Contamination Assessment of Soil and Groundwater within and around Semicontrolled Solid Waste Dumpsites in Port Harcourt, Nigeria
Department of Geology, College of Natural and Applied Sciences, University of Port Harcourt, Nigeria
- *Corresponding Author:
- Nwankwoala HO
Department of Geology
College of Natural and Applied Sciences,
Harcourt, Nigeria
Tel: +234-8036723009
E-mail: nwankwoala_ho@yahoo.com
Citation: Nwankwoala HO, Offor SC (2018) Contamination Assessment of Soil and Groundwater within and around Semicontrolled Solid Waste Dumpsites in Port Harcourt, Nigeria. J Waste Recycl Vol.3 No.2:8
Abstract
This study is aimed at assessing the contamination status of soil and groundwater within and around solid waste dumpsites in Port Harcourt, Nigeria. Standard sampling and analytical procedures were followed. In order to assess heavy metal enrichment and degree of contamination in water and soil, analytical data were subjected to pollution calculation methods. Results of soil analysis from the dumpsites shows that apart from Cadmium which exceeds DRP (1991) recommended limits. All other elements are within these limits. In Aluu dumpsite, the soil is slightly contaminated with Co, moderately contaminated with Pb, severely contaminated with Ni, Cr, and Cu, very severely contaminated with Zn and Mn and slightly polluted with Cd. In Iwofe, the soils are slightly contaminated with Pb and Co, moderately contaminated with Ni and Mn, severely contaminated with Cu, very severely contaminated with Zn and slightly polluted with Cd. Results of PLI shows that all the soils are in good condition. Result of enrichment factor (EF) shows no enrichment for all the analyzed metals except Cd, which shows minor enrichment in all three dumpsites. The dumpsites in Iguruta, Iwofe and Choba in Port-Harcourt are associated with little negative effects on the soils and groundwater in the surroundings. This study also revealed that all the analyzed geochemical parameters including cations, anions and heavy metals
Keywords
Dumpsites; Solid wastes; Contamination; Soil; Leachate; Groundwater
Introduction
Management of solid waste and related environmental impacts presents a challenge to both developing and developed countries. Rapid industrialization, growing population and changing lifestyle are the root causes for increasing rate of solid waste generation [1-3]. Areas near landfills have a greater possibility of groundwater contamination because of the potential pollution source of leachate originating from the nearby site. Such contamination of groundwater resource poses a substantial risk to local resource user and to the natural environment [4]. Ogundiran and Afolabi [5] stated that indiscriminate disposal of industrial and municipal waste can potentially contribute to elevated levels of various heavy metals in the environment and these metals are known to accumulate in soil and have long persistence time through interaction with soil component and consequently enter food chain through plants or animals. Similarly, continuous disposal of these wastes and particularly in unlined surfaces can enhance their mobility at environmentally hazardous levels [6]. Soils that have proximity to dumpsites are likely to have high concentrations of heavy metals which can be easily introduced into the groundwater of that area through infiltration.
Water resources around waste dump sites are prone to toxic pollutions if the waste dump is not properly managed. According to Pohland and Harper [7], leaching of inorganic and organic pollutants from solid waste dump site depend on different factors including pH, temperature, moisture content, type and properties of soil, hydrology of the area and the rate of progress through these stages is dependent on the physical, chemical and microbiological conditions developed within the landfill with time. The depreciating health of the humans around dumpsites, groundwater water contamination and poor agricultural produce are just some of the end result of an improper solid waste management system. Determination of adverse effects of various elements upon human health and the ecosystem has been gaining momentum recently, especially on scientific, social and emotional ground [5].
According to Ehirim and Ebeniro [8], “Port Harcourt and its environs including the study area has unconsolidated moderately porous and permeable sands with lenticular clays and shales at depth of up to 50 m.” Soils that are sandy in texture are very permeable which implies that they would drain quickly. Also, “the depth to the water table in Port Harcourt ranges between 3 to 15 m depending on the time of the year, while a percolation rate of 15 mm/hour has been reported for the Port Harcourt Area” [8]. The above description makes groundwater sources highly susceptible to contamination from poor sited and poorly managed dumpsites. The study areas; Aluu, Iguruta and Iwofe are communities in Port Harcourt where major dumpsites are cited, hence, groundwater sources in these areas must therefore be monitored continually to stall the wide spread of preventable diseases such as cholera, diarrhoea, typhoid, trachoma and others which have already been reported in the Niger Delta [9-11].
Port Harcourt is the nerve center of the Nigeria oil industry and other manufacturing industries. This industrial status of the city has resulted in the emergence of numerous and uncontrolled dumpsites which have been poorly managed. Presently, the Government has established the Rivers State Waste Management Authority (RIWAMA) to carry out a more organized waste management practice in the state. The authority as part of their strategies has designated waste collection points and dump sites throughout the city. This current waste management arrangement in the state has largely enhanced the sanitation of the environment, especially along major roads. But some of these dumpsites are poorly cited, some are full and others are mismanaged.
This study therefore is aimed at evaluating the impacts of solid waste and waste leachate on soil and groundwater quality in parts of Port Harcourt and to evaluate the magnitude of potential contamination in the different media, as well as assess any potential relationship between leachate, soil and groundwater.
Description of Study Area
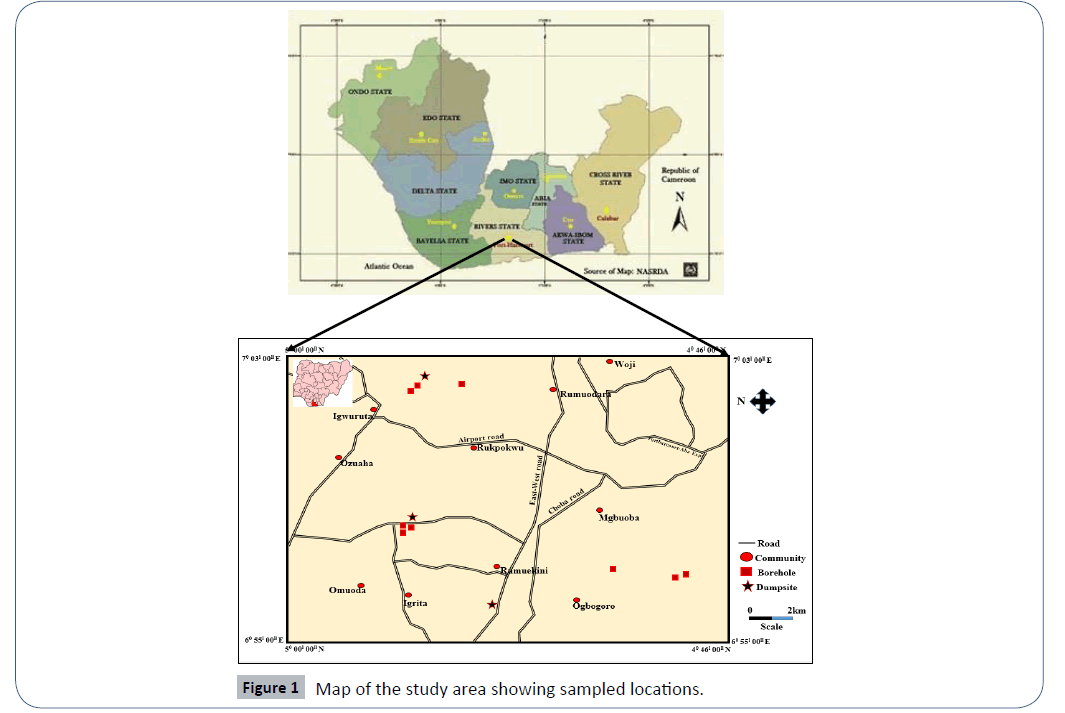
The area of study is located in Port Harcourt, Rivers State. The area is bounded geographically by latitudes 4°46’N to 5°00’N and longitudes 6°55’ E to 7°03’ E (Figure 1). The dumpsites within the study area include; Aluu, Igwuruta and Iwofe dumpsites.
The climate of the area is tropical and has an intimate relationship with its vegetation. There are two air masses which determine the two major seasons namely: (1) dry season and (2) wet season with the “August break” and (3) a short hammattan season. The climatic conditions characteristic of the raining season are associated with the southwest trade winds and those of the dry season are associated with the northeast trade winds. Sunshine hours vary with latitude and season even though the area is also experiencing the global climatic change. The distribution of temperatures depends on solar radiation. Low values of temperatures are recorded between May and September, and high values are recorded between February and May, November, December and January. Vegetation range from the rain forest to Guinea Savanna with its predominant grasses and low density trees [12].
The study area is located on sedimentary basin of the Southern Part of Nigeria with topographic configuration evolving from the sedimentary patterns of the last 75,000 years [13]. It consists of accumulations of cretaceous and tertiary sediments that are influenced by gravitational instability and tectonic forces [13]. The general surface feature of the Area is very unique. The areas fall within the coastal belt on the sedimentary environment that corresponds lithologically to the Agbada, Akata, and Benin formations from earliest to recent of the modern Niger Delta. The soils in the region are mainly sandy-loams, humus, alluvium and outer belt of salt water swamps, clay and mud. The soils are rich in phosphorus because of nutrients from the volcanic parents’ materials. The sands are fine, coarse grained, unconsolidated and granular in texture. The depth to the usable aquifer in the area is approximately 30 m to 45 m, which is penetrated by most burials in the vicinity of the dumpsite [14]. The vegetation found is thick mangrove forest, raffia palms, and light rainforest, due to drainage pattern. It also drains both fresh and salt water.
Methods of Study
The soils, leachates and groundwater samples were collected from Aluu dumpsite, Iwofe dumpsite and Iguruta dumpsite for the study. Three (3) groundwater samples, one (1) soil sample and one (1) leachate sample was collected from each of the three dumpsites. With the exception of leachates and soil samples that were collected at the dumpsite, all other groundwater samples were collected at various distances away from the dumpsite. The locations of all the sampling points were recorded with the aid of a garmin Global Positioning System (GPS).
Groundwater sampling
Groundwater sampling sites were selected at varying distances from the dumpsite where samples were taken. Details of the sampling points are presented in Table 1. Groundwater samples were collected using 1 litre plastic bottles which had been cleaned by soaking in 10% nitric acid and rinsed with distilled water. At the sampling site as well, the bottles were rinsed three times with groundwater to be sampled prior to filling and the bottles were labeled BH1 to BH9 respectively. Groundwater samples for the determination of cations were stabilized by adding few drops of diluted HCl after collection. Samples for heavy metal analysis were collected in new 500ml plastic containers and preserved by acidifying with a few drops of HNO3 acid to achieve a pH ≤ 2. The samples used for anions analysis were preserved in a refrigerator prior to analysis to exclude microbial activity. To maintain the integrity of the water samples, physico-chemical parameters sensitive to environmental changes such as pH and electrical conductivity were measured and recorded in-situ using portable digital meters. A total of nine (9) groundwater samples were collected from Aluu, Iwofe and Igwuruta respectively.
| Sn | Sample location | Sample code | GPS coordinates | Distance (m) and orientation from dumpsite |
|---|---|---|---|---|
| 1 | Iguruta | BH1 | 4° 55’ 83” N 7° 01’ 92” E |
2400 (South) |
| 2 | Iguruta | BH2 | 4° 56’ 27” N 7° 01’ 72” E |
450 (NW) |
| 3 | Iguruta | BH3 | 4° 56’ 40” N 7° 01’ 62” E |
740 (NW) |
| 4 | ALUU | BH4 | 4° 55’ 74” N 6° 57’ 35” E |
486 (NW) |
| 5 | ALUU | BH5 | 4° 55’ 88” N 6° 57’ 31” E |
205 (NW) |
| 6 | ALUU | BH6 | 4° 55’ 72” N 6° 57’ 53” E |
310 (NE) |
| 7 | Iwofe | BH7 | 4° 55’ 83” N 7° 01’ 92” E |
5760 (SE) |
| 8 | Iwofe | BH8 | 4° 55’ 83” N 7° 01’ 92” E |
8760 (SE) |
| 9 | Iwofe | BH9 | 4° 55’ 83” N 7° 01’ 92” E |
9197 (SE) |
Table 1 Water sampling locations and geographic coordinates.
Soil and leachate sampling
Soil samples were collected in sealed polythene bags from the various dumpsites. An auger was used for collecting soil samples at shallow depth of about 30 cm. For each sampling point, 3-5 samples were taken from the same area and mixed thoroughly to form a composite homogenous sample. Sampling tools were washed with water and dried before the next sample was collected. Over 1 kg of soil sample was collected at each sampling site in order to ensure that sufficient fine-grained material would be available for analysis. A total of three (3) soil samples were collected, each from a different dumpsite (Table 2a). Samples were labeled properly including date of collection, location and code number of soil samples.
| Sn | Sample location | Soil sample code | Leachate sample code | GPS coordinates | Distance from dumpsite |
|---|---|---|---|---|---|
| 1 | Iguruta Dumpsite | EN-S1 | EN-L | 4° 56’ 13” N 7° 01’ 92” E |
In-situ |
| 2 | Aluu Dumpsite | AL-S2 | AL-L | 4° 55’ 64” N 6° 57’ 39” E |
In-situ |
| 3 | Iwofe Dumpsite | IW-S3 | IW-L | 4° 53’ 20” N 6° 55’ 52” E |
In-situ |
Table 2a: Soil and leachate sampling locations and geographic coordinates.
Laboratory analytical methods
Atomic Absorption Spectrophotometer (AAS) were used in the determination of heavy metals as described in APHA 3111B and ASTM D3651. This involved direct aspiration of the sample into an air/acetylene or nitrous oxide/acetylene flame generated by a hollow cathode lamp at a specific wavelength peculiar only to the metal programmed for analysis. For every metal investigated, standards and blanks were prepared and used for calibration before samples were aspirated. Concentrations at specific absorbance displayed on the data system monitor for printing. The equipment limit of detection is <0.001 mg/L. Table 2b shows the analytical methods used for analysis.
| Parameter | Type of test | Equipment/Analytical method | Standard |
|---|---|---|---|
| pH | In-situ | Digital pH meter | APHA 4500H*B |
| Temperature | In-situ | Mercury-in-glass thermometer | |
| Conductivity | In-situ | Digital conductivity meter | APHA 2510B |
| Turbidity | Laboratory | HACH2100AN tubidimeter | APHA2130B |
| Calcium, Magnesium, Potassium, Alkalinity | Laboratory | Direct atomic absorption | ASTMD511-93 |
| Sodium, Hardness | Laboratory | Titration method | ASTM512B |
| Total Dissolved Solids | Laboratory | Filtration and evaporation | APHA 2510A |
| Sulphate and Phosphate | Laboratory | Turbidimetric method | ASTMS-516 |
| Chloride | Laboratory | Silver nitrate titration | ASTM512B |
| Nitrate | Laboratory | Brucine method | APHA 4500*E |
| Bicarbonate | Laboratory | Colorimetric method | |
| Heavy metals | Laboratory | Atomic absorption spectrophotometer | APHA 3111B |
Table 2b Equipment and analytical methods used for groundwater samples analysis.
Data analysis
In order to assess heavy metal enrichment and degree of contamination in water and soil, analytical data were subjected to pollution calculation methods as follows:
Contamination factor (CF) and pollution load index (PLI)
The contamination factor was derived by employing the contamination/pollution index as defined by Lacutusu [15]:
 (1)
(1)
Where Cn is the concentration of the metal in sample; Bn is the background concentration (DPR, (1991) standard is used for the background concentration of soil samples).
Contamination factor (CF) values greater than unity (1) defines the pollution range and when lowers than unity the contamination range (Tables 3-5).
| Element | Unit | IGURUTA | ALUU | IWOFE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IG-B1 | IG-B2 | IG-B3 | AL-B1 | AL-B2 | AL-B3 | IW-B1 | IW-B2 | IW-B3 | WHO (2011) |
NIS (2007) |
||
| pH | 4.36 | 5.02 | 4.47 | 4.7 | 5.04 | 4.5 | 4.82 | 4.41 | 4.1 | 6.5-8.5 | 6.5-8.5 | |
| EC | µS/cm | 19.5 | 26.9 | 30 | 13 | 18.8 | 12.3 | 29.6 | 30.2 | 114 | 1000.00 | 1000.00 |
| Salt | g/L | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | ||
| DO | mg/L | 3.36 | 3.44 | 3.49 | 3.49 | 3.13 | 3.25 | 3.73 | 3.58 | 3.84 | 14.00 | |
| TDS | mg/L | 11.7 | 16.2 | 18 | 7.8 | 11.3 | 7.38 | 17.7 | 18.2 | 68.4 | 1000.00 | 500.00 |
| Cl | mg/L | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 | 250.00 | 250.00 |
| NO3 | mg/L | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | 45.00 | 50.00 |
| PO4 | mg/L | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | 10.00 | |
| SO4 | mg/L | 1.51 | 0.85 | 2.3 | 3.9 | 3.4 | 0.14 | 4.27 | 3.52 | 4.65 | 200.00 | 100.00 |
| Mg | mg/L | 0.21 | 0.12 | 0.11 | 0.12 | 0.2 | 0.14 | 0.13 | 0.11 | 0.13 | 50.00 | 0.20 |
| Na | mg/L | <0.07 | <0.07 | <0.07 | <0.07 | <0.07 | <0.07 | 0.21 | 0.11 | 0.87 | 200.00 | 200.00 |
| Ca | mg/L | 1.81 | 0.35 | 0.9 | 0.32 | 0.64 | 0.69 | 0.81 | 1.04 | 0.57 | 75.00 | |
| As | mg/L | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.01 | |
| Cd | mg/L | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | 0.003 | 0.003 |
| Zn | mg/L | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | 5.00 | 3.00 |
| Mn | mg/L | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | 0.10 | 0.20 |
| Cu | mg/L | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.50 | 1.00 |
| Co | mg/L | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | 2.00 | |
| Cr | mg/L | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | 0.05 | |
| Ni | mg/L | <0.0006 | <0.0006 | <0.0006 | <0.006 | <0.006 | <0.006 | <0.006 | <0.006 | <0.006 | 0.07 | 0.02 |
| Pb | mg/L | <0.06 | <0.06 | <0.06 | >0.06 | >0.06 | >0.06 | >0.06 | >0.06 | >0.06 | 0.004 | 0.01 |
| TCC | cfu/100 ml | <0.008 | <0.008 | <0.008 | <0.008 | <0.008 | <0.008 | <0.008 | <0.08 | <0.08 | 10.00 | |
Table 3 Summary results of physical/chemical parameters and heavy metals composition of around dumpsites.
| Symbol | Unit | IGURUTA | ALUU | IWOFE |
|---|---|---|---|---|
| pH | 4.62 | 4.75 | 4.44 | |
| EC | µS/cm | 25.47 | 14.70 | 57.93 |
| Salt | g/L | 0.09 | 0.09 | 0.09 |
| DO | mg/L | 3.43 | 3.29 | 3.72 |
| TDS | mg/L | 15.30 | 8.83 | 34.77 |
| Cl | mg/L | 0.90 | 0.90 | 0.90 |
| NO3 | mg/L | 0.01 | 0.01 | 0.01 |
| PO4 | mg/L | 0.01 | 0.01 | 0.01 |
| SO4 | mg/L | 1.55 | 2.48 | 4.15 |
| Mg | mg/L | 0.15 | 0.15 | 0.12 |
| Na | mg/L | 0.06 | 0.06 | 0.40 |
| Ca | mg/L | 1.02 | 0.55 | 0.81 |
Table 4 Mean concentration of physical/chemical parameters of groundwater around dumpsites.
Symbol |
Unit | IGURUTA | ALUU | IWOFE |
|---|---|---|---|---|
| pH | 7.43 | 7.28 | 5.56 | |
| EC | µS/cm | 194 | 889 | 15700 |
| Salt | g/L | 0.08 | 0.45 | 9.2 |
| DO | mg/L | 2.14 | 2.32 | 2.67 |
| TDS | mg/L | 117 | 533 | 10.9 |
| Cl | mg/L | 92.4 | 145 | 1408 |
| NO3 | mg/L | 8.45 | 1.08 | 0.04 |
| PO4 | mg/L | 9.93 | 1.13 | <0.02 |
| SO4 | mg/L | 4.8 | 3.11 | 764 |
| Mg | mg/L | 6.43 | 3.75 | 103 |
| Na | mg/L | 54.2 | 82.4 | 678 |
| Ca | mg/L | 0.8 | 19.1 | 3464 |
| As | mg/L | <0.0001 | 0.001 | 0.0152 |
| Cd | mg/L | <0.002 | <0.002 | <0.002 |
| Zn | mg/L | 0.09 | <0.02 | 0.87 |
| Ba | mg/L | <0.03 | <0.03 | <0.03 |
| Mn | mg/L | <0.05 | 2.19 | 11.7 |
| Cu | mg/L | <0.02 | <0.02 | 0.17 |
| Co | mg/L | <0.03 | <0.03 | <0.03 |
| Cr | mg/L | <0.006 | <0.006 | <0.006 |
| Ni | mg/L | >0.06 | >0.06 | >0.06 |
| Pb | mg/L | <0.008 | <0.008 | <0.008 |
| TCC | cfu/100 ml | 75 | 4 | 0 |
Table 5 Summary results of physical/chemical parameters and heavy metals composition of leachate from dumpsites.
In order to give further assessment on the degree of contamination, attempts were made to calculate the PLI using Tomlinson et al. [16]. The PLI is obtained using eqns. (1) and (2);
 (2)
(2)
Where
CF: Contamination Factor; and n: Number of Metals.
Enrichment factor (EF)
Enrichment Factor was used to assess the relative contributions of natural and anthropogenic heavy metal inputs in the various dumpsites. Based on Rubio et al. [17] EF is defined as:
 (3)
(3)
Where,
[X/Zn]sample is the ratio of heavy metal
[X] to Zn in the samples, and
[X/Zn]background/control is the natural background/control value of the metal-Fe ratio.
Result Interpretation
Leachate
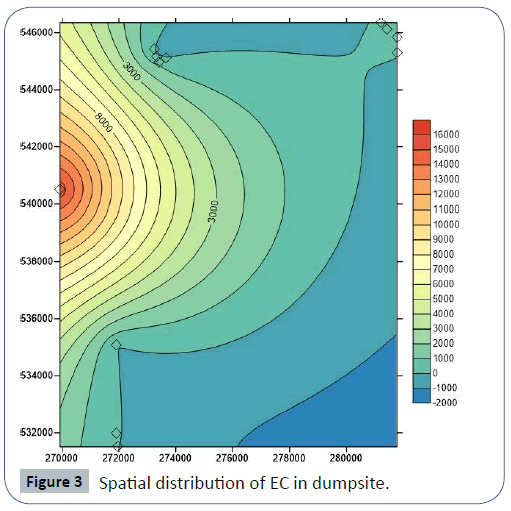
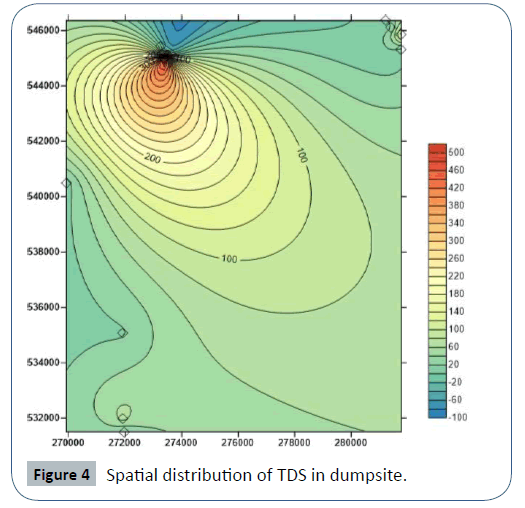
The pH value of leachates samples from Iguruta and Aluu dumpsites shows basic composition of 7.43 and 7.28 and acidic composition in Iwofe dumpsite with a value of 5.56 (Table 4). The concentrations of EC, Salinity, DO and TDS in Iguruta, Aluu and Iwofe dumpsites are 194, 889 and 15700 μS/cm; 0.08, 0.45 and 9.2 mg/L; 2.14, 2.32 and 2.67 mg/L; 117, 533 and 10.9 mg/L respectively (Table 5). Electrical conductivity is highest in Iwofe dumpsite and TDS is highest in Aluu dumpsite.
The concentrations of major anions in Iguruta, Aluu and Iwofe dumpsites including Cl, NO3, PO4 and SO4 are 92.4, 145 and 1408 mg/L; 8.45, 1.08 and 0.04 mg/L; 9.93, 1.13 and <0.02 mg/L; 4.8, 3.11 and 764 mg/L respectively (Table 3). The concentration of Mg, Na and Ca in Iguruta, Aluu and Iwofe are 6.43, 3.75 and 103 mg/L; 54.2, 82.4 and 678 mg/L; 0.8, 19.1 and 3464 mg/L respectively. These results show that cationic and anionic concentrations are highest in Iwofe dumpsite. Barium content is <0.03 mg/L in all the samples.
Heavy metals concentration in Iguruta, Aluu and Iwofe including Cd, Co, Cr, Ni and Pb are <0.002, <0.03, <0.03, <0.006, <0.06 and <0.008 mg/L respectively. Arsenic is <0.0001 mg/L in Iguruta, 0.0001 in Aluu and 0.015 in Iwofe dumpsite. Zinc and Mn are 0.09 and <0.05 mg/L in Iguruta, <0.02 and 2.19 mg/L in Aluu and 0.87 and 11.7 mg/L in Iwofe dumpsite. Copper is <0.02 mg/L in Iguruta and Aluu dumpsites and 0.17 mg/L in Iwofe dumpsite (Tables 6 and 7). Total coliform count is 75 cfu/100ml in Iguruta, 4 cfu/100ml in Aluu and absent in leachate sample from Iwofe dumpsite.
Groundwater
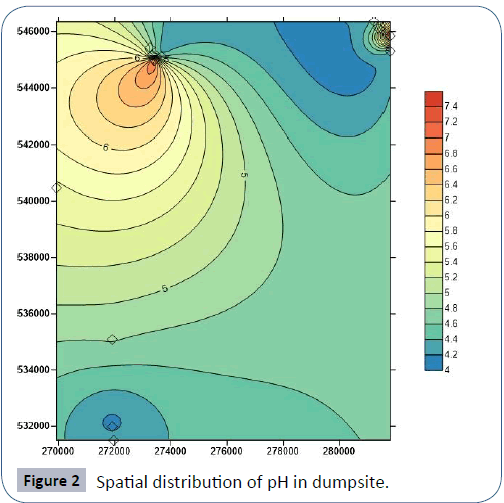
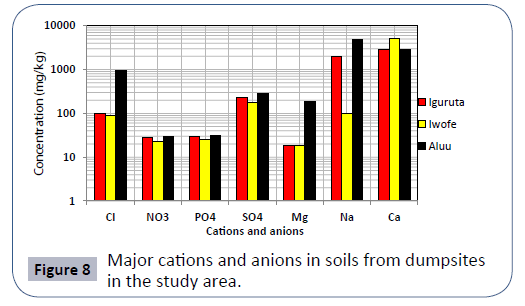
In Iguruta, Aluu and Iwofe, groundwater pH ranges from 4.36- 5.02, 4.5-5.02 and 4.1-4.82 with mean values of 4.62, 4.75 and 4.44 respectively (Table 6). Electrical conductivity ranges from 19.5-30, 12.3-18.8 and 29.6-114 μS/cm with mean values of 25.47, 14.70 and 57.93 μS/cm. Salinity and chloride values are <0.10 and <1.0 mg/L in all the groundwater samples respectively. Dissolved oxygen ranges from 3.36 to 3.49 mg/L in Iguruta, 3.13-3.49 mg/L in Aluu and 3.58-3.84 mg/L in Iwofe with mean values of 3.43, 3.29 and 3.72 mg/L (Table 6). The ranges and mean values of TDS in Iguruta, Aluu and Iwofe are 11.7-18, 7.38-11.3 and 17.7-68.4 mg/L with mean values of 15.3, 8.83 and 34.77 mg/L respectively. Nitrate and phosphate are very low and have concentrations <0.02 mg/L in all the samples. Sulphate and magnesium ranges from 0.85-2.3 and 0.11- 0.21 mg/L in Iguruta, 0.14-3.9 and 0.12- 0.2 mg/L in Aluu and 3.52-4.65 mg/L in Iwofe (Table 7). Sodium is <0.07 in Iguruta and Aluu but ranges from 0.11-0.87 mg/L in Iwofe. Calcium ranges from 0.35-1.81 in Iguruta, 0.32-0.69 in Aluu and 0.57-1.04 in Iwofe with mean values of 1.02, 0.55 and 0.81 mg/L respectively. Barium content is <0.03 in all the samples. The concentration of metals in the groundwater samples including As, Cd, Zn, Mn, Cu, Co, Cr, Ni and Pb are <0.0001, <0.0002, <0.02, <0.05, <0.02, <0.03, <0.006, <0.06 and <0.008 mg/L respectively (Table 7). Total coliform count is 1 cfu/100ml in samples from Iguruta and absent in samples from Aluu and Iwofe. Figure 2 shows the major cations and anions in the groundwater system.
| Element | IGURUTA | ALUU | IWOFE |
|---|---|---|---|
| Cd | 1.25 (Slight pollution) | 1.25 (Slight pollution) | 3 (Slight pollution) |
| Zn | 0.67 (Severe contamination) | 0.94 (Very severe contamination) | 0.77 (Very severe contamination) |
| Mn | 0.12 (Severe contamination) | 0.80 (Very severe contamination) | 0.42 (Moderate contamination) |
| Cu | 0.42 (Moderate contamination) | 0.73 (Severe contamination) | 0.57 (Severe contamination) |
| Co | 0.1 (Slight contamination) | 0.23 (Slight contamination) | 0.21 (Slight contamination) |
| Cr | 0.12 (Slight contamination) | 0.64 (Severe contamination) | 0.73 (Severe contamination) |
| Ni | 0.54 (Severe contamination) | 0.67 (Severe contamination) | 0.49 (Moderate contamination) |
| Pb | 0.31 (Moderate contamination) | 0.33 (Moderate contamination) | 0.17 (Slight contamination) |
| Pollution load index | 0.31 (Perfect) | 0.62 (Perfect) | 0.54 (Perfect) |
Table 6 Contamination factor (CF) and PLI of heavy metals in soils from dumpsites.
Element |
IGURUTA | ALUU | IWOFE |
|---|---|---|---|
| Cd | 1.87 (Minor enrichment) | 1.34 (Minor enrichment) | 3.89 (Minor enrichment) |
| Zn (Normalizer) | 1.00 | 1.00 | 1.00 |
| Mn | 0.19 (No enrichment) | 0.85 (No enrichment) | 0.55 (No enrichment) |
| Cu | 0.63 (No enrichment) | 0.78 (No enrichment) | 0.73 (No enrichment) |
| Co | 0.15 (No enrichment) | 0.24 (No enrichment) | 0.27 (No enrichment) |
| Cr | 0.17 (No enrichment) | 0.69 (No enrichment) | 0.94 (No enrichment) |
| Ni | 0.81 (No enrichment) | 0.71 (No enrichment) | 0.63 (No enrichment) |
| Pb | 0.46 (No enrichment) | 0.35 (No enrichment) | 0.22 (No enrichment) |
Table 7 Enrichment factors (EF) for heavy metals in soils from dumpsites.
Soil
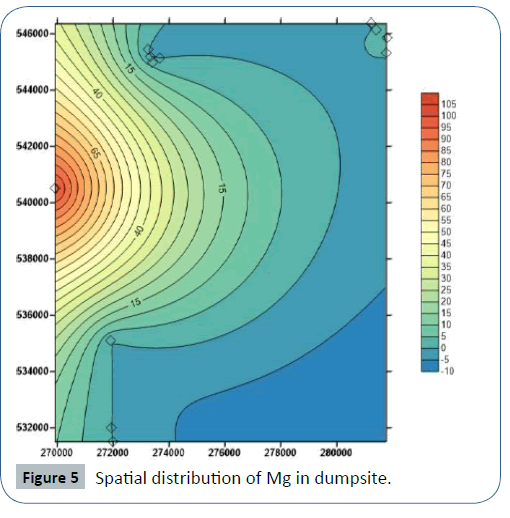
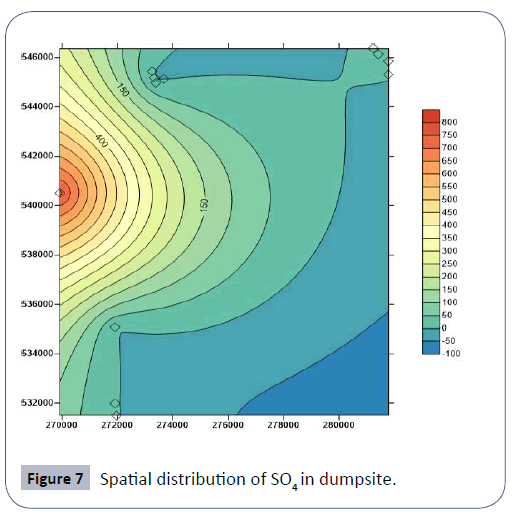
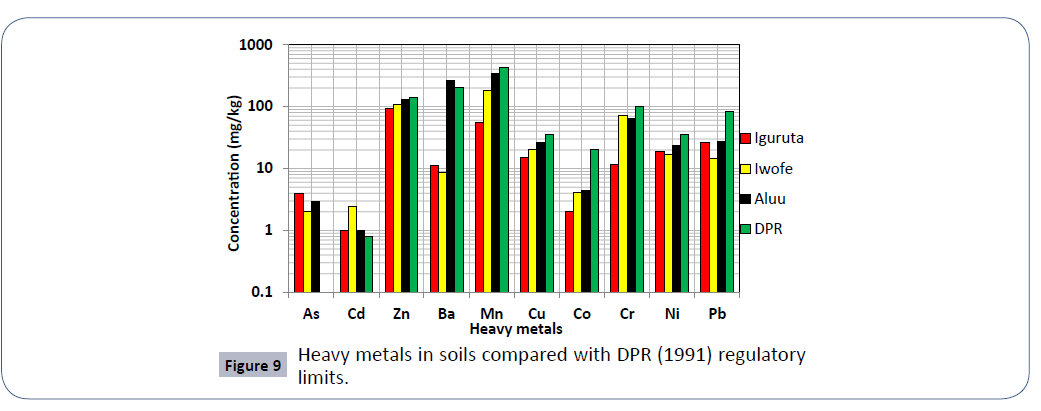
Results of soil analysis from the dumpsites shows that apart from Cadmium which exceeds DRP (1991) recommended limits, all other element are within these limits. The higher concentration of metals in dumpsite soils than in leachate and groundwater is due to the clayey nature of the soils which acts as a receptor but prevents seepage of these metals to the subsurface groundwater Figures 3-8.
Soil pollution indices shows that Cadmium is the only source of pollution with minor enrichment in the soils from the dumpsites. In all three dumpsites, the dumpsite in Iwofe has the highest concentration of cations, anions and heavy metals in leachates while Igwuruta dumpsite has the least concentrations. The concentration of metals in leachate and groundwater are similar in all the dumpsites with no observable trend. This trend suggests that there is no correlation existing between the direction of groundwater flow and the concentration of metals in groundwater.
The Contamination Factor (CF), Pollution load Index (PLI) and Enrichment factor (EF) were used to determine the heavy metal contamination in soils of the area of study. The combined use of these different approaches for evaluating soil metal contamination facilitates a comprehensive interpretation of the soils characteristics in terms of the background influences (Tables 6 and 7).
Results of CF shows that the soil in Iguruta dumpsite is slightly contaminated with Cr and Co, moderately contaminated with Pb and Cu, severely contaminated with Ni, Mn and Zn and slightly polluted with Cd (Table 7). In Aluu dumpsite, the soil is slightly contaminated with Co, moderately contaminated with Pb, severely contaminated with Ni, Cr, and Cu, very severely contaminated with Zn and Mn and slightly polluted with Cd. In Iwofe, the soils are slightly contaminated with Pb and Co, moderately contaminated with Ni and Mn, severely contaminated with Cu, very severely contaminated with Zn and slightly polluted with Cd. Results of PLI shows that all the soils are in good condition. Result of EF shows no enrichment for all the analyzed metals except Cd, which shows minor enrichment in all three dumpsites (Figure 9). Figure 10 shows the classification of groundwater and leachate samples in the study area based on the plot of metal load and pH [18].
Figure 10: Classification of groundwater and leachate samples in the study area based on Figure 10 the plot of metal load and pH (after Edet et al. [18]).
Conclusion
This study shows that all the analyzed geochemical parameters including cations, anions and heavy metals in groundwater sources in the vicinity of three major dumpsite situated in Aluu, Igwuruta and Iwofe are low and within WHO (2011) and NIS (2007) recommended limits for potable drinking water.
Results of soil analysis from the dumpsites shows that apart from Cadmium which exceeds DRP (1991) recommended limits, all other element are within these limits. The higher concentration of metals in dumpsite soils than in leachate and groundwater is due to the clayey nature of the soils which acts as a receptor but prevents seepage of these metals to the subsurface groundwater (Figure 10).
Soil pollution indices shows that Cadmium is the only source of pollution with minor enrichment in the soils from the dumpsites. In all three dumpsites, the dumpsite in Iwofe has the highest concentration of cations, anions and heavy metals in leachates while Igwuruta dumpsite has the least concentrations. The concentration of metals in leachate and groundwater are similar in all the dumpsites with no observable trend. This trend suggests that there is no correlation existing between the direction of groundwater flow and the concentration of metals in groundwater.
It is obvious from this study that dumpsites in Iguruta, Iwofe and Choba in Port-Harcourt are associated with little negative effects on the soils and groundwater in the surroundings. The dumpsite soils are polluted with only cadmium while the groundwater sources are all acidic in composition. This study therefore suggested the need for treatment of the groundwater sources in these areas with lime that can neutralize the amount of acid in the
water. Also, regular assessment of soil and groundwater quality should be carried out in these areas surrounding dumpsites so as to ensure that there are within regulatory guidelines. This is because the composition of these media changes over time.
Acknowledgement
Authors thank immensely the editors and gratefully acknowledge the reviewers whose editorial and substantive/thoughtful comments, contributions and recommendations improved the quality and scientific objectivity of this paper.
References
- Amadi AN, Olasehinde PI, Okosun EA, Okoye NO, Okunlola IA, et al. (2012) A Comparative Study on the Impact of Avu and Ihie Dumpsites on Soil Quality in Southeastern Nigeria. Am J Chem 2: 17-23.
- Amadi AN, Nwankwoala HO (2013) Evaluation of Heavy Metal in Soils from Enyimba Dumpsites in Aba, Southeastern Nigeria Using Contamination Factor and Geo-Accumulation Index. Energ Environ Res 3: 125-134.
- Kamble SB (2016) Characterization of Leachate and its Effects on Ground Water Quality around Jawaharnagar Municipal Open Dumpsite, Rangareddy, Telangana. Curr World Environ 11: 114-125.
- Smith SE (2012) What is groundwater pollution. Wise GEEK, Wallace O (ed.) Last modified date: 7 March, 2012.
- Ogundiran OO, Afolabi TA (2008) Assessment of the physicochemical parameters and heavy metals toxicity of leachates from municipal solid waste open dumpsite. Int J Environ Sci Tech 5: 243-250.
- Uba S, Uzairu A, Harrison GFS, Balarabe ML, Okunola OJ (2008) Assessment of heavy metals bioavailability in dumpsites of Zaria metropolis, Nigeria. Afr J Biotechnol 7: 122-130.
- Pohland FG, Harper SR (1985) Critical Review and Summary of Leachate and Gas Production from landfills, US Environmental Protection Agency, Cincinnati; EPA/600/2-86/073.
- Ehirim CN, Ebeniro JO (2013) 2-D Electrical Resistivity Monitoring of a Municipal Solid waste Dumpsite in Port Harcourt, Nigeria. IOSR J Environ Sci Toxicol Food Technol 2: 29-34.
- Oladeji OS (2002) Preliminary Assessment of the Environmental impacts of land use Patterns on Ground Water Quality. Glob J Environ Sci 1: 35-42.
- Erah PO, Akujieze CN, Oteze GE (2002) The Quality of Groundwater in Benin City: A baseline study on inorganic chemicals and microbial contaminant of health importance in borehole and open wells. Trop J Pharm Res 1: 75-82.
- Nwidu LL, Ebong OO, Wariso BA (2006) The health implication of underground Water used for domestic consumption in oil Producing area, Port Harcourt City, Nigeria. J Med Pharm Sci 2: 39-45.
- Nwajide CS (2013) Geology of Nigeria’s Sedimentary Basins. C.S.S Bookshops 347-518.
- Short KC, Stauble AJ (1967) Outline of Geology of Niger Delta. AAPG Bull 51: 761-779.
- Ehirim CN, Ebeniro JO, Olanegan OP (2009) A geophysical investigation of Solid Waste Landfill Using 2-D Resistivity Imaging and Vertical Electrical Sounding Methods in Port Harcourt Municipality, Rivers State, Nigeria, Pac J Sci Technol 10: 12-22.
- Lacutusu R (2000) Appraising levels of soil contamination and pollution with heavy metals. In: Heinike HJ, Eckselman W, Thomasson AJ, Jones RJA, Montanarella L, et al. (eds.) Land information systems for planning the sustainable use of land resources. European Soil Bureau Research Report No. 4. Office of Official Publication of the European Communities, Luxembourg 393-402.
- Tomlison DC, Wilson DJ, Harris CR, Jeffrey DW (1980) Problem in heavy metals in estuaries and the formation of pollution index. Helgol Wiss Meeresunlter 33: 566-575.
- Rubio B, Nombela MA, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain). Mar Pollut Bull40: 968-980.
- Edet AE, Merkel BJ, Offion OE (2004) Contamination Risk Assessment of Fresh Groundwater Using the Distribution and Chemical Speciation of Some Potentially Toxic Elements in Calabar (Southern Nigeria). Environ Geology 45: 1025-1035.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences