Cryptogenic Hemoptysis in Children: A Case Report
1Department of Paediatrics, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
2National Institute of Preventive and Social Medicine (NIPSOM), Dhaka, Bangladesh.
- *Corresponding Author:
- Rahman Atiar
Department of Paediatrics, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
E-mail: atiar777@yahoo.com
Received date: April 02, 2018; Accepted date: April 26, 2018; Published date: May 03, 2018
Citation: Atiar R, Abdullahel A, Khainoor Z (2018) Cryptogenic Hemoptysis in Children: A Case Report. J Birth Defects. 1:4
Abstract
Hemoptysis in children is a rare but potentially life-threatening condition of various underlying respiratory tract abnormalities. The diagnosis is challenging as it is difficult to elicit a clear history and perform thorough physical examination in a child. However, despite the use of currently available diagnostic tools such as bronchoscopy and computed tomography, the etiology cannot be determined in up to 25% of cases, termed as cryptogenic. Here we report a 5½ years old girl having history of intermittent, moderate-volume hemoptysis for one year. Her coagulation profile, bronchoscopy and CT aorta pulmonary angiogram findings were normal. Her CT scan of chest revealed an ill-defined in homogenous opacities and Tc-99m MAA (micro aggregated albumin) revealed defective perfusion in upper lobe of left lung which may be due to healing lession of previous infection. Ultimately she was diagnosed having cryptogenic hemoptysis and was transfered to higher Centre for bronchial artery embolization.
Keywords
Cryptogenic; Hemoptysis; Children
Introduction
Hemoptysis is defined as the expectoration of blood or blood tinged sputum from the lower respiratory tract [1]. Although common in adults, hemoptysis is a rare presenting symptom in children. As children often swallow their sputum; therefore, hemoptysis may go unnoticed, diagnosis of pediatric hemoptysis can be challenging unless the bleeding is substantial [2].
Hemoptysis may be confused with hematemesis in children. As the diagnostic and treatment strategies differ markedly, the two sources must be differentiated. The blood in hemoptysis is bright red in color and may be admixed with sputum and frothy. The blood in hematemesis is dark red or brown and may be mixed with food particles and is commonly preceeded by vomiting or retching [1,2]. The pH of the hemoptysis is generally alkaline, while the pH of hematemesis is acidic [1]. Once hemoptysis is confirmed, the underlying causes then are to be explored.
The bleeding may occur from the large or small pulmonary vessels. Bleeding from the small vessels is known as diffuse alveolar hemorrhage [3]. There are various etiologies of hemoptysis in children, including- infection (pneumonia, tracheobronchitis, tuberculosis), foreign body aspiration, cystic fibrosis, bronchiectasis, idiopathic pulmonary hemosiderosis, congenital heart disease (Endocardial cushion defects, complex heart diseases, tetralogy of Fallot, truncus arteriosus, Transposition of great arteries), pulmonary vascular malformation (e.g bronchial artery pseudoaneurysm), pulmonary arteriovenous fistula [2,4]. However in some cases, the cause remains unknown even after clinical evaluation and detailed investigations, including radiologic imaging and bronchoscopy. These cases are defined as Cryptogenic Hemoptysis (CH) [5,6]. Nearly 20-25% of hemoptysis cases are reported to be diagnosed as cryptogenic [4,7].
Infection accounts for up to 40% of hemoptysis cases where destruction of lung parenchyma and erosion of blood vessels results in hemoptysis [2]. The infection is usually bacterial in nature and consists of Streptococcus pneumonie, Staphylococcus aureus, M. catarrhalis, klebsiella species, or Pseudomonas aeruginosa. Hemoptysis from aspergillus infection of the lungs either in the form of Allergic Broncho Pulmonary Aspergillosis (ABPA) or invasive aspergilliosis have also been reported in children [8].
Congenital heart disease can cause hemoptysis in children. Here hemoptysis occurs most frequently from resultant pulmonary vascular obstructive disease. The other pahology found are enlarged collateral bronchial circulation, erosion of a tortuous dilated bronchial artery into a bronchus, from rupture of an atherosclerotic bronchial artery plaque, or from localized pulmonary infarction at the bronchopulmonary anastomosis [1,9]. Isolated bronchial artery pseudo aneurysm was also identified as a source of hemoptysis [3]. Foreign body aspiration is another important cause of pediatric hemoptysis. Bleeding here results from the mechanical trauma to the respiratory epithelium or the ensuing inflammatory reaction, especially to vegetable matter [10].
Approximately 5% of patients with cystic fibrosis may present with massive hemoptysis due to bronchiectasis. The pathology here found are-hyperplasia, tortuosity and dilatation of bronchial arteries due to chronic inflammation and subsequent erosion of these dilated, thin walled bronchial vessels after successive pulmonary infections [1]. Idiopathic pulmonary hemosiderosis is a rare cause of small and recurrent hemoptysis, resulting from diffuse alveolar hemorrhage. It has a variable natural history and has been reported to have a high mortality [11]. Many patients develop iron deficiency anemia secondary to deposition of hemosiderin iron in the alveoli. In one study it was found that, about 58% of childhood hemoptysis results from pulmonary hemosiderosis [12]. Hemosiderin-laden alveolar macrophages (siderophages) are found on examination of sputum & broncho alveolar lavage and also the lung biopsy shows numerous siderophages in the alveoli, without any evidence of pulmonary vasculitis, nonspecific/granulomatous inflammation, or deposition of immunoglobulins. Normo-complement urticarial vasculitis has been observed in some children which is supposed to be the cause of hemoptysis [13].
Malignancy of respiratory tract, like endobronchial or pulmonary parenchymal malignancies, may rarely cause of hemoptysis in children. Tumors that may cause hemoptysis include bronchial carcinoid, bronchial adenoma, endobronchial metastasis, mediastinal teratomas, tracheal tumours, or bronchial arteriovenous malformations in children [14].
Hemoptysis was found in approximately 10% of the patients with long-term tracheostomy [15] about 15.5% of the hemoptysis was described to relate with tracheotomy [16]. In these cases, pink or red-tinged secretions are found on suctioning the tracheobronchial tree.
Factitious hemoptysis is considered when the suggestive medical history or abnormal behavior is found in the child [17]. Covert biting of the buccal mucosa has been attributed to be the cause of bleeding in a study [18]. There are some other less common causes causing hemoptysis in children, such as bleeding from localized lesions in upper airways or bleeding into the lungs as like part of a systemic disease systemic lupus erythematosis, Goodpasture’s syndrome, pulmonary thromboembolism, hydatid cyst, and even duplication cyst of the stomach [19-22]. An isolated pulmonary arteritis or endobronchial endometriosis can lead to massive hemoptysis in children [23,24]. The amount of hemoptysis was categorized as mild (<20 mL/day), moderate (20-99 mL/day), and massive (≥ 100 mL/day or bronchial blood loss that causes hemodynamic or respiratory compromises) [1]. Hemoptysis, when severe and untreated, has a mortality rate of more than 50% [1] with interventional radiological procedures and surgery, this rate has dropped to 7%-18% [25].
While hemoptysis is usually identified with the currently available diagnostic tools such as Computed Tomography (CT) scan and fibroptic bronchoscopy, 20-25% of cases remain classified as cryptogenic [26]. It represents a challenge to identify the etiology of cryptogenic hemoptysis and manage the patient with massive cryptogenic hemoptysis in emergency. Up to now, multidisciplinary approach, such as medical management especially together with bronchial artery embolization, has been effectively applied to the treatment of massive hemoptysis, and surgical treatment has seldom been employed [4].
Case Presentation
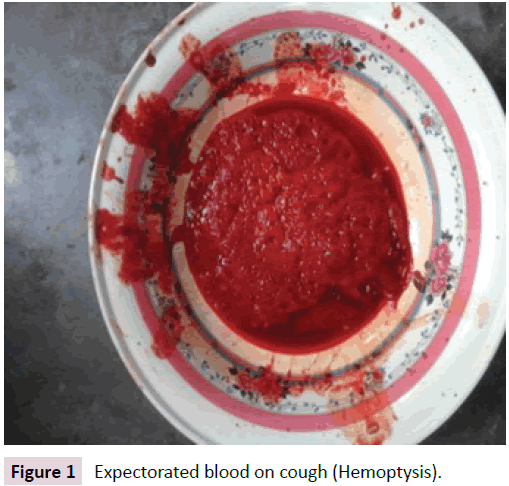
Nuha 5 and ½ year old girl, only issue of her devorced, nonconsanguineous, parents admitted with the complaints of recurrent episodes of hemoptysis for one year, having amount more than 1 cup per episode (Figure 1).
Each episode was preceeded by cough and bubbling sensation in chest, followed by passage of copious blood mixed sputum. The sputum was bright red, fresh not associated with fever or vomiting. The bleeding was so severe that she had to take 4 units of blood transfusion following the start of the event. She remained symptom free in other time. She had no feature of infection, had no history of contact with tuberculosis patient or foreign body aspiration.
She was diagnosed as having a Patent Ductus Arteriosus (PDA) at her 3½ years of age and got PDA closure by a device immediately after then. About 9 months after the implantation of the device, it was displaced and emergency open heart surgery was done. At that time, excision of a pseudo aneurysm of pulmonary artery and sealing of the PDA device was done.
On examination, she was ill-looking, mildly pale, afebrile, acyanotic, BCG mark was present, there is a vertical scar, about 10 cm in length in mid chest, for the previous surgery. Her vitals were within normal limit (respiratory rate 24 breaths/min, Temp. 98°F, heart rate 88 b/min) and she was anthropometrically well thriving. Her systemic examinations, including respiratory system revealed no abnormality.
Her investigation revealed mild anemia (Hb-9.1 gm/dl) with normal diffrentials and platelet count and red cell indices (Hb%- 9.1 g/dl, total leucocyte count: 8000/mm3, polymorphs-42%, lymphocytes-53%, eosinophil-2%. platelet-300,000/mm3, ESR 06 mm 1st hr. MCV-80fl, MCH-28pg. MCHC-32gm/dl). Her PBF revealed moderate normocytic normochromic anaemia.
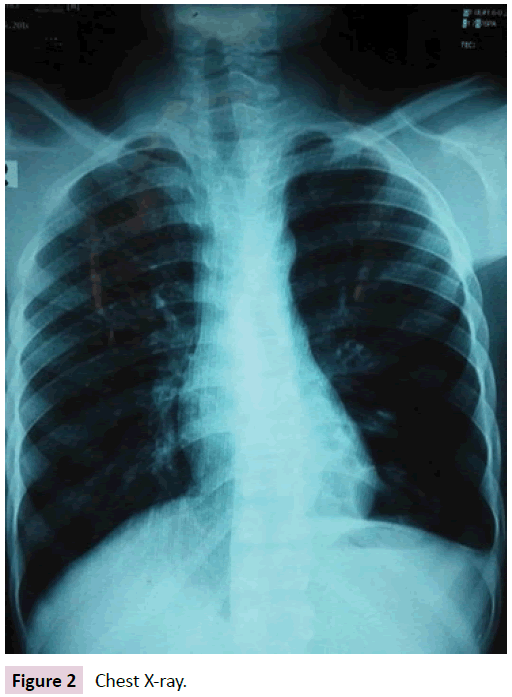
Her coagulation profile was also normal (ProthombinTimepatient 15 sec, control -12 sec.INR-1.1 & Activated Partial Thromboplastin Time-34 sec). Vasculitis was excluded by normal findings of antinuclear antibody, pANCA, cANCA. Her chest radiograph (Figure 2) was normal and tuberculosis was excluded by the negative result of MT & multiplex PCR of sputum.
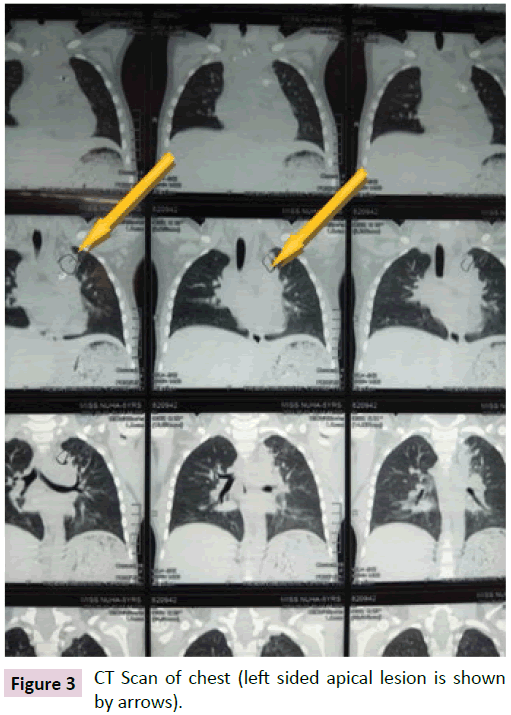
Her echocardiographic findings were normal with good LV systolic function. PDA device was in situ without any residual flow. Doppler Study of portal vein was normal. Her CT scan of chest revealed a consolidation in the apical segment of left upper lobe (Figure 3).
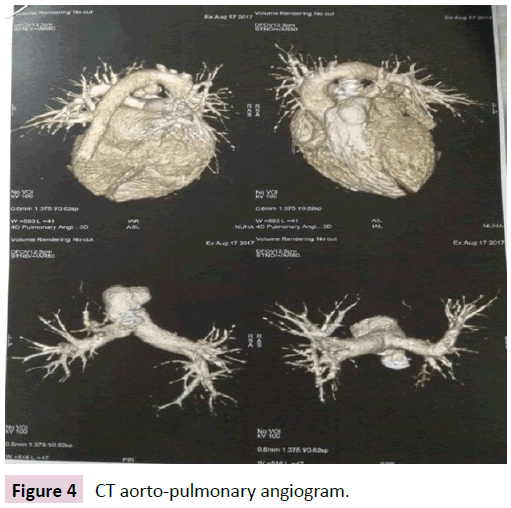
Bronchoscopic finding was normal. CT aorto pulmonary angiogram revealed that, all pulmonary arteries and veins were normal (Figure 4).
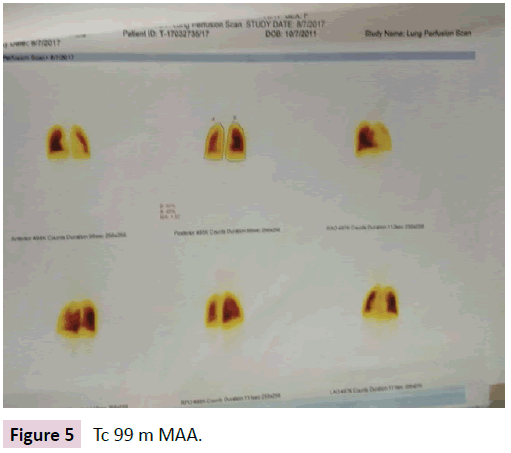
Subsequently a Tc-99m MAA (micro aggregated albumin) was done which revealed that perfusion was defective in upper lobe of left lung (Figure 5).
Bornchial Arterial Embolization (BAE) could not be done due to lack of facility. She was finally diagnosed having cryptogenic hemoptysis.
Discussion
Hemoptysis in children should be evaluated systematically begining with taking a detailed medical history and physical examination. First, the hemoptysis has to be differentiated from hematemesis. Patient history also can help to identify the anatomic site of bleeding, differentiation between hemoptysis and pseudohemoptysis, and to narrow the differential diagnosis.
Once true hemoptysis is suspected, the investigations should focus on the respiratory system. The possibility of foreign body aspiration should be suspected if there is history of choking or coughing episodes, and new onset wheezing. A history of chronic lung disease or CHD is also important. This is followed by thorough examination of the neck and head. Special attention is to be given in to the oral cavity and nasopharynx as these are potential sources of bleeding. Lung examination may reveal localized wheezing, suggesting foreign body, or rales or decreased breath sounds, which may be associated with an infectious process [1].
Routine study of blood & sputum has to be done in all the children to exclude infectous etiology and to identify the pathogens and sensitivities to various antibiotics [1]. Chest radiography is a valuable investigation where unilateral air trapping with hyperinflation suggests foreign body aspiration and parenchymal infiltrate suggests infection [27] other helpful findings are hilar adenopathy, pleural effusion and cardiomegaly. In approximately one third of children with hemoptysis, chest radiographs may be normal.
High Resolution Computed Tomography (HRCT) is useful in further delineation of chest radiography findings. Contrast studies are helpful to differentiate between vascular structures and solid masses [28]. In recent years, HRCT scan of thorax has become the most accurate and sensitive noninvasive diagnostic tool for the evaluation of bronchiectasis [29].
If the etiology of hemoptysis remains undiscovered after these workup, and if the bleeding is recurrent, bronchoscopy which may be rigid or fibreoptic, is indicated to identify source of bleeding [30]. Fibreoptic bronchoscopy can be performed with sedation and allows more detailed evaluation of distal bronchial tree. However, it does not permit effective ventilation and removal of blood clots. In contrast, rigid bronchoscope offers ventilation and helps localize site of bleeding. It is also ideal for suctioning of clotted blood and is also more effective for removal of airway foreign bodies [31]. The various other bronchoscopic findings includs: mucosal inflammation, purulence, tracheal abrasions and bronchial mass. The diagnostic yield of bronchoscopy in hemoptysis ranges from 40% to 100% in various studies [30].
If etiology still remains unexplained, then cardiac evaluation cardiac evaluation is considered even in the absence of overt cardiac symptoms. Echocardiography is performed for the evaluation of any suspected congenital cardiac disease [32]. Pulmonary thromboembolism is a rare cause of hemoptysis in children, and a combination of diagnostic procedures must be used to identify a suspected or confirmed case of pulmonary thromboembolism in children, including ventilation perfusion studies [20].
When no other cause is found for pulmonary hemorrhage, the presumed diagnosis is idiopathic pulmonary hemosiderosis. In these patients, sputum and bronchoalveolar lavage fluid can disclose haemosiderin-laden alveolar macrophages (siderophages) [12].
Bronchial arteriography and Bronchial Artery Embolization (BAE) may provide an effective means of rapid diagnosis and treatment of severe, life-threatening hemoptysis, and has revolutionized the management of the disease, providing a reliable, minimally invasive tool with excellent diagnostic and therapeutic outcomes. Embolization is often lifesaving; it may postpone or replace surgery, and in some situations it is the treatment of choice [33].
Life-threatening hemoptysis is one of the most serious emergencies in pulmonary medicine. The initial approach is no different than for any other bleeding or hemodynamically unstable patient. According to standard management protocols, the physician's primary goals include stabilizing the patient and securing the airway, identifying the bleeding site and efficiently containing the hemorrhage [5].
Localization of the bleeding site is usually accomplished with imaging studies (chest x-ray, CT) and bronchoscopy. Bronchoscopy plays a central role in the evaluation and management of hemoptysis. Direct inspection allows localization of the bleeding site and isolation of bleeding segment to prevent flooding of nonbleeding lung and asphyxiation. Rigid bronchoscope is preferred over flexible bronchoscope for management of massive hemoptysis but its utility is limited by lack of trained personnel in majority of medical centers. In the absence of facilities for rigid bronchoscopy, it is prudent to secure airway with a large endotracheal tube, perform early flexible bronchoscopy, and initiate aggressive resuscitative measures. Several bronchoscopic techniques such as balloon tamponade, topical application of cold saline, vasoconstrictors, and pro-coagulant substances may be applied for temporary control of bleeding [34]. Bronchoscopy provides more information regarding vascular malformation if done with endobronchial ultrasound [34].
Pulmonary and bronchial arteriography are important methods to diagnose the etiology of recurrent hemoptysis, e.g. bronchial artery pseudo aneurysm in children [34]. Surgery was once regarded as the treatment of choice in operable patients with massive hemoptysis, but inability to localize the bleeding site makes it a poor option. Even with the advent of modern diagnostic tools such as multidetector CT and flexible bronchoscopy, as many as 25% of patients presenting with hemoptysis remain classified as having cryptogenic hemoptysis (CH). Here recurrent, minor to severe life threatening bleeding may happen [4]. Therefore, a substantial portion of patients with cryptogenic hemoptysis may require an immediate hemostatic procedure. Bronchial arterial embolization (BAE) has been accepted as the first-line nonsurgical treatment of massive or recurrent hemoptysis of various etiologies. It has been shown to be an effective therapy for controlling massive hemoptysis from a large spectrum of causes, including tuberculosis, bronchiectasis, bronchogenic carcinoma, aspergilloma, and bronchial inflammation. In 90% of cases, embolization stops the bleeding immediately, and 70% of patients do not experience recurrences during 1 year of follow-up.6 In some studies, about 85% of patients were found having abnormal angiographic findings and most common vascular abnormalities found were bronchial artery enlargement and hypervascularity [35,36]. However in case of cryptogenic hemoptysis where the patients have angiographically normal bronchial arteries on the side of bronchial bleeding, it is difficult to decide whether to perform embolization or not. In such cases, embolization of the ipsilateral bronchial artery based on bleeding localization by CT can result in immediate cessation of bleeding. Therefore, it is surmised that angiographically normal bronchial arteries can even be responsible for hemoptysis, and BAE is can be the choice in such cases [36-38].
In our patient, from the pulmonologist point of view, we have no limitation at all, because we did all the investigations to explore the diagnosis. But ultimately the case remained underdiagnosed. That’s why, we labeled the case as cryptogenic hemoptysis. The next course of action should be bronchial arterial embolization which should be done by thoracic surgeon. Moreover, the facility of this intervention is not available in our institute. This might be our limitation.
References
- Batra PS, Holinger LD (2001) Etiology and Management of Pediatric Hemoptysis. Arch Otolaryngol Head Neck Surg 127: 377-382.
- Gaude GS (2010) Hemoptysis in children. Indian Pediatr 47: 245-254.
- Patel R, Uchida D, Peter GF, Jeremy DM (2014) Bronchial Artery Pseudoaneurysm as an Unsuspected Cause of Hemoptysis in a Pediatric Patient. Ann Otol Rhinol & Laryngol 123: 591-595.
- Samara KD, Tsetis D, Antoniou KM, Protopapadakis C, Maltezakis G, et al. (2011) Bronchial artery embolization for management of massive cryptogenic hemoptysis: a case series. J Med Case Rep 5: 58-63.
- Corder R (2011) Hemoptysis. Emerg Med Clin North Am 21: 421-435.
- Mal H, Rullon I, Mellot F, Brugière O, Sleiman C, et al. (1999) Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest 115: 996-1001.
- Santiago S, Tobias J, Williams AJ (1991) A reappraisal of the causes of hemoptysis. Arch Intern Med 151: 2449-2451.
- Crassad N, Halden H, Piens MA, Pondarre C, Hadden R, et al. (2008) Invasive aspergillosis in a pediatric hematologic department: a 15 year review. Mycosis 51: 109-116.
- Haroutunian LM, Neill CA (1972) Pulmonary complications of congenital heart disease: hemoptysis. Am Heart J 84: 540-549.
- Dore ND, Landar LI, Hallam L(1997) Hemoptysis in healthy children due to unsuspected foreign body. J Pediatric Child Health 33: 448-450.
- Dearborn DG (1997) Pulmonary hemorrhage in infants and children. Curr Opin Pediatric 9: 219-224.
- Kabra SK, Bhargava S, Lodha S, Satyavani A, Walia M (2007) Idiopathic pulmonary hemosiderosis: clinical profile and follow up of 26 children. Indian Pediatr 44: 333-338.
- Yukset H, Yilmaz O, Saras R, Kirmaz C, Sogut A, et al. (2007) Pulmonary hemosiderosis with normocomplentemic urticarial vasculitis in a child. Monaldi Arch Chest Dis 67: 63-67.
- Hancock BJ, Dilorenzo M, Youssef S, Yazbeck S, Marcotte JE, et al. (1993) Childhood primary pulmonary neoplasms. J Pediatric Surg 28: 1133-1136.
- Wetmore RF, Handler SD, Patsic WP (1982) Pediatric tracheostomy: experience during the past decade. Ann Otol Rhinol Laryngol 91: 628-632.
- Fabian MC, Smitheringale (1996) Hemoptysis in children: the hospital for sick children experience. J Otolaryngol 25: 44-45.
- Baktari JB, Tashkin DP, Small GW (1994) Factitious hemoptysis: adding to the differential diagnosis. Chest 105: 943-945.
- Sood M, Clarke JR, Murphy MS (1999) Covert biting of buccal mucosa masquerading as haemetemesis or hemoptysis in children. Acta Paediatr 88: 1038-1040.
- Godfrey S (2004) Pulmonary hemorrhage/hemoptysis in children. Pediatric Pulmonol 37: 476-484.
- Baby NPS, Gahunia HK, Massicotte P (2005) Pulmonary thromboembolism in children. Pediatric Radiol 35: 258-274.
- Bousseta K, Siala N, Brini I, Aloui N, Sammoud A, et al. (2005) The hydatid of lung in children: 54 cases. Tunis Med 83: 24-27.
- Menon P, Rao KL, Saxena AK (2004) Duplication cyst of the stomach presenting as hemoptysis. Eur J Pediatric Surg 14: 429-431.
- Chan EY, Avcin T, Manson D, Cutz E, Scneider R, et al. (2007) Massive hemoptysis in a 11 year old girl with isolated pulmonary arteritis. Pediatr Pulmonol 42: 177-180.
- Martire B, Loizzi M, Cimmino A, Perazzi S, De Mattin D, et al. (2007) Catamenial hemoptysis from endobronchial endometriosis in a child with type I von Willebrand disease. Pediatr Pulmonol 42: 386-388.
- Fernando HC, Stein M, Benfield JR, Link DP (1998) Role of bronchial artery embolization in the management of hemoptysis. Arch Surg 133: 862-886.
- Lee YJ, Lee SM, Park JS, Yim JJ, Yang SC, et al. (2012) The clinical implications of bronchoscopy in hemoptysis patients with no explainable lesions in computed tomography. Respir Med 106: 413-419.
- Pianosi P, Al Sadoon H (1996) Hemoptysis in children. Pediatric Rev 19: 344-348.
- Turcios NL, Vega M (1987) The child with hemoptysis. Hosp Prac 22: 217-218.
- Tsao PC, Lin CY (2002) Clinical spectrum of bronchiectasis in children. Acta Paediatr Taiwan 43: 271-275.
- Ulong KS, Wang CR, Lim TY (1998) Hemoptysis in children. Chang Gung Med J 21: 57-62.
- Miller J (1996) Rigid bronchoscopy. Chest Surg Clin N Am 6: 161-167.
- Sritippayawan S, Margetis MF, Machaughlin EF, Achermann R, Wells WS, et al. (2002) Cor triatriatum: A cause of hemoptysis. Pediatr Pulmonol 34: 405-408.
- Tom LM, Palevsky HI, Holsclaw DS (2015) Recurrent bleeding, survival and longitudinal pulmonary function following bronchial artery embolization for hemoptysis in a U.S. adult population. J Vasc Interv Radiol 26: 1806-1813.
- Mhanna L, Noel-Savina E, Pontier S, Jaffro M, Guibert N, et al. (2017) Use of endobronchial ultrasound in the management of cryptogenic haemoptysis. Rev Mal Respir 34: 770-773.
- Kervancioglu S, Bayram N, Gelebek Yilmaz F, Sanli M, Sirikci A (2015) Radiological findings and outcomes of bronchial artery embolization in cryptogenic hemoptysis. J Kor Med Sci 30: 591-597.
- Lee H, Yoon CJ, Seong NJ, Jeon CH, Yoon HI, et al. (2017) Cryptogenic Hemoptysis: Effectiveness of Bronchial Artery Embolization Using N-Butyl Cyanoacrylate. J Vasc Interv Radiol 28: 1161-1166.
- Delage A, Tillie-Leblond I, Cavestri B, Wallaert B, Marquette CH (2010) Cryptogenic hemoptysis in chronic obstructive pulmonary disease: characteristics and outcome. 80: 387-392.
- Menchini L, Remy-Jardin M, Faivre JB (2009) Cryptogenic haemoptysis in smokers: angiography and results of embolisation in 35 patients. EurRespir J 34: 1031-1039.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences