ISSN : 2393-8854
Global Journal of Research and Review
Comparative Morphological Study of Leptospira interrogans Serovar Ranarum and Shermani by Scanning and Transmission Electron Microscopy
Krittika Keeratikunakorn1, Duangjai Suwancharoen2, Kleophant Thakerngpol3, Paisal Parichatikanond3, Duangporn Phulsuksombati4, Kasem Kerdpuech1, Chaiyapruk Pilakasiri4 , Narakorn Senglek1 and Kajee Pilakasiri1*
1 Department of Anatomy, Faculty of Medicine,Siriraj Hospital, Mahidol University, Bangkok, Thailand
2 Department of Livestock Development, Leptospirosis Center, National Institute Animal of Health, Bangkok, Thailand
3 Department of Pathology, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand
4 Armed Forces Research Institute of Medical Science, Bangkok, Thailand
- *Corresponding Author:
- Kajee Pilakasiri
Department of Anatomy,
Faculty of Medicine,
Siriraj Hospital,
Mahidol University,
Bangkok,
Thailand,
Email: kpilakasiri5@yahoo.com
Received Date: July 21, 2020; Accepted Date: August 07, 2020; Published Date: August 14, 2020
Citation: Keeratikunakorn K, Suwancharoen D, Thakerngpol K, Parichatikanond P, Phulsuksombati D, Kerdpuech K, Pilakasiri C, Senglek N, Pilakasiri K (2020) Comparative Morphological Study of Leptospira Interrogans Serovar Ranarum and Shermani by Scanning and Transmission Electron Microscopy. Glob J Res Rev Vol.7 No.2:51.DOI: 10.36648/2393-8854.7.1.52
Copyright: © 2020 Keeratikunakorn K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Leptospirosis is an important zoonotic disease caused by the spirochete bacteria of the genus Leptospira. Leptospirainterrogansis pathogenic to both humans and animals. The bacteria are classified into more than 200 serovars by their antigen-antibody reaction in an agglutination test. There is currently no standard procedure to classify leptospiretypes morphologically. Therefore, this research sought to study the types of leptospires through the differences in their morphologies revealed by scanning and transmission electron microscopes.This research investigated the morphological differences between 2 leptospires using scanning and transmission electron microscopy. LeptospirainterrogansserovarRanarum and Shermani were chosen for the comparative study. There were significant differences in all 4 parameters (length, width, wavelength, and amplitude of the spiral body) of the 2 serovars that were measured. At the same time, the transmission electron microscopic study of the 2 serovars revealed 3 major components of the body: an enveloping membrane, 2 axial filaments, and a protoplasmic cylinder, with some detailed differences. The enveloping membrane of the Ranarum had an irregular, serrated shape and was loosely attached to the protoplasmic cylinder, whereas that of the Shermani was smooth and intimately attached to the protoplasmic cylinder. In addition, the protoplasmic cylinder of the Ranarum showed loosely formed components, providing a lower electron density than that of the Shermani.These morphological differences might be used as an alternative means of identifying the leptospiral type in conjunction with an agglutination test, until such time as further, more definitive studies on leptospires are available.
Keywords
Leptospirainterrogans; Serovar; Electron microscope
Introduction
Leptospirosis, a major zoonotic disease in both humans and animals, is caused by pathogenic Leptospirainterrogans [1]. Leptospires usually enter the body through wounds and the mucous membrane of the conjunctiva, oral cavity, and genital surfaces. Humans can be infected by either direct or indirect contact with infected urine [2]. Typically, direct transmission occurs through contact with the urine of infected animals, while indirect contact results from exposure to water or soil that has been contaminated with urine infected with leptospires [3]. Since Leptospiraspp can survive in water and in a suitable environment, the majority of infections occur through indirect contact [4-5].
Leptospires are bacteria in the phylum Spirochaetes, class Spirochaetes, order Spirochaetales, family Leptospiraceae, and genus Leptospiraspp. Leptospira spp. is divided by its antigenantibody reaction in an agglutination test into 2 species, Leptospirainterrogans and Leptospirabiflexa, which are pathogenic and non-pathogenic strains, respectively. The nonpathogenic, saprophytic strain is isolated from the environment [6]. Each species is also subdivided into serovars by the agglutination test: Leptospirabiflexainto more than 60 serovars, and Leptospirainterrogans into more than 200. Furthermore, each serovar is grouped into serogroups, with a total of 24 serogroups [6-7]. The agglutination test is normally used to identify the type of serovar for an epidemiological study whenever an outbreak of leptospirosis occurs [6-7]. In addition, each serovar is associated with different animal reservoirs [8].
The major antibodies detected in a serological survey of the livestock in Thailand [9] were LeptospirainterogansserovarsRanarum, Sejroe, and Mini, all of which needed cattle as their specific host. Sheep and goats are the hosts for the LeptospirainterogansserovarsShermani and Ranarum; however, pigs are also the host for Ranarum, Pomona, and Bratislava [9]. In 2007, there was a disease outbreak in the south of Thailand at Satun Province, the cause of which was Ranarum, which corresponded with the serovar detected in cattle and dogs in the area [10].
Concerning the detailed morphology of the leptospiral bodies, some studies have demonstrated the external and internal structural appearances using different approaches. Leptospires are tightly coiled spirochetes with pointed hooks at one or both ends [11]. Leptospires cannot be studied using either Gram or Wright staining for visualization witha light microscope; they can only be visually displayed by silver staining and examined under a dark-field microscope [12]. In 1942, Morton and Anderson [11] studied the morphology of L. icterohemorrhagiaeand L. canicolaas revealed by an electron microscope. They reported that the body length varied from 4-10 μm and the width from 0.07-0.14 μm, that the height of the coil was 0.25 μm, and that the distance from one pitch to the adjacent one ranged from 0.3-0.6 μm [11].
Subsequently, ultrastructural research using transmission electron microscopy was conducted. Leptospires consist of 3 main elements, namely, the enveloping membrane (the outer sheath), 2 axial filaments (the periplasmic flagella), and the protoplasmic cylinder. The enveloping membrane forms the most external layer and has a trilaminar [13-17]. The axial filaments are located between the enveloping membrane and the protoplasmic cylinder, and each axial filament intertwines around a spiral-shape protoplasmic cylinder [17-20]. In addition, a study of L.pomonaby an electron microscope showed that the ribosome is located in the protoplasm of the protoplasmic cylinder [14] and that the lucid region inside the protoplasmic cylinder contains the nucleus [13-14].
Regarding the classification of leptospires, their serovars are usually identified by the agglutination test, which involves the antigen-antibody reaction. To date, no data on the morphological differences between serovars have been reported. Therefore, this work aimed to study the morphology of both the external and internal structures of serovars in detail, using scanning and transmission electron microscopes. Two serovars of L. interroganswere chosen for the comparative study:the Ranarum and Shermani, being representative of the Ranarum and Shermaniserogroups, respectively. The morphological data so obtained may provide a deeper understanding of the relationship between the morphological and serological approaches. In addition, any identified morphological differences between these 2 serovars from different sero-groups might be able to be used as an alternative way to identify leptospire type in parallel with the agglutination test.
Material and Methods
Microorganisms
The leptospires, LeptospirainterrogansserovarRanarum and Shermani, were supplied by the Leptospirosis Center, National Institute of Animal Health, Department of Livestock Development, Ministry of Agriculture and Cooperatives, Thailand. The reference stock of the leptospires of the 2 serovars were cultured in Ellinghausen-McCullough-Johnson-Harris (EMJH) semisolid culture media and kept at 30°C. The presence of leptospires in the culture media was checked under a dark field microscope every week for 12-16 weeks. They were then sub-cultured in EMJH media and purified by passing through a 0.2 μm filter. Each one millimeter of the EMJH media, containing each selected serovar at a density of 108 cells per millimeter, was put in an Eppendorf tube and carried from the Leptospirosis Center to the laboratory of the Department of Anatomy, Faculty of Medicine, Siriraj Hospital, Mahidol University. Specimens were washed by centrifugation at 3,000 rpm for 15 minutes with a 0.1 M cacodylate buffer, pH 7.2, 3 times. They were subsequently fixed in 2.5% glutaradehyde in a 0.1 M cacodylate buffer, pH 7.2, for 24 hours. Finally, thespecimens were dividedequally in preparation for scanning and transmission electron microscopic processing.
Scanning electron microscopy
After fixation, the specimens were washed with a 0.1 M cacodylate buffer, pH 7.2, by centrifugation at 3,000 rpm for 15 minutes each. After that, 1% osmium tetroxide was dropped into the Eppendorf tube containing the samples and put aside for one hour. They were then washed with the same buffer, using the same procedure, 3 times before a dehydrating process using a graded series of ethanol. The supernatant of each sample was discarded, and the precipitation at the bottom of the Eppendorf tube was dropped and smeared evenly on a glass slide. The smeared slide, which was cut to almost the same size as that of the stub surface, was fixed on the stub. The stubs containing the samples were coated with gold particles for 3 minutes before being examined under the scanning electron microscope (JEOL JSM-6510LV). Scanning electron micrographs of all the organisms needed for the study were taken.
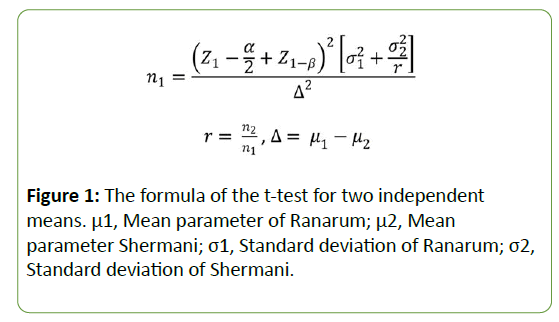
A statistical calculation determined that a sample size of 40 organisms each of L. interrogansserovar Ranarum and Shermani were needed for study purposes. These 2 organism sample sizes were obtained from a pilot study using n=5, and using the formula of the t-test for two independent means; the equation is presented at (Figure 1) [21].
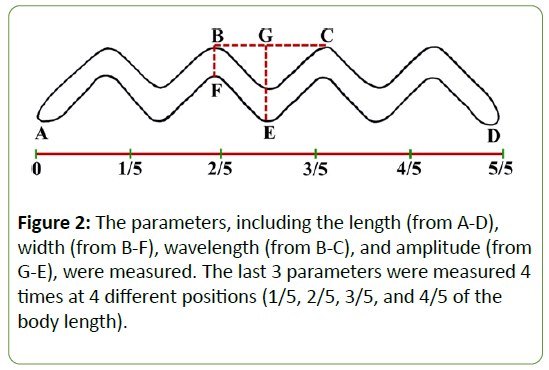
The scanning electron micrographs of each serovar were studied and 4 parameters-the length, width, wavelength, and amplitude of the leptospire body-were measured (Figure 2). The width, wavelength, and amplitude were measured at 4 different positions, namely, at the 1/5, 2/5, 3/5, and 4/5 parts of the body length. PASW Statistics for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis of the significant differences between the 2 serovars. In the case of normally distributed data, the unpaired (2-sample) t-test was chosen, while the Mann–Whitney U (Wilcoxon’s rank sum) test was used for non-normally distributed data. A p-value <0.05 was considered as statistically significant [22].
Transmission electron microscopy
After fixation, the specimens were washed, and 1% osmium tetroxide was added in the same manner as for scanning electron microscopy. They were further processed for conventional transmission electron processing, as follows. Firstly, they were washed with a 0.1 M cacodylate buffer, pH 7.2, 3 times, followed by distilled water. The samples were stained with 2% uranyl acetate for 30 minutes and then washed with distilled water, followed by a graded series of ethanol. After that, propylene oxide was added to the sample and put aside for 5 minutes. They were centrifuged twice, and the propylene oxide was discarded. Next, a mixture of propylene oxide and araldite resin, in the ratio of 2:1, was added to the samples for an infiltrating process for 1 hour, followed by a mixture of propylene oxide and araldite resin in the ratio of 1:2. They were left in the Eppendorf tube overnight at room temperature, after which the tube was opened for 6 hours to allow the propylene oxide to completely evaporate and the resin to set. Next, the sediment samples were embedded in the araldite resin in an embedding capsule. The resin blocks containing the samples were left to polymerize at 70̊ C overnight.
Ultra-thin sections (about 60-90 nm thick) were cut with an ultramicrotome (Leica EM UC6) using glass knives. The ultra-thin sections were placed on copper grids and double stained with uranyl acetate and lead citrate. The specimens in the grid were examined using a transmission electron microscope (JEOL 1230), and transmission electron micrographs were taken.
Results
Scanning electron microscopy
Both the Ranarum and Shermani were spiral-shaped, with a hook-like appearance at each end. The Ranarum displayed a longer body length and looser spiral coils than those of the Shermani. The results of a statistical analysis of the significant differences of the 4 parameters (the length, width, wavelength, and amplitude) of L. interrogansserovarRanarum and Shermani are at Table 1. The parameters were obtained by measuring pictures of the organisms on scanning electron micrographs; significant differences between all parameters of the Ranarum and Shermani were demonstrated.
| Parameters (µm) | Mean ± SD, Median (Min, Max) | |
|---|---|---|
| L. interrogansserovar Ranarum | L. interrogansserovar Shermani | |
| Length of organism | 13.09 ± 2.66* | 11.26 ± 3.57* |
| Width of organism | 0.14 (0.12, 0.23)* | 0.16 (0.12, 0.27)* |
| Wavelength | 0.54 ± 0.05† | 0.44 ± 0.05† |
| Amplitude | 0.30 ± 0.05† | 0.34 ± 0.05† |
n=40; *Significant differences within the same parameter at p-value <0.05; †Significant differences within the same parameter at p-value <0.001
Table 1: The statistical differences of 4 parameters of L. interrogansserovar Ranarum and Shermani measured under the scanning electron microscope.
The length and wavelength of the Ranarum were significantly longer than those of the Shermani, with p-values of 0.006 and 0.001, respectively, while the width and amplitude of the former were significantly smaller than those of the latter (the p-values were 0.003 and 0.001, respectively).
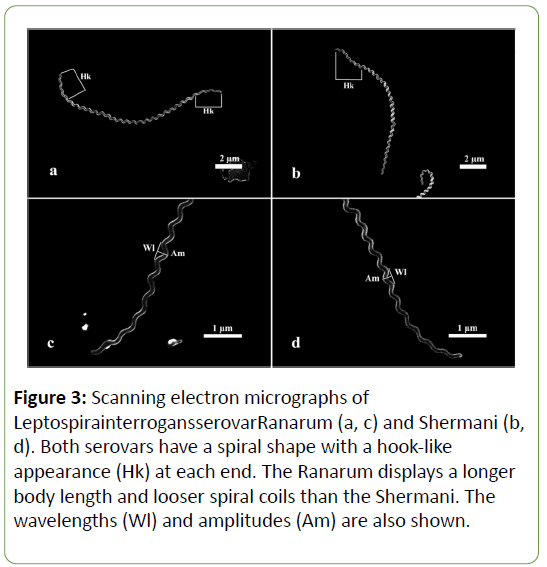
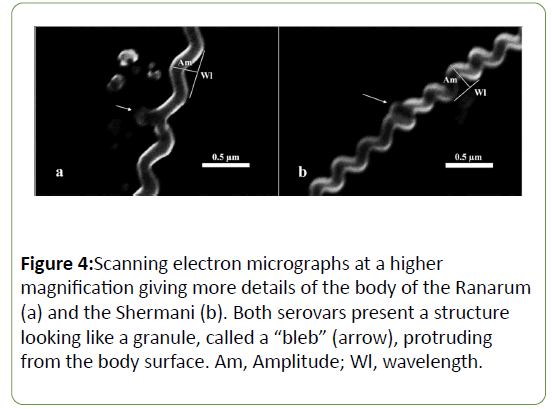
A study of the scanning electron micrograph (Figure 3) of each serovar revealed that they were long, spiral-shaped bacteria with a hook-like appearance at each end. However, there were some morphological differences. The Ranarum (Figures 3a and c) displayed a visually longer body length and obviously looser spiral coils than those of the Shermani (Figures 3b and d). Although the statistical analysis showed significant dissimilarities in the other 3 parameters, differences in the visual appearances of those aspects were not apparent. Furthermore, the pictures of the serovars taken by the scanning electron microscope revealed structures which looked like granules, or “ blebs ” , protruding from the body surface (Figure 4). Blebs of different sizes were found in both serovars. No information on the differences in the blebs of each serovar was revealed using the scanning electron microscope (SEM) technique.
Figure 3: Scanning electron micrographs of LeptospirainterrogansserovarRanarum (a, c) and Shermani (b,d). Both serovars have a spiral shape with a hook-like appearance (Hk) at each end. The Ranarum displays a longer body length and looser spiral coils than the Shermani. The wavelengths (Wl) and amplitudes (Am) are also shown.
Transmission electron microscopy
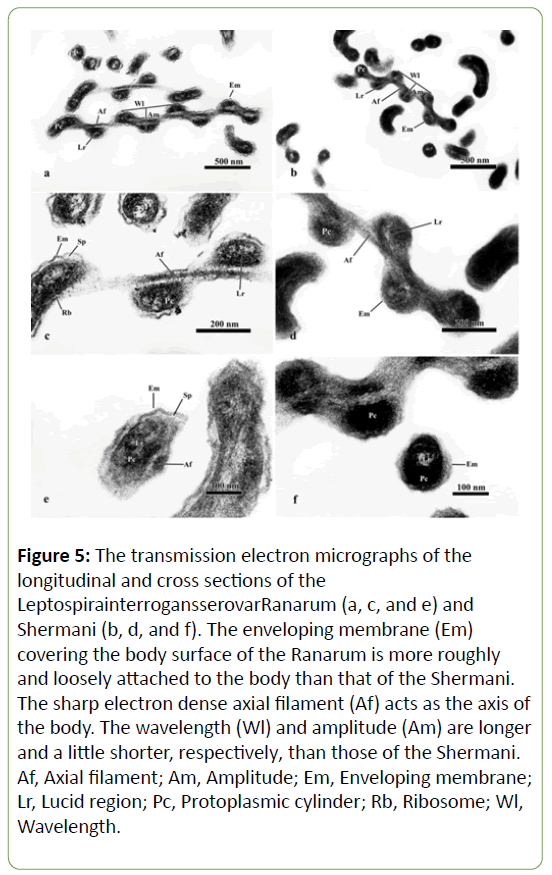
A transmission electron microscope study of the ultrastructure of L. interrogansserovarRanarum (Figures 5a, c, and e) and Shermani (Figures 5b, d, and f) revealed that they were composed of the same 3 main structures-an enveloping membrane, 2 axial filaments and a protoplasmic cylinder-with few differences.
Figure 5: The transmission electron micrographs of the longitudinal and cross sections of the LeptospirainterrogansserovarRanarum (a, c, and e) and Shermani (b, d, and f). The enveloping membrane (Em)covering the body surface of the Ranarum is more roughly and loosely attached to the body than that of the Shermani. The sharp electron dense axial filament (Af) acts as the axis ofthe body. The wavelength (Wl) and amplitude (Am) are longer and a little shorter, respectively, than those of the Shermani.Af, Axial filament; Am, Amplitude; Em, Enveloping membrane;Lr, Lucid region; Pc, Protoplasmic cylinder; Rb, Ribosome; Wl,Wavelength.
The enveloping membrane appeared to be trilaminar and was the most external part, covering the entire body. In the Ranarum, this structure had a rough, irregular appearance, and it was loosely attached to the protoplasmic cylinder, leaving an obvious space between them (Figures. 5a, c, and e). By contrast, the enveloping membrane of the Shermani was smooth and tightly attached to the protoplasmic cylinder, providing a barely discernible space between the enveloping membrane and protoplasmic cylinder (Figures 5b, d, and f).
The axial filaments lay between the enveloping membrane and the protoplasmic cylinder (Figure 5). They were intertwined with the protoplasmic cylinder (Figures 5a-d). Those of the Ranarum (Figures 5a, c, and e) were more clearly demonstrated than those of the Shermani (Figures 5e, d, and f), which were difficult to be identified.
The protoplasmic cylinder of the Ranarum had loosely formed components that resulted in a less electron dense appearance than that of the Shermani. This made the ribosome and lucid region of the protoplasmic cylinder more clearly identified Figures 5a, c, and e). The more electron dense protoplasmic cylinder of the Shermani made it hard to identify the ribosome, whereas its lucid region was obvious (Figures 5b, d, and f).
Moreover, the wavelengths of the Ranarum (Figures 5a and c) were obviously longer than those of the Shermani (Figures 5b and d).
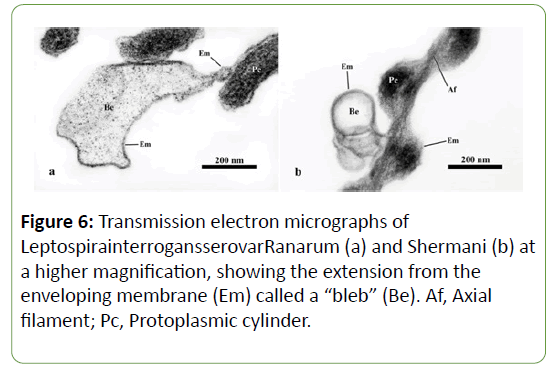
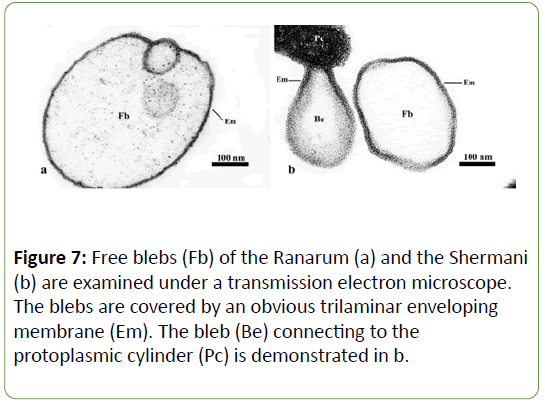
In addition, the organisms of both serovars illustrated blebs, which were extensions of the enveloping membrane that formed pouch-like structures (Figures 6 and 7). Two kinds of bleb were identified. Those showing no connection to the enveloping membrane were called “free blebs”, while those with a connection were simply termed “blebs” or “connecting blebs” (Figure 7). The free blebs and blebs were round, ovoid, or irregularly shaped, and they were covered with the trilaminar enveloping membrane.
Discussion
Scanning electron microscopy
The general appearances of L. interrogansserovarRanarum and Shermani were alike. Both were long, spiral-shaped bacteria, and a hook-like terminal end was visualized on one or both sides. A hook in the terminal area of leptospires was also mentioned by Noguchi, 1918, who said that it had a semicircular The general appearances of L. interrogansserovarRanarum and Shermani were alike. Both were long, spiral-shaped bacteria, and a hook-like terminal end was visualized on one or both sides. A hook in the terminal area of leptospires was also mentioned by Noguchi, 1918, who said that it had a semicircular shape and might not be found while the bacteria were moving [23].
Differences between the 2 serovars were found when measuring the 4 parameters: length, width, wavelength, and amplitude of the bacterial body. All were statistically different. The mean ± SD of the length of the Ranarum was 13.09 ±2.66 μm, which was significantly longer than the 11.26 ± 3.5 μm of the Shermani (p-value=0.006). There have been numerous studies of the length of many kinds of leptospires, all of which have displayed differences. For example, the Icterohaemorrhagiae was found to be 3-40 μm long [23]; the Illini was 13-21 μm [24]; the Icterohaemorrhagiae together with the Canicola was 4-10 μm [11]; and the Icterohaemorrhagiae together with the Pomona,Canicola, and Autumnalis was 6-9 μm [25]. These variable lengths might be because of the serovars themselves, or might be due to whether the leptospires were static or motile. However, the length of an organism could be an unstable parameter since it depends on the age of organism [26]. Still, the difference in the lengths of the Ranarum and Shermani demonstrated in this study should be reliable since they were all the same age, having been obtained on the same day of culturing.
The median (min, max) width of the Ranarum was statistically significantly narrower than that of the Shermani, being 0.14 (0.12, 0.23) and 0.16 (0.12, 0.27), respectively, with a pvalue= 0.003. Despite the width of the Ranarum being significantly narrower than that of the Shermani, the scanning electron micrograph images of them showed no difference. The widths of other serovars have been reported by many researchers: 0.1 μm for the Illini [24]; 0.07-0.14 μm for the Icterohaemorrhagiae and Canicola [11]; and 0.2-0.4 μm for the Icterohaemorrhagiae, Pomona, Canicola, and Autumnalis [25]. In spite of the widths mentioned having some differences, they were in the same range. However, the study by Morton and Anderson [11] was carried out by studying the Icterohaemorragiae together with the Canicola, while Simpson and White [25] studied the Icterohaemorrhagiae, Pomona, and Canicola together with Autumnalis without making comparisons between each serovar; therefore, the information obtained might not be trustworthy.
The wavelength of the Ranarum was 0.54 ± 0.05 μm, which was statistically significantly longer than the 0.44 ± 0.05 μm for the Shermani (p-value<0.001). The scanning electron micrographs of the 2 serovars clearly showed a difference in their spiral coils in that those of the Ranarum were looser than those of the Shermani. As a result, the wavelength of the loosely-coiled Ranarum was statistically longer than that of the tightly-coiled Shermani. There have been a number of reports on the wavelengths of many other kinds of leptospires; for instance, the reported wavelength of the Icterohaemorrhagiae was 0.5 μm [23], the Pomona was 0.5-0.6 μm [14], the Illini was 0.6 μm [24], and the Icterohaemorrhagiae and Canicola was 0.3-0.6 μm [11]. In addition, the work of Morton and Anderson [11] did not compare the difference between the 2 serovars studied. However, the wavelengths of the 2 serovars obtained from the present study corresponded with, or were in the same range as, others.
The amplitude of the Ranarum was 0.30 ± 0.05 μm, which was highly significantly shorter than the 0.34 ± 0.05 μm for the Shermani (p-value<0.001). In addition, the amplitudes at the terminal parts of the 2 serovars appeared shorter than at the other parts of the body. This finding corresponded to the work of Noguchi [23], who studied the Icterohaemorrhagiae. Again, many researchers have studied the amplitudes of other leptospires; their findings include 0.1 μm for the Illini [24] and 0.25 μm for the Icterohaemorrhagiae and Canicola[11]but without any comparisons being madebetween them.
The blebs, or granule-like structures, were found in both serovars. They were similar to those that have been demonstrated by SEM in the Icterohaemorrhagiae and Canicola [27] as well as in the Icterohaemorrhagiae, Pomona, Canicola, and Autumnalis [25]. Czekalowski and Eaves [27], who studied the Icterohaemorrhagiae and Canicola, reported that either granules or free granules were seen, and that large granules were found at the terminal parts of the cells. Moreover, Babudieri[28] reported that small granules were often found in older leptospires. Simpson and White [25], who studied the Icterohaemorrhagiae, Pomona, Canicola, and Autumnalis, demonstrated that there were bulbs or knobbed processes in the terminal parts of the cells. Those bulbs resembled the blebs seen in the current study. The granular structure was found to be not a product of cell degeneration, but rather a part of the leptospiral life cycle [27]. Because of the technical limitations of scanning electron microscopy, which reveals only the 3- dimensional body surface, there was no information on the differences between the blebs of each serovar.
Transmission electron microscopy
Both the Ranarum and Shermani comprised 3 main structures-the enveloping membrane, the protoplasmic cylinder and 2 axial filaments-with some small differences. This finding corresponded to studies of the Icterohemorrhagiae [11,25,27], the Canicola [11,25,27,29], the Hebdomadis [15], the Autumnalis [25], and the Pomona [13,14,17,18,25].
The enveloping membrane was the most external structure of the organisms. The enveloping membranes of both serovars were trilaminar, which corresponded with the findings of the earlier studies ofthe Canicola [29] and the Pomona [17]. However, Ziegler and Vaneseltine [17] reported a 5-layered, rather than a 3-layered, enveloping membrane. This might because of an artifact in their pictures that made the membrane appear to be 5- instead of 3-layered. The enveloping membrane of the Ranarum was loosely attached to the protoplasmic cylinder, creating an easily discernible space between the membrane and the cylinder. This feature also appeared in the Pomona studied by Ritchie and Ellinghausen (1965) and Birch- Andersen (1973) [13,14]. However, there were some differences in the enveloping membranes of the 2 serovars. Firstly, the Ranarummembrane was rough, whereas the Pomona membrane was smooth. As well, the Shermani membrane was intimately attached to the protoplasmic cylinder, making it difficult to identify the space between the membrane and the cylinder. The Shermani membrane was also smooth like that of the Pomona.
The axial filaments lying between the enveloping membrane and the protoplasmic cylinder were more clearly revealed in the Ranarum than in the Shermani. This was because the enveloping membrane of the Ranarum was rough and loosely attached to the protoplasmic cylinder, providing an obvious space between them, while that of the Shermani was smooth and tightly attached to the protoplasmic cylinder; as a result, the space between the membrane and the cylinder was barely discernible. As the axial filaments were located in this space, the filaments of the Ranarum were more clearly identified than those of the Shermani. There have been some reports on axial filaments, such as Czekalowski and Eaves (1955) [30] and Czekalowski (1963) [19]. Studying the Icterohemorrhagiae and Canicola, Czekalowski and Eaves [30] reported that the axial filaments were close to the surface of the protoplasmic cylinder. Czekalowski [19] also reported that the axial filaments not only formed the backbone of the body but were an important structure for locomotion.
The protoplasmic cylinders of the Ranarum and Pomona were much less electron dense than that of the Shermani. As well, the Pomona cylinder appeared more electron dense than that of the Ranarum. In other words, the electron density of the protoplasmic cylinder progressed from the Ranarum, to the Pomona, and finally to the Shermani. This resulted in a more obviously identified ribosome and lucid region in the Ranarum than in the Pomona and Shermani.
The Ranarun and Shermani displayed extensions of the enveloping membrane called “blebs”. They corresponded to the negative stained cells of the Pomona [13]and the Canicola[30]. The large blebs were located at the terminal parts [13,30] and the middle of the organisms [1]. Small blebs were also found throughout the organism with no specific location [13,30]. The blebs were either circular or irregular in shape [30]. Sometime, free blebs were found in both serovars; no connection between the free blebs and the enveloping membrane was seen. Identical structures were also found in the Canicola[30].
The structures extending from the enveloping membrane have been reported under different names, including “granule” [28], “bulb” or “knobbed process” [25], and “bleb” [13,30].
Conclusion
As to the knowledge obtained, there are some obvious differences in the morphologies and ultrastructures of the Ranarum and Shermani. These structural differences may be used to identify the serovar of leptospires. Usually, the serovars are identified by the agglutination test.In addition, the morphological differences of the 2 serovars might be used as an alternative way to identify the leptospiral type in parallel with the agglutination test, which should provide a complete understanding of the relationship between the morphological and serological approaches. However, this approach can only be done if there are studies of many other serovars or leptospires.
Acknowledgments
The authors thank the Leptospirosis Center, National Institute of Animal Health, Department of Livestock Development, Ministry of Agriculture and Cooperatives, Thailand, for providing the leptospire samples, and the Electron Microscopy Unit, Department of Pathology, Faculty of Medicine, Siriraj Hospital, Mahidol University, for making the transmission electron microscope available. We also appreciatethe support of the Department of Anatomy, Faculty of Medicine, Siriraj Hospital, Mahidol University, which made this research successful.
References
- Adler B. History of Leptospirosis and Leptospira In: B Adler (eds) Leptospira and Leptospirosis (1 edn), Springer-Verlag Berlin Heidelberg publisher, Germany. 2005; pp: 1-10.
- Haake D A, Levett P N. Leptospirosis in humans. In: B Adler (Ed) Leptospira and leptospirosis. (1st edn) Springer-Verlag Berlin Heidelberg, Germany. 2005; pp: 65-80.
- Goldstein SF, Charon NW. Motility of the spirochete Leptospira. Cell Motil. Cytoskeleton. 1988;9(2): 101-110.
- Evangelista KV, Coburn J. Leptospira as an emerging pathogen: a review of its biology, pathogenesis and host immune responses. Future microbiol. 2010;5(9): 1413-1425.
- Levett P N. Leptospirosis. Clinical microbiology reviews, 2001;14: 296-326.
- Dutta T, Christocher M. Leptospirosis - an overview. JAPI. 2005;53: 545-551.
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. The Lancet infectious diseases. 2003;3(12): 757-771.
- Suwancharoen D, Chaisakdanugull Y, Thanapongtharm W, Yoshida S. Serological survey of leptospirosis in livestock in Thailand. Epidemiol Infect. 2013;141(11): 2269-2277.
- Jitdamrong S, Mungaomklang A, Chaimongkol E, Suwancharone D, Tangkanakul W. The outerbreak of leptospirosis among Thai eco-tourist in a southern rural Thai province, 2007. J Health Sci, 2011;20: 104-114.
- Morton HE, Anderson TF. The Morphology of Leptospira icterohemorrhagiae and L. canicola as Revealed by the Electron Microscope. J Bacteriol. 1943;45(2): 143.
- Jacobs R. Leptospirosis. West J Med. 1980;132(5): 440-450.
- Ritchie AE, Ellinghausen HC. Electron Microscopy of Leptospires I. Anatomical Features of Leptospira pomona. J Bacteriol. 1965;89(1): 223-233.
- Birch‐Andersen A, Hougen KH, Borg‐Petersen C. ELECTRON MICROSCOPY OF LEPTOSPIRA: 1. Leptospira Strain Pomona. Acta path. microbiol. scan. 1973;81(6): 665-676.
- Takeya K, Mori R, Toda T. Studies on the structure of Leptospira as revealed by the electron microscope. Japan. J. Microb. 1957;1(2): 99-104.
- Nauman RK, Holt SC, Cox CD. Purification, ultrastructure, and composition of axial filaments from Leptospira. Journal of bacteriology. 1969 Apr 1;98(1): 264-280.
- Zeigler JA, VanEseltine WP. Isolation and chemical characterization of outer envelope of Leptospira pomona. Can. J. Microbiol. 1975;21(7): 1102-1112.
- Grinyer I. An electron microscopic study of Leptospira pomona. Can Vet Jour. 1962;3(4): 112.
- Czekalowski JW. Electron microscope study ofLeptospira. Antonie Van Leeuwenhoek. 1963;29(1): 29-34.
- Holt SC. Anatomy and chemistry of spirochetes. Microbiological reviews. 1978;42(1): 114.
- Rosner B. Fundamentals of biostatistics (7 edn). Boston: Cengage Learning. 2010.
- McDonald J H. Hanbook of biological statistic. Maryland: Sparky House Publishing, USA. 2008.
- Noguchi H. Morphological characteristics and nomenclature of Leptospira (Spirochaeta) icterohaemorrhagiae (Inada and Ido). The Journal of experimental medicine. 1918;27(5): 575-592.
- Hovind-Hougen KA. Leptospiraceae, a new family to include Leptospira Noguchi 1917 and Leptonema gen. nov. Int. J. Sysy. Bacteriol. 1979;29(3): 245-251.
- Simpson CF, White FH. Electron microscope studies and staining reactions of leptospires. J Infect. 196: 243-250.
- Hovind-Hougen K. Morphology of leptospires electron microscopic studies in relation of to the classification of Leptospira. In: W A Ellis, T W A Little (Eds) The present state of leptospirosis diagnosis and control. Martinus nijhoff publishers. 1986; pp: 1-12.
- Czekalowski J, Eaves G. Formation of granular structures by Leptospirae as revealed by the electron microscope. J Bacteriol. 1954;67(6): 619.
- Babudieri B. The morphology of the genus Leptospira as shown by the electron microscope. Epidemiology & Infection. 1949;47(4): 390-392.
- Auran NE, Johnson RC, Ritzi DM. Isolation of the outer sheath of Leptospira and its immunogenic properties in hamsters. Infection and immunity. 1972;5(6): 968-975.
- Czekalowski J W, Eaves G (1955). The structure of Leptospira as revealed by electron microscope. J Path Bact. 1955;69: 129-132.
- Anderson DL, Johnson RC. Electron microscopy of immune disruption of leptospires: action of complement and lysozyme. Journal of bacteriology. 1968;95(6): 2293-2309.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences