8H, Arâ€ÂÂH), 4.86-4.81 (dd, 1H, Hx pyrazoline), 3.80 (s, 3H, -OCH3), 3.36-3.42 (dd, 1H, HB pyrazoline), 3.20-3.18 (dd, 1H, HA pyrazoline), 2.84-2.20 (q, 6H, CH3-CH2-), 2.41- 2.35 (t, 4H, -CH2-CH3), MS: m/z 435 (M+1).
(7c): 4,6-diethoxy-N-(4-(4,5-dihydro-5-(2,3,4-trimethoxyphenyl)-1H-pyrazol-3-yl) phenyl)-1,3,5-triazin-2-amine
Yield 75%; M.P. 132°C: Elemental analysis Calcd for (C25H30N6O5); C, 60.72; H, 6.11; N, 16.99; found: C, 60.68; H, 6.09; N, 19.80%; IR (KBr pellets cmâ€ÂÂ1): 3265 (N-H), 3078 (Ar-H), 2965 Ali(C-H), 1500 (C=N), 1330 (C-N): 1H NMR (DMSO-d6, 400 MHz), δ 8.04 (s, 2H, N-H), 7.98â€ÂÂ6.85 (m, 6H, Arâ€ÂÂH), 4.86-4.81 (dd, 1H, Hx pyrazoline), 3.85 (s, 6H, 2X -OCH3), 3.50 (s, 3H, -OCH3), 3.35-3.40 (dd, 1H, HB pyrazoline), 3.22-3.16 (dd, 1H, HA pyrazoline), 2.78-2.16 (q, 6H, CH3-CH2-), 2.40-2.35 (t, 4H, -CH2-CH3), MS: m/z 495 (M+1).
(7d): 4,6-diethoxy-N-(4-(4,5-dihydro-5-(3,4,5-trimethoxyphenyl)-1H-pyrazol-3-yl)phenyl)-1,3,5-triazin-2-amine
Yield 78%; M.P. 130°C: Elemental analysis Calcd for (C25H30N6O5); C, 60.72; H, 6.11; N, 16.99; found: C, 60.68; H, 6.08; N, 16.90%; IR (KBr pellets cmâ€ÂÂ1): 3280 (N-H), 3012 (Ar-H), 2945 Ali(C-H), 1520 (C=N), 1325 (C-N): 1H NMR (DMSO-d6, 400 MHz), δ 8.06 (s, 2H, N-H), 7.95â€ÂÂ6.65 (m, 6H, Arâ€ÂÂH), 4.85-4.80 (dd, 1H, Hx pyrazoline), 3.85 (s, 6H, 2X -OCH3), 3.50 (s, 3H, -OCH3), 3.36-3.40 (dd, 1H, HB pyrazoline), 3.20-3.18 (dd, 1H, HA pyrazoline), 2.80-2.19 (q, 6H, CH3-CH2-), 2.41-2.35 (t, 4H, -CH2-CH3), MS: m/z 495 (M+1).
(7e): 4,6-diethoxy-N-(4-(4,5-dihydro-5-(4-fluorophenyl)-1H-pyrazol-3-yl)phenyl)-1,3,5-triazin-2-amine
Yield 82%; M.P. 124°C: Elemental analysis Calcd for (C22H23FN6O2); C, 62.55; H, 5.49; N, 19.89; found: C, 62.52; H, 5.47; N, 19.70%; IR (KBr pellets cmâ€ÂÂ1): 3270 (N-H), 3086 (Ar-H), 2970 Ali(C-H), 1510 (C=N), 1320 (C-N), 740 (Câ€ÂÂF): 1H NMR (DMSO-d6, 400 MHz), δ 8.02 (s, 2H, N-H), 7.88â€ÂÂ6.80 (m, 8H, Arâ€ÂÂH), 4.85-4.80 (dd, 1H, Hx pyrazoline), 3.36-3.40 (dd, 1H, HB pyrazoline), 3.20-3.18 (dd, 1H, HA pyrazoline), 2.81-2.20 (q, 6H, CH3-CH2-), 2.41- 2.35 (t, 4H, -CH2-CH3), MS: m/z 423 (M+1).
(7f): 4,6-diethoxy-N-(4-(4,5-dihydro-5-(2-chlorophenyl)-1H-pyrazol-3-yl)phenyl)- 1,3,5-triazin-2-amine
Yield 80%; M.P. 160°C: Elemental analysis Calcd for (C22H23ClN6O2); C, 60.20; H, 5.28; N, 19.15; found: C, 60.18; H, 5.25; N, 19.13%; IR (KBr pellets cmâ€ÂÂ1): 3260 (N-H), 3019 (Ar-H), 2975 Ali(C-H), 1502 (C=N), 1310 (C-N), 840 (Câ€ÂÂCl): 1H NMR (DMSO-d6, 400 MHz), δ 8.08 (s, 2H, N-H), 7.98â€ÂÂ6.84 (m, 8H, Arâ€ÂÂH), 4.85-4.81 (dd, 1H, Hx pyrazoline), 3.38-3.42 (dd, 1H, HB pyrazoline), 3.22-3.20 (dd, 1H, HA pyrazoline), 2.80-2.19 (q, 6H, CH3-CH2-), 2.40- 2.35 (t, 4H, -CH2-CH3), MS: m/z 439 (M+1).
(7g): 4,6-diethoxy-N-(4-(4,5-dihydro-5-(4-chlorophenyl)-1H-pyrazol-3-yl)phenyl)- 1,3,5-triazin-2-amine
Yield 82%; M.P. 122°C: Elemental analysis Calcd for (C22H23ClN6O2); C, 60.48; H, 4.84; N, 19.24; found: C, 60.45; H, 4.80; N, 19.22%; IR (KBr pellets cmâ€ÂÂ1): 3262 (N-H), 3081 (Ar-H), 2981 Ali(C-H), 1502 (C=N), 1329 (C-N), 842 (Câ€ÂÂCl): 1H NMR (DMSO-d6, 400 MHz), δ 8.02 (s, 2H, N-H), 7.99â€ÂÂ6.86 (m, 8H, Arâ€ÂÂH), 4.86-4.81 (dd, 1H, Hx pyrazoline), 3.41-3.40 (dd, 1H, HB pyrazoline), 3.22-3.19 (dd, 1H, HA pyrazoline), 2.81-2.19 (q, 6H, CH3-CH2-), 2.41- 2.35 (t, 4H, -CH2-CH3), MS: m/z 439 (M+1).
(7h): 4,6-diethoxy-N-(4-(4,5-dihydro-5-(2,4-dichlorophenyl)-1H-pyrazol-3-yl)phenyl)-1,3,5-triazin-2-amine
Yield 78%; M.P. 120°C: Elemental analysis Calcd for (C22H22C12N6O2); C, 55.82; H, 4.68; N,17.75; found: C, 55.80; H, 4.64; N, 17.70%; IR (KBr pellets cmâ€ÂÂ1): 3262 (N-H), 3088 (Ar-H), 2987 Ali(C-H), 1515 (C=N), 1320 (C-N), 845 (Câ€ÂÂCl): 1H NMR (DMSO-d6, 400 MHz), δ 8.08 (s, 2H, N-H), 7.94â€ÂÂ6.82 (m, 7H, Arâ€ÂÂH), 4.85-4.81 (dd, 1H, Hx pyrazoline), 3.36-3.40 (dd, 1H, HB pyrazoline), 3.20-3.18 (dd, 1H, HA pyrazoline), 2.88-2.17 (q, 6H, CH3-CH2-), 2.40- 2.33 (t, 4H, -CH2-CH3), MS: m/z 473 (M+1).
Biological activity
Antimicrobial activity
The newly synthesized compounds were screened for their antibacterial activity against E. coli, Salmonella typhi and Staphylococcus aureus by disc diffusion method [22,23] using Penicillin as standard and antifungal activity against Aspergillus niger, Aspergillus flavus, Penicillium chrysogenum by poison plate method [24] using Griseofulvin as reference standard and DMSO as control solvent. The investigation of antibacterial screening results indicates that few of the compounds shows significant property and some of the compounds are moderately active. The investigation of antifungal activity data revealed that some compounds have promising and some showed no antifungal activity. The results are shown in Tables 1 and 2 respectively.
| S. No. | Compounds | E. coli | Salmonella typhi | Staphylococcus aureus |
|---|---|---|---|---|
| 1 | 7a | 13 | 12 | 16 |
| 2 | 7b | 15 | 16 | 14 |
| 3 | 7c | 18 | 17 | 17 |
| 4 | 7d | 15 | 14 | 15 |
| 5 | 7e | 19 | 20 | 24 |
| 6 | 7f | 14 | 17 | 22 |
| 7 | 7g | 12 | 19 | 19 |
| 8 | 7h | 16 | 20 | 14 |
| 9 | Penicillin | 22 | 25 | 35 |
| 10 | DMSO | -ve | -ve | -ve |
Table 1: Antibacterial screening results of the compounds 7a-7h.
| S. No. | Compounds | Aspergillus niger | Aspergillus flavus | Penicillium chrysogenum |
|---|---|---|---|---|
| 1 | 7a | RG | +ve | -ve |
| 2 | 7b | +ve | RG | -ve |
| 3 | 7c | -ve | +ve | -ve |
| 4 | 7d | -ve | -ve | -ve |
| 5 | 7e | -ve | -ve | -ve |
| 6 | 7f | +ve | -ve | -ve |
| 7 | 7g | -ve | -ve | +ve |
| 8 | 7h | -ve | -ve | -ve |
| 9 | Greseofulvin | -ve | -ve | -ve |
| 10 | DMSO | +ve | +ve | +ve |
| -ve: No growth Antifungal activity present; +ve: Growth Antifungal activity absent; RG: Reduced growth | ||||
Table 2: Antifungal screening results of the compounds 7a-7h.
Conclusion
In summary, it describes the synthesis of new Series of 2-Pyrazoline Containing s-triazine and their derivatives. Due to the presence of two pharmacologically active structural i.e., 2-Pyrazoline and s-triazine and owing to the biological significance, it was thought of interest to merge both 2-Pyrazoline and s-triazine moieties which may enhance the drug activity. The antimicrobial activity of these compounds was evaluated against various Gram-positive, Gram-negative bacteria and fungi. In the course of this study, particularly derivatives which possess chloro, fluoro and methaoxy groups exhibiting potent groups for antimicrobial activity against tested microorganisms. Thus, it may be considered as a promising lead for further design and development of new chemical entities.
Acknowledgement
The authors gratefully acknowledge SAIF and CIL CHAndigarh, for IR, 1HNMR spectra. The authors thank to Principal Milind College of Science, Aurangabad for providing research facility. The authors also thank to Head Department of Biotechnology Milind College of Science, Aurangabad for microbial activity.
References
- Sączewski F, Bułakowska A, Bednarski P (2006) Synthesis, structure and anticancer activity of novel 2,4-Diamino-1,3,5-Triazine derivatives.Eur J Med Chem 41: 219-225.
- Yaguchi SC, Chin Fukui Y, Koshimizu I (2006) Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst98: 545-556.
- Sączewski F, Bułakowska A (2006) Synthesis, Structure and Anticancer Activity of Novel Alkenyl-1,3,5-Triazine Derivatives. Eur J Med Chem 41: 611-615.
- Lin YM, Zhou Y, Flavin MT, Zhou LM, Nie W, et al. (2002) CHAlcones and flavonoids as antituberculosis agents. BioorgMed Chem 10: 2795-2802.
- Raval JP, Rai AR, Patel NH, Patel HV, Patel PS (2009) Synthesis and in vitro antimicrobial activity of N'-(4-(arylamino)-6-(pyridin-2-ylamino)-1,3,5-triazin-2- yl)benzohydrazide. IntJ Chem Tech Res 1: 616-620.
- Nielsen SF, Boesen M, Larsen K, Schonning HK (2004) Antibacterial CHAlcones-bioisosteric replacement of the 4'-hydroxy group. BioorgMedChem12: 3047-3054.
- Ghaib A, Menager S, Verite P, Lafont O (2002) Synthesis of variously 9,9-dialkylated octahydropyrimido [3,4-a]-s-triazines with potential antifungal activity. FarmacO57: 109-116.
- Singh UP, Bhat HR, Gahtori P (2012) Antifungal activity, SAR and physicochemical correlation of some clubbed thiazole-1,3,5-triazine derivatives. JMycol Med 22: 34-141.
- Melato S, Prosperi D, Coghi P, Basilico N, Monti D (2008) A Combinatorial Approach to 2,4,6-Trisubstituted Triazines with Potent Antimalarial Activity: Combining Conventional Synthesis and Microwave-Assistance. ChemMedChem3: 873-876.
- Agarwal A, Srivastava K, Puri SK, CHAuhan PMS (2005) Syntheses of 2, 4, 6-trisubstituted triazines as antimalarial agents. BioorgMedChemLett15: 531-533.
- Xiong YZ, Chen FE, Balzarini J, De Clercq E, Pannecouque C (2008) Non-nucleoside HIV-1 reverse transcriptase inhibitors. Part 11: structural modulations of diaryltriazines with potent anti-HIV activity. Eur J Med Chem43: 1230-1236.
- Garmouna M, Blanchoud H, Teil M, BlanCHArd M, Chevreuil M (2001) Triazines in the Marne and the Seine rivers (France), Longitudinal evolution and flows. Water Air Soil Poll132: 1-17.
- Daukshas VK, Ramamauskas Y, Udrenaite AB, Brukshtus VV, Lapinskas RS, et al. (1984) Synthesis and local-anesthetic activity of 6-[ω-amino-ω)-arylalkyl] benzo-1,4-dioanes. PharmChemJ18: 471-475.
- ZaCHArie B, Fortin D, Abbott SD, Bienvenu JF (2010) 2,4,6-trisubstituted triazines as protein a mimetics for the treatment of autoimmune diseases. J Med Chem53: 1138-1145.
- Seham YH (2013) Synthesis, Antibacterial and Antifungal Activity of Some New Pyrazoline and Pyrazole Derivatives. Molecules18: 2683-2711.
- Mahew A, Mary Sheeja TL, Kumar AT, Radha K (2011) Design, Synthesis and Biological evaluation of Pyrazole analogues of Natural Piperine. HygeiaJDMed3: 48-56.
- Padmaja A, Rajasekhar C, Muralikrishna A, Padmavathi V (2011) Synthesis and antioxidant activity of oxazolyl/thiazolylsulfonylmethyl pyrazoles and isoxazoles. Eur J MedChem46: 5034-5038.
- Sahu SK, Banerjee M, Samantray A, Behera C, Azam MA (2008) Synthesis, analgesic, anti-inflammatory and antimicrobial activities of some novel pyrazoline derivatives. Trop J Pharm Res 7: 961-968.
- Jadhav SB, Rathod SD (2015) Synthesis, CHAracterization and in-vitro anti-inflammatory, antimicrobial activities of some novel2,4-disubstituted-1,5-benzothiazepine derivatives. Der ChemSiN6: 13-18.
- Jadhav SB, Rathod SD (2016) Synthesis of Some Novel 3, 5-Diaryl-N-CHAlcone-2-pyrazoline Derivatives and Evaluation of their antimicrobial Activity. Chem Sci Trans5: 109-116.
- KarunakaramD, Govindarajan R,Jupudi S, Talari S, Udhayavani S (2012) Synthesis and biological evaluation of some newer s-triazine derivatives. Asian JPharmRes2: 51-56.
- Cruickshank R, Duguid JP, Marion BP, Swain RHA (1975) MedicinalMicrobiology, Vol. II, 12th edn, Churchill Livingstone, London, UK. pp: 196-202.
- Collins AH (1976) Microbiological Method, 2nd ed. Butterworth, London, UK.
- Cruickshank RJ, Duguid P, Swain RR (1975) Medicinal Microbiology,Vol. I, 12th edn, Churchill Livingstone, London, UK.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences