ISSN : ISSN: 2576-1412
Journal of Applied Microbiology and Biochemistry
Cyclocondensation, Characterization and Antibacterial Activities of Novel 5-Chloro-1HIndole-2,3-Dione Derivatives
Z Tribak1*, MK Skalli1, A Haoudi1, Y Kandri Rodi2, O Senhaji3 and EM Essassi4
1Laboratory of Applied Chemistry, Sidi Mohamed Ben Abdellah University, Fes, Morocco
2Laboratory of Applied Organic Chemistry, Sidi Mohamed Ben Abdellah University, Fes, Morocco
3Laboratory of Applied Physical Chemistry, Moulay Ismail University, Meknes, Morocco
4Laboratory of Heterocyclic Organic Chemistry, Mohammed V University, Rabat, Morocco
- *Corresponding Author:
- Tribak Zineb
Faculty of Science and Technology
Laboratory of Applied Chemistry
Sidi Mohamed Ben Abdellah University
Fes 30050, Morocco
Tel: +212 659899497
E-mail: tribak.zineb@gmail.com
Received Date: May 08, 2018; Accepted Date: May 17, 2018; Published Date: May 24, 2018
Citation: Tribak Z, Skalli MK, Haoudi A, Rodi YK, Senhaji O, et al. (2018) Cyclocondensation, Characterization and Antibacterial Activities of Novel 5-Chloro-1H-Indole-2,3-Dione Derivatives. J Appl Microbiol Biochem. Vol.2 No.2:7
DOI: 10.21767/2576-1412.100023
Abstract
In our ongoing research to identify effective and simple compounds, we studied the cyclocondensation of 5-Chlorosatin derivatives by the action of Diamino-5- bromo-pyridine to give heterocyclic possessing Pyrido [2,3-b] Pyrazines, in a moderate to good yield. The synthesized products were characterized by 1H NMR and 13C NMR. In addition, the antibacterial properties in vitro were tested against certain microorganisms using the disk diffusion technique. The final results revealed that the majority of the compounds exhibited good antibacterial activity against Bacillus cereus and Staphylococcus aureus.
Keywords
Cyclocondensation; 5-Chlorosatin derivatives; Diamino-5-bromopyridine; Pyrido [2,3-b] Pyrazines; 1H NMR; 13C NMR; Antibacterial properties; Bacillus cereus; Staphylococcus aureus
Introduction
Isatin is a valuable starting material for the synthesis of a large number of fused ring systems, like the pyridine ring which can be considered as one of the simplest yet most important heteroaromatic structures, it is utilized in many pharmaceutical actives and possibly even more commonly found in agrochemical products [1,2].
Isatin shows a wide range of pharmacological activities reported in the literature, including antiviral [3], spermicidal [4], anticorrosive [5-7], analgesic [8], anticonvulsant [9], antioxidant [10], antitubercular [11], transthyretin fibrillogenesis inhibitory activity [12], antidepressant [13] and antiepileptic [14]. Tribak et al. [15] synthesized a series of 5-Chloroisatin derivatives and screened them for their anti-bacterial activity [16].
In view of this we become interested in the syntheses of some new 5-Chloroisatin derivatives. We have previously reported the cyclocondensation reactions of amino group with heterocyclic carbonyl are the subject of our study because of the possibility of their use for preparation of various heterocycles [17,18]. However, only one heterocyclic carbonyl group is able to undergo to cyclocondensation, the product of this reaction can be easily predicted.
The purpose of this paper is to focus on the study of cyclo-condensation of 5-Chloroisatin derivatives with diamino-5- bromo-pyridine, to obtain new six-membered heterocyclic rings and also to investigate the possibility of using these amino ketones for the synthesis of new nitrogen-containing heterocyclic with conventional procedures.
Materials and Methods
Chemistry
Melting points were determined using a Buchi apparatus in glass capillary tubes or a Kofler hot-stage apparatus and are uncorrected. NMR spectra of solutions in CDCl3 (TMS as internal standard) were measured on a Bruker Avance 300 spectrometer AC 300 (1H) or 75 MHz (13C). The solvents were removed under reduced pressure using standard rotary evaporators. The purity of compounds was done by column chromatography was carried out, while thin-layer chromatography was carried out on silica gel (230-400 Mesh).
General procedure of the cyclocondensation with diamino-5-bromo-pyridine
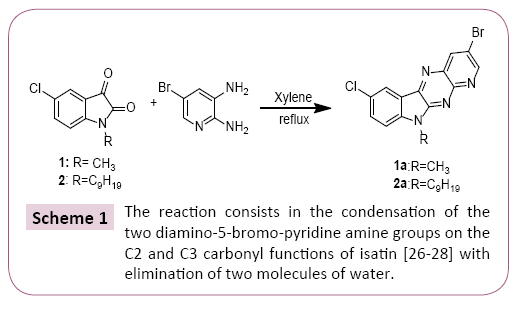
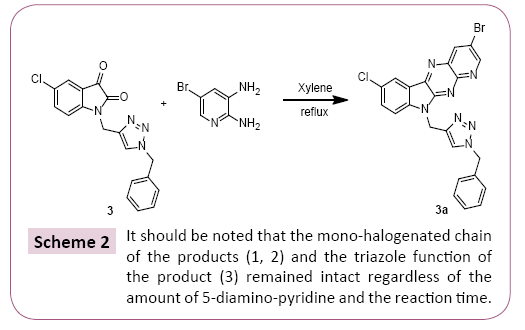
A mixture of 1 equivalent of 5-chloro-1-methylindoline-2,3-dione and 1.2 equivalent of Diamino-5-bromopyridine are added and 20 mL of xylene. The mixture is brought to reflux for 24 hours. After cooling of the reaction, the reaction mixture was concentrated under vacuum, the precipitate obtained is treated and separated by column chromatography and then recrystallized from ethanol (Scheme 1). All the prepared compounds are practically insoluble in water and only partially soluble in most organic solvents. These properties aggravated their characterization and study in solution with use of NMR spectroscopy (Scheme 2).
Spectral data
3 - b r o m o - 7 - c h l o r o - 1 0 - m e t h y l - 1 0 H - p y r i d o [ 3 ' , 2 ' : 5 , 6 ] pyrazino[2,3-b]indole (1a): yield: 88%; mp >220°C; Rf= 0.85; (Ethyl acetate/Hexane (2:1)) 1H NMR (CDCl3; 300 MHz): δ (ppm) 8.68 (d, H, HAr, 4JH-H =3Hz); 8.52 (d, H, HAr, 4JH-H =3Hz); 7.63- 7.88 (m, 2H, HAr); 7.34 (d, H, HAr, 4JH-H=3Hz); 3.80 (s, 3H, CH3,). 13C NMR (CDCl3; 75MHz): δ (ppm) 152.79 (NC=N); 149.40, 136.04, 134.35, 133.29, 127.99, 126.71, 121.20, 112.51 (Cq); 126.29, 121.63, 118.02, 112.93 (CHAr); 44.46 (CH3).
3-bromo-7-chloro-10-nonyl-10H-pyrido[3',2':5,6]pyrazino[2,3-b] indole (2a): yield: 88%; mp >250°C; Rf= 0.9; (Ethyl acetate/ Hexane (3:1)) 1H NMR (CDCl3; 300 MHz): δ (ppm) 8.45(d, H, HAr, 4JH-H =3Hz); 8.24(d, H, HAr, 4JH-H =3Hz); 7.57-7.61(m, 2H, HAr); 6.76(d, H, HAr, 4JH-H=3 Hz); 4.27(t, 3H, CH2, 3JH-H =6Hz, 4JHH =3Hz); 3.65(t, 3H, CH2, 3JH-H=6Hz, 4JH-H=3 Hz); 1.18(m, 12H, CH2); 0.81(m, 3H, CH3). 13C NMR (CDCl3; 75MHz): δ (ppm) 156.18 (NC=N); 147.91, 135.83, 134.14, 133.07, 127.99, 126.50, 126.29, 120.99, 112.30 (Cq); 129.51, 121.63, 117.26, 112.72 (CHAr); 60.99,41.06, 36.61, 29.82, 29.40, 27.49, 22.62(CH2); 13.29 (CH3).
10-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-3-bromo-7-chloro- 10H pyrido [3',2':5,6] pyrazino [2,3-b]indole (3a): yield: 68%; mp>250°C; Rf=0.8; (Ethyl acetate/Hexane (4:1)). 1H NMR (CDCl3;300 MHz): δ (ppm) 9.09 (d, H, HAr, 4JH-H=3 Hz); 8.77 (d, H, HAr, 4JH-H=3 Hz); 8.36 (d, H, HAr, 4JH-H =3Hz); 7.67-7.70 (m, 5H, HAr ); 7.49-7.52 (m, 3H, HAr ) 4.27 (t, 3H, CH2, 3JH-H =6Hz, 4JH-H =3Hz); 5.77 (s, 2H, CH2,); 5.40 (s, 2H, CH2); 13C NMR (CDCl3; 75 MHz): δ (ppm) 154.49 (NC=N); 146.86, 140.07, 136.47, 136.26, 134.14, 133.71, 127.45, 126.50, 121.23, 111.24 (Cq); 128.62, 128.41, 126.08, 125.63, 12.49, 121.34, 118.17, 113.14 (CHAr); 63.60, 53.14 (CH2).
Biology
Antibacterial activity: The antibacterial activity of the synthesized compounds was tested against two bacteria Gram negative: Pseudomonas aeruginosa, Escherichia coli, and two others Gram positive : Bacillus cereus and Staphylococcus aureus using LB medium (Luria Bertani medium: yeast extract 5.0 g, peptone 10.0 g, sodium chloride 5.0 g, distilled water 1000 mL).
Disc diffusion test: It consists of depositing a sterile disk imbibed by the test product on a bacterial mat at the very beginning of its growth and measuring the area where the bacteria could not develop. The inhibition diameter, which reflects the antibacterial activity of the product tested, is thus determined. The antibacterial activity of 5-Chloro-1H-indole-2,3-dione derivatives was evaluated using the disc diffusion method [19] using different microorganisms, including three types of bacteria: Bacillus cereus, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Mueller Hinton agar medium (MHA) was used for bacteria. Plates were preincubated at 37°C for 24h.
A sterile paper disk (6 mm in diameter) was placed on the surface of each agar plate and impregnated with 5 μL of each solution of 5-chloroisatin derivatives at a final concentration of 10 mg/ mL. Then, the Petri dishes are incubated at 37°C for 24 h for the bacteria.
Minimum inhibitory concentration determination (MIC): As a second step, we have proceeded to the determination of MICs of the products which have given positive results in the first test. The MIC was defined as the lowest concentration of compound giving complete inhibition of visible growth. All experiments were conducted in triplicates. MICs values were determined in 96 well-microplates using the micro-dilution assay according to the protocol described by Ismaili et al. [20] with some modifications.
Briefly, a stock solution of each product was prepared in 2% DMSO. Then, serial dilutions, of the antimicrobial agent were prepared in Mueller Hinton Broth (MHB) at final concentrations ranged between 5 mg/mL and 0.004 mg/mL. Then, each well is inoculated with a microbial inoculum prepared in the same medium at a final concentration of 106 CFU/mL. The 12th tube was considered as growth control, because no extracts solutions were added. Then, 50 μL of bacterial inoculum was added to each well at a final concentration of 106 CFU/mL. The final concentration of the extracts was included from 5 mg mL-1 (3rd well) to 0.019 mg mL-1 (11th well). After incubation at 37°C for 24 hours, 10 μL of rezasurin were added to each well as mycobacterial growth indicator. After further incubation at 37°C for 2 hours, the bacterial growth was revealed by reduction of blue dye resazurin to pink resorufin.
Minimum bactericidal concentrations (MBC): In order to determine the CMB, a bactericidal control is carried out 24 hours earlier by streaking on a platelet agar, after micro dilution to the broth by spreading 5 μL of the negative wells on Luria Bertani agar plates (Luria Bertani medium: Yeast extract 5.0g, peptone 10.0g, sodium chloride 5.0g, distilled water 1000 mL). The MBC will be the lowest concentration whose transplant shows a growth of germ less than or equal to 0.01% of survivors [21].
Results and Discussion
Herein, we report an efficient and economic synthesis of new products containing 6-membered heterocycles from 5-Chloroisatin. The starting material for preparation of these precedent compounds were 5-chloro-1-methylindoline-2,3- dione (1) and 5-chloro-1-nonylindoline-2,3-dione (2), which were obtained by N-alkylation reaction under phase transfer catalysis condition [22-24] and also 1-((1-benzyl-1H-1,2,3-triazol- 4-yl)methyl)-5-chloroindoline-2,3-dione (3) that was prepared by cycloaddition reaction catalyzed by Cu(I) [25].
The cyclocondensation of these compounds 3-5 was given by the action of the diamino-5-bromo-pyridine on a stoichiometric amount of the 5-chloroisatin derivatives in xylene at reflux for 12 hours resulted in a single product reclosing the pyrido [2,3- b] pyrazine structure, to give desired products in good to better yields, purity of which was declared by NMR methods.
The reaction consists in the condensation of the two diamino- 5-bromo-pyridine amine groups on the C2 and C3 carbonyl functions of isatin [26-28] with elimination of two molecules of water. It should be noted that the mono-halogenated chain of the products (1,2) and the triazole function of the product (3) remained intact regardless of the amount of 5-diamino-pyridine and the reaction time [29]. The synthesis of compounds 1a-3a is shown in Schemes 1 and 2.
Table 1 shows that compound 2 exercised an excellent inhibitory activity against Staphylococcus aureus (Gram positive bacteria), with a MIC value of 0.01 mg/mL, while It displayed comparable activity against Bacillus cereus with a MIC value of 0.078mg/mL, Indeed, It showed no antibacterial effect against Escherichia coli and Pseudomonas aeruginosa. Then, the compound 1 displayed moderate to good activity against Gram positive bacteria studied, on the other hand it did not present any activity against Gram-negative bacteria. Moreover, the compounds 3 did not show any antibacterial effects against all tested strains. Generally, Grampositives bacteria are much more susceptible to antibacterial agents than Gram-negatives bacteria, whose resistance is attributed to the structures of their cell wall. Gram-negative bacteria have a thick lipid bilayer which is selectively permeable [30].
Table 1 MICs and MBCs of the compounds against the microbes used.
| Compounds | MIC/MBC (mg/mL) | |||
|---|---|---|---|---|
| Gram+ | Gram- | |||
| Bacillus cereus | Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | |
| 1 | 0.156/0.156 | 0.313/0.313 | - | - |
| 2 | 0.078/0.078 | 0.01/0.01 | - | - |
| 3 | - | - | - | - |
Conclusion
In this paper, we have developed successfully a straightforward and an efficient single-step reaction between diamino-5-bromopyridine and 5-Chloroisatin derivatives to synthesize new products containing pyrido [2,3-b] pyrazine, with moderate to good yield under mild conditions, the structures of compounds obtained were confirmed by 1H-NMR, 13C-NMR. These derivatives were evaluated for their antibacterial activity in vitro against four bacteria including two Gram positive and two Gram negative. It is clear that the results revealed that the 2 compounds were biologically active with different spectral activity across all bacteria studied, whereas compound 3 showed no antibacterial effect. Whereas the other compounds 1 exhibited a moderate inhibitory effect. These encouraging results indicate that these organic compounds should be studied more widely in order to explore their potential in the treatment of infectious diseases as well as in the inhibition of bacterial growth.
Acknowledgement
The authors would like to thank yasir zaid who helped to carry out this work such as 1H NMR, 13C NMR in France.
References
- Kiuru P, Yli-Kauhaluoma J, Majumdar K, Chattopadhyay SK (2011) Heterocycles in Natural Product Synthesis.
- Baumann M, Baxendale IR, Beilstein J (2013) An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Org Chem 9: 2265-2319.
- Kang IJ, Wang LW, Hsu TA, Yueh A, Lee CC, et al. (2011) Isatin-thiosemicarbazones as potent herpes simplex virus inhibitors. Bioorg Med Chem Lett 21: 1948-1952.
- Paira P, Hazra A, Kumar S, Paira R, Sahu KB, et al. (2009) Efficient synthesis of 3,3-diheteroaromatic oxindole analogues and their in vitro evaluation for spermicidal potential. Med Chem Lett 19: 4786-4789.
- Quraishi MA, Ahamad I, Singh AK, Shukla SK (2008) N-(Piperidinomethyl)-3-[(pyridylidene)amino] isatin: A new and effective acid corrosion inhibitor for mild steel. Mater Chem Phys 3: 1035-1039.
- Tribak Z, Haoudi A, Skalli MK, Kandri Rodi Y, El Azzouzi M, et al. (2017) 5-Chloro-1H-indole-2,3-dione derivative as corrosion inhibitor for mild steel in 1M H3PO4 weight loss, electrochemical and SEM studies. J Mater Environ Sci 8: 298-309.
- Tribak Z, Kandri Rodi Y, Elmsellem H, Abdel-Rahman I, Haoudi A, et al. (2017) 5-chloro-1-octylindoline-2,3-dione as a new corrosion inhibitor for mild steel in hydrochloric acid solution. Sci J Mater Environ Sci 8: 1116-1127.
- Figueiredo GS, Zardo RS, Silva BV, Violante FA, Pinto AC, et al. (2013) Convolutamydine A and synthetic analogues have antinociceptive properties in mice. Pharmacol Biochem 3: 431-439.
- Nagihan B, Kocyigit-Kaymakcioglu B, Salih G, Aricioglu F (2013) Synthesis and anticonvulsant activity of some 2-pyrazolines derived from chalcones. Arab J Chem 7: 37.
- Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, et al. (2010) New isatin derivatives with antioxidant activity. Eur J Med Chem 45: 1374-1378.
- Kumar K, Carrere-Kremer S, Kremer L, Guerardel Y, Biot C, et al. (2013) 1H-1,2,3-triazole-tethered isatin-ferrocene and isatin-ferrocenylchalcone conjugates: synthesis and in vitro antitubercular evaluation. Organometallics 32: 110-116.
- Gonzalez A, Quirante J, Nieto J, Almeida MR, Saraiva MJ, et al. (2009) Isatin derivatives, a novel class of transthyretin fibrillogenesis inhibitors. Bioorg Med Chem Lett 19: 5270-5273.
- Manley-King CI, Bergh JJ, Petzer JP (2011) Inhibition of monoamine oxidase by selected C5- and C6-substituted isatin analogues. Bioorg Med Chem 19: 261-274.
- Prakash CR, Raja S (2011) Design, synthesis and antiepileptic properties of novel 1-(substitutedbenzylidene)-3-(1-(morpholino/piperidinomethyl)-2,3-dioxoindolin-5-yl)urea derivatives. Eur J Med Chem 46: 6057-6065.
- Tribak Z, El Amin O, Skalli MK, Senhaji O, Kandri Y, et al. (2017) N-alkylation methods, characterization and evaluation of antibacterial activity of some novel 5-chloroisatin derivatives. Int J Eng Res Appl 7: 21-24.
- Tribak Z, El Amin O, Skalli MK, Senhaji O, Kandri Y, et al. (2017) Synthesis, characterization, and antibacterial activity of some novel 5-chloroisatin derivatives. Int J Eng Res Appl 7: 66-70.
- Tribak Z, Kandri Y, Haoudi A, Skalli MK, Mazzah A, et al. (2016) HeterocyclCycloaddition 1,3-dipolaire des derives de la 5-chloro-1H-indole-2,3 dione: vers de nouvelles isoxazolines et spirodioxazolines.J Mar Chim Heterocycl 16: 58-65.
- Tribak Z, Ghibate R, Skalli MK, Kandri Rodi Y, Mrani D, et al. (2017) Synthesis and characterization of a new cationic surfactant derived from 5-Chloro-1H-indole-2,3-dione In Aqueous Systems. Int J Eng Res Appl 7: 04-08.
- Balouiri M, Sadiki M, Ibnsoud SK (2014) Methods for in vitro evaluating antimicrobial activity: A review. J Pharmaceutical Analysis 6: 6-9.
- Ismaili H, Milella L, Fkih-Tetouani S, Ilidrissi A, Camporese A, et al. (2004) In vivo topical anti-inflammatory and in vitro antioxidant activities of two extracts ofThymus satureioides leaves. J Ethnopharmacol 91: 31-36.
- Bouhdid S, Abrini J, Zhiri A, Espuny MJ, Manresa A (2009) Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil. J Appl Microbiol 106: 1558-1568.
- Tribak Z, Haoudi A, Kandri Rodi Y, Elmsellem H, Skalli MK, et al. (2016) Synthesis and reactivity of new heterocyclic systems derived from 5-chloro-1H-indole-2,3-dione. Mor J Chem 4: 1157-1163.
- Tribak Z, Kandri Rodi Y, Haoudi A, El Mokhtar E, Capet F, et al. (2016) IUCrData1-Benzyl-5-chloroindoline-2,3-dione.
- Tribak Z, Kandri Rodi Y, Haoudi A, El Mokhtar E, Capet F, et al. (2016) IUCrData 1-(12-Bromododecyl)-5-chloroindoline-2,3-dione.
- Wannassi N, Rammah MM, Boudriga S, Rammah MB, Monnier-Jobé K, et al.(2010) Regio and stereoselective 1,3-dipolar cycloaddition of C-aryl-N-phenylnitrones over (E)-arylidene-(2H)-indan-1-ones: Synthesis of highly substituted novel spiro-isoxazolidines. Heterocycles 81: 2749-2762.
- Tribak Z, Kandri Rodi Y, Haoudi A, El Mokhtar E, Capet F, et al. (2016) IUCrData 1-Allyl-5-chloroindoline-2,3-dione.
- Tribak Z, Kandri Rodi Y, Haoudi A, El Mokhtar E, Capet F, et al. (2016) IUCrData 5-Chloro-1-methylindoline-2,3-dione.
- Tribak Z, Ghibate R, Skalli MK, Kandri Y, Rodi O, et al. (2016) Experimental and theoretical study for corrosion inhibition in 1m HCl solution by new 5-chloroisatin derivative.Inter J Sci Tech Eng 3: 257-262.
- Bonacorso HG, Lourega RV, Righi FJ, Deon ED, Zanatta N, et al. (2008) Preparation of new 2â€ÂÂamino†and 2,3â€ÂÂdiaminoâ€ÂÂpyridine trifluoroacetyl enamine derivatives and their application to the synthesis of trifluoromethylâ€ÂÂcontaining 3Hâ€ÂÂpyrido[2,3â€ÂÂb][1,4] diazepinols. J Heterocyclic Chem 45: 1679-1686.
- Iqbal MS, Bukhari IH, Arif M (2005) Preparation, characterization and biological evaluation of copper(II) and zinc(II) complexes with schiff bases derived from amoxicillin and cephalexin. Appl Organomet Chem 19: 864-869.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences