Delivery of a Tolvaptan (JINARC) Service for Adults with Autosomal Dominant Polycystic Kidney Disease (ADPKD) in a UK Renal Centre
Aaron Acquaye1* and Sunil Bhandari2
1Renal Pharmacist, Hull University Teaching Hospitals NHS Trust, Anlaby Road, Hull, HU3 2JZ, UK
2Department of Nephrologist and Honorary, Hull University Teaching Hospitals NHS Trust, Anlaby Road, Hull, HU3 2JZ, UK
- *Corresponding Author:
- Aaron Acquaye
Renal Pharmacist, Hull University Teaching Hospitals NHS Trust,
Anlaby Road, Hull, HU3 2JZ,
UK,
Tel: +01482-675050;
E-mail: aaron.acquaye@nhs.net
Received Date: October 22, 2021; Accepted Date: November 05, 2021; Published Date: November 12, 2021
Citation: Acquaye A, Bhandari S (2021) Delivery of a Tolvaptan (JINARC) Service for Adults with Autosomal Dominant Polycystic Kidney Disease (ADPKD) in a UK Renal Centre. J Nephrol Urol Vol.5 No.5: 21
Abstract
Background: Tolvaptan slows down disease progression in ADPKD; however, its side effect and monitoring profile has implications for service provision and compliance.
Aims: To assess the
• Retention rate among patients on tolvaptan
• Compliance and tolerability of side effects.
• Monitoring process for as indicated.
• Average rate of change of eGFR pre-tolvaptan vs. post tolvaptan treatment
• Change in urate level, proBNP and haemoglobin after tarting tolvaptan
Design: A prospective questionnaire-based service evaluation of atients and a retrospective observational study of eGFR change, haemoglobin, roBNP and urate before and after tolvaptan treatment.
Method: We collected demographic data, eGFR change 12 months before tolvaptan initiation and compared it to eGFR change after initiation. We carried out a questionnaire that explored patient education, side effects andcompliance.
Results: The retention rate during the period of the study was 92.6%.
55% indicated that they were 100% compliant with treatment. 80% indicated that the side-effects were tolerable. 65.8% of appointments were attended and 69.2% of blood tests were done at the required frequency. Rate of eGFR decline reduced by 1 ml/min/year after commencing tolvaptan.
The median urate level rose by 6.5% (26 μmol/L) but reverted to baseline after 18 months. The median proBNP (68 ng/L) increased by 26%. Median haemoglobin levels remained stable.
Conclusion: Compliance rates were comparable to patients on treatment for other long-term conditions.
Keywords
Tolvaptan; Polycystic kidney disease; Jinarc; Pharmacist; Kidney function
Introduction
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is the most common inherited condition that affects the kidneys and ultimately leads to the need for renal replacement therapy [1]. In most cases, ADPKD is caused by a mutation in the PKD1 or PKD2 genes and occasionally a spontaneous mutation [2]. This leads to the formation of multiple cysts in the kidneys but also other tissues including the liver and spleen. Cyst formation is thought to be due to a two-hit mechanism on tubules leading to monoclonal proliferation of cells causing cysts with the subsequent accumulation of fluid via a complex pathway involving cAMP [3,4].
Treatment historically has largely been based on lifestyle changes to smoking and diet [1], reduced salt consumption, tight blood pressure control and treatment of infections when present until licencing of tolvaptan; which received European marketing authorisation in May 2015. Tolvaptan [JINARC®] is the first drug approved for management of ADPKD [5].
In 2015, NICE approved tolvaptan following the results from the TEMPO 3:4 trial (Tolvaptan efficacy and safety in the management of PKD and its outcomes) [6] and later the REPRISE study [5,7]. Based on the current published data, tolvaptan slows the progression of ADPKD and delays the rate at which kidney failure may develop, thereby improving the quality and increasing the longevity of the lives of those affected [8].
Due to the noted elevated serum liver enzymes in 0.4% (n=6) of patients during the TEMPO 3:4 trial, the NICE/TEMPO steering committee recommended monthly liver enzyme monitoring of patients to minimise the risk of drug-induced liver injury [5,9]. Monitoring monthly was recommended for the first 18 months of treatment; and then 3 monthly thereafter [6].
A post-hoc analysis of the TEMPO 3:4 trial showed that 7.5% of patients who had been randomly assigned to receive tolvaptan discontinued due to the aquaretic adverse effects of tolvaptan [4,10].
Current local practice
The need for regular monitoring and follow-up has necessitated new ways of working to optimise patient care and involving pharmacist input. Therefore, revised practice has been instituted in many UK renal centres.
Our tolvaptan clinic is currently run as a pharmacist-led clinic with consultant oversight. When patients are referred to the clinic, the first appointment is with the nephrologist, who determines if patients referred would benefit from tolvaptan based on a combination of the NICE eligibility criteria and patient decision after information.
During the consultant review, there is a focus on tight blood pressure control and patient education. Target blood pressure is set at less than 130/80 mmHg [11]. The potential impact of tolvaptan on the lifestyle of the patient and need to maintain adequate hydration is explained to the patient.
All patients receive a comprehensive explanation of the merits and side-effects of tolvaptan with written literature and a copy of their initial assessment consultation prior to commencement of therapy. Once every 12 months, each patient is referred to the consultant for a review of progress with their kidney disease and therapy with tolvaptan, or sooner if there are any unexplained acute abnormalities in patient’s biochemical profile.
Once the patient is commenced on tolvaptan at the lowest dose, they are then referred to the pharmacist clinic for ongoing management which includes dose escalation to the maximum tolerated dose and the necessary monitoring.
Aims
To assess the
• Retention rate among patients commenced on tolvaptan therapy
• Compliance of tolvaptan and tolerability of the side effects.
• Completeness of the monitoring process for tolvaptan as indicated by NICE.
• Average rate of change of eGFR pre-tolvaptan vs. post tolvaptan treatment
• Change in urate level, proBNP and haemoglobin since starting tolvaptan treatment
Materials and Methods
We carried out a prospective semi-quantitative questionnairebased quality improvement project on all patients who had been prescribed tolvaptan for ADPKD. The questionnaire asked a series of questions covering patient education, compliance to treatment, side effects, effect of tolvaptan on quality of life and monitoring.
We also completed a retrospective analysis of patient’s annual change in eGFR the 12 months before and after starting tolvaptan, urate level, proBNP and haemoglobin since starting tolvaptan. Data was gathered from the hospital’s electronic patient record system.
Over a 6-month period, patients were given the questionnaire prior to one of their clinic visits with the consultant/pharmacist and asked to complete it independently on their own. The questionnaire was anonymised with no identifiable data. The questionnaire was then handed in after the consultation to an independent third party not linked to the study for collation of all answers from the questionnaires. Each patient was only allowed to fill in one questionnaire on a single occasion (indicated by a note in the record that it had been completed).
A retrospective review of clinic attendance and measurement of biochemical parameters were analysed as part of tolvaptan treatment review.
This project was a service improvement/evaluation project and approved by the Hull University Teaching Hospitals (HUTH) NHS Trust Clinical Audit and Effectiveness Team (Governance and Audit Department) with a signed Clinical Governance Form 1 from 01/10/2018 (CG1).
Statistical analysis
Analysis was descriptive for the questionnaire with percentages given for outcomes. For laboratory measures means or median values and ranges were calculated over the study period. P values were not calculated due to the nature and size of the study.
Results
In total, 27 patients, mean baseline eGFR 53.3 ml/min/1.73 m2 (SEM: 2.304), consisting of 18 males and 9 females on tolvaptan therapy were evaluated. Median age was 46 years (range 21-63 years). All patients were Caucasian. The median duration of treatment for the whole group was 10 months (range 1 month-69 months). The majority of patients at the time of the study (n=22) were on monthly follow-up. All 27 patients approached from this cohort completed the questionnaire giving a 100% response rate.
Guidance information
During the initial assessment for possible introduction of tolvaptan therapy, all 27 patients indicated that they had received the guidance information before commencement of therapy which included.
• A standard leaflet about the drug
• An explanation of the expected side effects from the clinician
• Adetailed explanation of the potential benefits of therapy
• Information and advice on the need to drink plenty of fluid during the day and potentially at night if they were on tolvaptan
• Compliance with treatment
During the study period, two patients (7.4%) dropped out of treatment with tolvaptan: one patient opted out 2 months after starting treatment due to polyuria interfering with their job requirements and the other had relocated to another country 7 months after starting treatment; giving a retention rate of 92.6%. However, the latter patient, who emigrated, planned to continue therapy, if possible, while abroad, if the drug was available.
Review of the clinical records of attendance found that 65.8% of clinic appointments were attended and 69.2% had the required blood tests performed at the required frequency based on NICE.
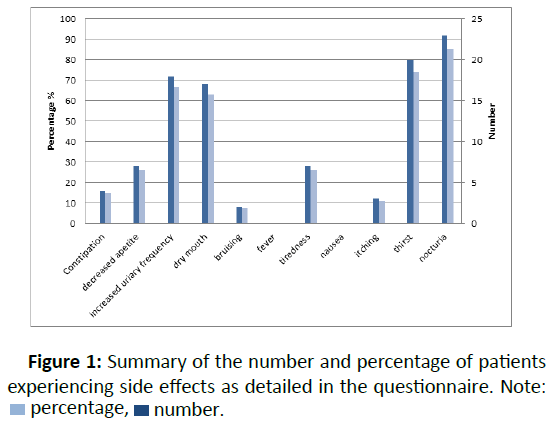
Fifty-five per cent of patients indicated that they had not missed a dose of drug at any time during the treatment period despite all patients indicating they had experienced one or more of the expected recognised side-effects (Figure 1).
Two patients indicated that they had purposely missed a dose of medication due to an upcoming social event and not wanting the inconvenience on polyuria and/or thirst. Other reasons for missing a dose of drug included:
• Being on holiday
• Simply forgetting to take the medication
• Being extremely tired
• When going out for the day (pre-planned)
• Temporary cessation due to a tooth infection in one case
Adverse effects
All patients porvided details any side effects they had experienced while taking tolvaptan on the questionnaire.
In total, 92.6% (n=25) of patients were on the full dose of 90 mg in the morning and 30 mg after 8 hours. 80% indicated that tolvaptan did not impact on their quality of life. Two patients “felt like stopping treatment” due to nocturia and the subsequent sleepless nights that they experienced.
Renal function
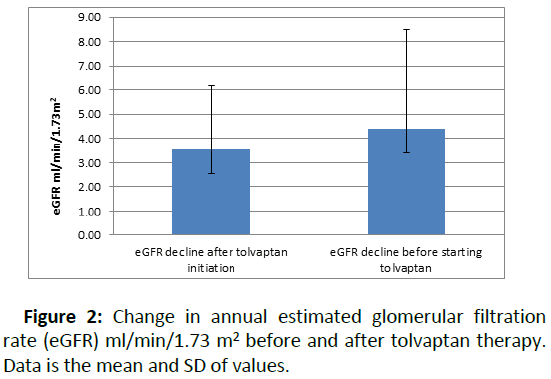
Twelve patients (44%) had a reduced rate of eGFR decline per year after starting tolvaptan compared to eGFR decline pretolvaptan. Overall, the median eGFR decline was 4.4 ml/min/ 1.73 m2 (range: 0–10 ml/min/1.73 m2) in the 12 months before tolvaptan treatment. This reduced to 3.5 ml/min/1.73 m2 (range: 0–9.3 ml/min/1.73 m2) annually after starting tolvaptan (Figure 2).
Two patients (7%), both males with an eGFR pre tolvaptan >70 ml/min.1.73 m2, did not record any change in the eGFR post tolvaptan therapy. One patient (3%) had an increase in eGFR post tolvaptan as compared to his average eGFR pre tolvaptan.
Other biochemical parameters
No patient experienced acute liver injury, as defined by a threefold rise in liver enzymes. The median urate level rose by 6.5% (26 μmol/L) six months after initiation of tolvaptan, but this reverted to baseline after 18 months. One patient was on medication for management of gout before starting tolvaptan (Allopurinol 100 mg daily); the dose of allopurinol was increased to 200 mg daily in the first year of tolvaptan treatment in this case. No other patient required medication for treatment of symptomatic gout or suffered an episode of gout during the study period.
The median proBNP (68 ng/L) rose after the first year by 11% and then increased by 26% from baseline (to 115 ng/L) after 24 months. However overall, these values remained in the normal reference range and were not indicative of cardiac compromise or fluid retention.
The median haemoglobin levels remained stable during tolvaptan therapy. Pre therapy the median value was 134 g/L (range 112 g/L–159 g/L) and 128 g/L (range 111 g/L–128 g/L) post therapy amongst the patients.
Discussion
The introduction of tolvaptan following NICE approval for appropriate patients with ADPKD has presented challenges to both patients and NHS Trusts.
Our data confirms that despite development of a comprehensive service for delivery of the program to identify and treat appropriate patients only 65.8% of appointments were attended. Qualitative investigation from the questionnaire survey found that factors including work, holidays, and university schedules impacted patients’ ability to attend clinic appointments, even with a degree of flexibility from the service. This was before the challenges of COVID-19 which may add further complexities to the service currently. This added complexity in addition to the current findings might present future challenges in ensuring adequate monitoring occurs in the first 18 months to detect the rare complication of liver failure. The health foundation estimated in 2020 that missed NHS appointments currently stand at approximately 22% of outpatient appointments [12]. Also, with the current concern about the cost of missed NHS appointments, NHS centres offering tolvaptan treatment could benefit from using digital online portals where patients manage their own appointments.
Compliance to treatment is critical to maximum benefit from therapy. The adherence rate in this study was 55%. This compares with the WHO report, Adherence to Long Term Therapies 2003 [13], which stated that adherence to medication for chronic long-term conditions in the developed world was at 50%. Although this is comparable, it is recognised that maximum benefit occurs with sustained therapy [6]. The main reason for intentional missed doses of tolvaptan in our study was the aquaretic side effects like the results seen in the landmark studies [6]. We did not however measure urine osmolality as a possible means of testing for compliance.
The most common side effects reported were polyuria, nocturia and increased thirst; however, patients having been educated on these side effects, consequently, did not drop out of treatment and this perhaps indicates the importance of patient education initially and their awareness of these potential side effects. The retention rate of 92.6% measures favourably with most centres in the UK. Although not objectively studied, it could be attributed to the strong emphasis on patient education and coordinated management to reassure patients.
Modelling by the ADPKD Outcomes Model (ADPKD-OM) suggests that tolvaptan use in this patient cohort can delay ESKD by between 2.7 and 4.7 years [14]. This is supported by the reduced rate of eGFR decline when patients initiate tolvaptan treatment and remain on therapy for a sustained time. This represents a potentially significant future cost saving for renal replacement therapy, its complications and the socio-economic implications of ESKD. The TEMPO 3:4 trial also demonstrated that tolvaptan reduced significantly the rate of increase in Total Kidney Volume (TKV) by approximately 50% compared with a placebo: 2.80% per year vs. 5.51% per year [6]. Our study did not examine whether there was a change in total kidney volume.
Other biochemical measurements – Haemoglobin, proBNP and Urate levels
Haemoglobin (Hb) remained stable during therapy. Changes in Hb have correlated with a poor prognosis and renal progression in retrospective and prospective cohort studies in part via effects on hypoxia-inducible factor impacting cyst [15,16]. One could speculate that changes in haemoglobin could results from a reduction in median eGFR with time; blood loss from ruptured cysts, and possibly the increased oral fluid intake due to tolvaptan may lead to a degree of haemodilution. More studies will be needed to establish the relationship between haemoglobin levels and tolvaptan.
We also measured proBNP to see if this was impacted by tolvaptan due to fluid changes from intake and loss. In the EVEREST trial, tolvaptan was shown to help with decongestion of the body as part of the treatment for heart failure [17]. In our study, there was an upward trend of pro BNP. One could speculate that this might suggest generous fluid intake by patients leading to a degree of cardiac enlargement but more studies that focus on the use of tolvaptan in patients with ADPKD as well as heart failure are needed to explore this further.
Theoretically tolvaptan can cause a rise in urate level due to its aquauretic effect. In this study however, there was no significant difference in urate level over the course of 36 months or symptoms of possible gout. This could be due to the counter effect of increased thirst but is reassuring for patients considering therapy in the future.
Limitations
This study has several limitations including the small sample size, single centre nature of the study and relatively short followup. In addition, it was retrospective and observational, therefore confounding factors remains in this study and firm conclusions cannot be drawn except around the delivery and success of the service.
Conclusion
This study has demonstrated that despite the side effects of tolvaptan, our pharmacist led clinic with consultant oversight has led to high rates of patient retention on therapy and few issues in compliance beyond reasonable “excuses”. In our population acute liver injury did. This has highlighted an effective model of service delivery involving other health care staff.
Conflict of Interest
None declared by any authors
References
- Blair HA, Keating GM (2015) Tolvaptan: a review in autosomal dominant polycystic kidney disease. Drugs 75(15): 1797-1806.
- Kidney Research UK (2019) Chronic kidney disease-Kidney research UK. Retrieved.
- Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, et al. (1998) OPC-41061, a highly potent human vasopressin V2-receptor antagonist: Pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther 287(3): 860-867.
- Torres VE, Harris PC, Pirson Y (2007) Autosomal dominant polycystic kidney disease. Lancet 369(9569): 1287-1301.
- NICE (2015) Tolvaptan for treating autosomal dominant polycystic kidney disease.
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, et al. (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367(25): 2407-2418.
- Nomigolzar S, Patel H (2018) Review of the reprise trial: Tolvaptan in later-stage autosomal dominant polycystic kidney disease. J Nephrol 4(1): 07-12.
- Vicente ET (2019) Pro: Tolvaptan delays the progression of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 34(1): 30-34.
- Ong A, Ashley C, Harris T, Sandford R, Sayer J, et al (2016) Tolvaptan for ADPKD: Interpreting the NICE decision.
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, et al. (2017) Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377(20): 1930-1942.
- Ingelfinger JR (2017) Tolvaptan and autosomal dominant polycystic kidney disease. N Engl J Med. 377(20): 1988-1989.
- Richards R, Reed S (2020) How can unnecessary outpatient appointments be reduced? The Health Foundation website.
- World Health Organization (2003) Adherence to long-term therapies: Evidence for action. Geneva.
- Hayley B, Phil ME, Karina H, Karl OR (2019) Modelling the long-term benefits of tolvaptan therapy on renal function decline in autosomal dominant polycystic kidney disease: An exploratory analysis using the ADPKD outcomes model. BMC Nephrol 20(1): 136.
- Ushio Y, Kataoka H, Sato M, Manabe S, Watanabe S, et al. (2020) Association between anemia and renal prognosis in autosomal dominant polycystic kidney disease: A retrospective study. Clin Exp Nephrol 24(6): 500-508.
- Uchiyama K, Mochizuki T, Shimada Y, Nishio S, Kataoka H, et al. (2021). Factors predicting decline in renal function and kidney volume growth in autosomal dominant polycystic kidney disease: A prospective cohort study (Japanese polycystic kidney disease registry: J-PKD). Clin Exp Nephrol 25(9): 970-980.
- Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, et al. (2013). Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur Heart J 34(11): 835-843.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences