Clinical and Urine Scoring in Assessing Long-Term Outcome of Acute Kidney Injury/Acute Kidney Disease (AKI/AKD) with Risk of Progression to Chronic Kidney Disease (CKD)
Saugat Das Gupta1*, Arpita Ray Chaudhury1, Koushik Bhattacharjee1, Atanu Pal1, Abhishek Kumar1, Smartya Pulai1, Debabrata Sen1, Keya Basu2 and Moumita Sengupta2
1Department of Nephrology, IPGME and R, SSKM Hospital, Kolkata, India
2Department of Pathology, IPGME and R, SSKM Hospital, Kolkata, India
- *Corresponding Author:
- Saugat Das Gupta
Department of Nephrology,
IPGME and R,SSKM Hospital,
Kolkata,
India,
Tel: 8876388540;
E-mail: dasgupta.saugat13@gmail.com
Received date: October 19, 2022, Manuscript No. IPJNU-22-14824; Editor assigned date: October 24, 2022, PreQC No. IPJNU-22-14824 (PQ); Reviewed date: November 08, 2022, QC No. IPJNU-22-14824; Revised date: January 02, 2023, Manuscript No. IPJNU-22-14824 (R); Published date: January 10, 2023, DOI: 10.36648/ IPJNU.7.1.001
Citation: Gupta SD, Chaudhury AR, Bhattacharjee K, Pal A, Kumar A, et al. (2023) Clinical and Urine Scoring in Assessing Long-Term Outcome of Acute Kidney Injury/Acute Kidney Disease (AKI/AKD) with Risk of Progression to Chronic Kidney Disease (CKD). J Nephrol Urol Vol:7 No:1
Abstract
Background: AKD represents an important transition period for patients who have suffered an episode of AKI and AKI is a significant risk factor for the development of CKD.
Methods: Single centre observational study conducted in the department of nephrology, IPGME and R and SSKM Hospital of 430 AKI patients from May 2019 to November 2020. Six-variable risk index for advanced chronic kidney disease is using Advanced CKD after AKI RISK Index’ as proposed by MT James, Alberta, Canada and Urine Cast Scoring Index (CSI) was calculated. Scheduled follow up visits for each participant done during the subsequent six months.
Results: Most patients were in the age group 21-30 years (23.5%), 55.3% were male, 76% from rural background, 27.8% smoker with majority (62.7%) had community acquired acquired AKI. 97% KDIGO stage 3 with 88.8% being oligo-anuric at presentation. 95.3% had medical cause of AKI, amongst which top 3 causes of AKI were snake bite (34.8%), tropical infection (15.3%), sepsis (14%). 85.8% progressed to AKD. Patient recovery at discharge, 1 m, 3 m and 6 m were 16.3%, 47.8%, 58.8%, 60% respectively. Peak creatinine and urine score was found to have significance with recovery at 3 months, while only urine score was found to have significance with recovery at 6 months.
Conclusion: Snake bite, tropical infection, sepsis still represent important cause of RRT requiring AKI in our part of Indian subcontinent with substantial proportion not recovering upto 6 months of follow up and contributing to overall CKD burden. Persistence of AKI or AKD should be a wakeup call for the clinician to further assess and evaluate treatment options in order to improve long-term outcome. Combining comprehensive clinical evaluation with use of “risk scoring” and urine sediment analysis would provide new insights into AKI for better patient risk stratification and prognostication and resurrect the ancient tradition of looking at the urine to open the window of the body.
Keywords
AKD acute kidney disease; CSI cast scoring index; CA AKI community acquired AKI; HA AKI Hospital acquired AKI; TPR true positive rate; TNR true negative rate
Introduction
AKI as an abrupt decrease in kidney function that occurs over a period of 7 days or less and CKD as abnormalities in kidney structure or function that persist for >90 days. AKI survivors are at increased risk of developing Chronic Kidney Disease (CKD) and End Stage Kidney Disease (ESKD) [1]. It is increasingly recognized that AKI and CKD are not always discrete entities and likely represent a continuum with patients who have sustained an episode of AKI having an increased risk of developing either de novo CKD or worsening of underlying CKD. The term Acute Kidney Disease (AKD) has been proposed to define the course of disease after AKI among patients in whom the renal pathophysiologic processes are ongoing. AKD is conceptualized not as pre-CKD but rather, as post-AKI [2]. In addition, the risk factors for AKI and CKD, such as advanced age, diabetes and hypertension, often overlap [3].
Materials and Methods
The study was a single centre observational study conducted in the department of nephrology, IPGME and R and SSKM Hospital, Kolkata, India with total of 430 patients with either community or hospital acquired AKI along with those attending OPD services from May 2019 to November 2020 [4]. Scheduled follow up visits for each participant occured during the subsequent six months.
Inclusion criteria
• Patients with AKI (community or hospital acquired) willing for regular follow-up.
• Age ≥ 18 years.
Exclusion criteria
• Inability to provide informed or surrogate consent.
• Acute GN diagnosed clinically or by biopsy.
• Clinically significant urinary tract obstruction, confirmed by imaging.
• Pregnant or breastfeeding mothers.
• H/O prior chronic hemodialysis or peritoneal dialysis (lasting >3 months) or known case of CKD f) H/O solid organ and/or haematopoietic transplant.
• H/O multiple myeloma, past nephrectomy, metastatic cancer receiving active treatment NYHA class IV heart failure prior to index hospitalization.
• Patients who are unable to complete at-least one month of follow up post discharge.
Six variable risk index for advanced chronic kidney disease was calculated using advanced CKD after AKI RISK index’ as proposed by MT James, Alberta, Canada.
Urine Cast Scoring Index (CSI) was calculated by viewing entire slide under bright light microscopy at low power (× 10) to search for granular and RTE casts. High power (× 40) was available to confirm the type of cast; grading was done as proposed by L SChawla, et al.
All patients included in the study subjected to a uniform evaluation. Demographic data, history, detailed clinical examination was recorded in a proforma. Informed written consent was obtained from all the patients at the time of enrolment. Adult patients with AKI and AKD will be identified during the index hospitalization and screened for initial eligibility. During this inpatient visit, enrolled AKI and AKD patients will undergo urinalysis with microscopy through the hospital clinical laboratory and provide at least one sample of blood and urine for testing within 96 hours of the episode of AKI [5]. Patients categorized into the following groups:
• Transient AKI complete and sustained reversal of AKI episode within 48 hours.
• Persistent AKI continuance of AKI by urine output or serum creatinine criteria beyond 48 hrs of AKI onset.
• AKD (Acute kidney disease)-AKI is present for >7 days after an AKI initiating event.
Patients with persistent AKI and AKD categorized into two groups, RRT requiring-2 subgroups, RRT dependent, recovery from RRT dependence sustained independence from RRT for a minimum of 14 days, non RRT requiring, recovery from AKI will be assessed by urine output criteria and/or creatinine value. All AKI and AKD patients will be staged as per scheme proposed by KDIGO and ADQI 16 work group respectively.
Follow up
All patients will be followed up at 1 month, 3 months, 6 months after the AKI initiating event to assess link of loss of kidney function associated with AKI with that of new onset hypertension, cardio-vascular disease, chronic kidney disease.
Results
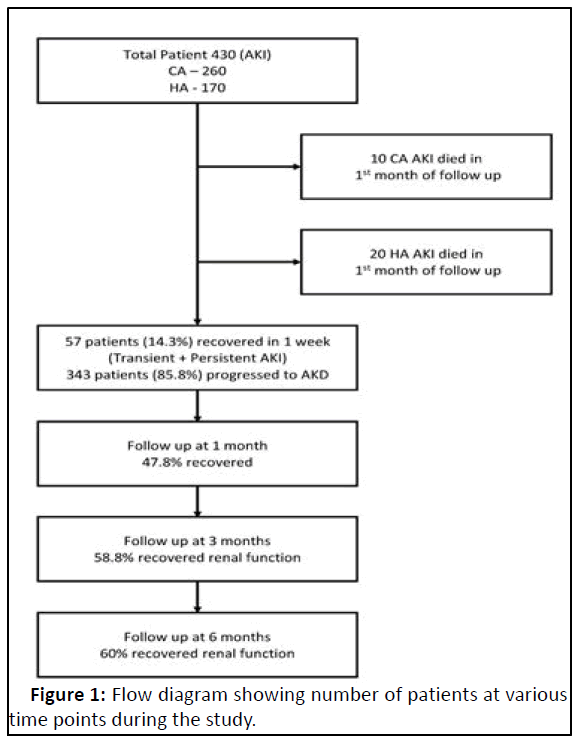
In this study, total 430 AKI patients were taken from nephrology indoor, Outpatient Department (OPD) and Dialysis unit, but 30 (6.9%) patients died during the first month of follow up, with remaining 400 patients subsequent follow up was undertaken till 6 months (Figure 1).
Baseline characteristics of hospitalized patients with reversible AKI surviving initial month and alive 180 days after discharge.
Demographic characteristic
Most patients were in the age group 21-30 years (23.5%) and 41-50 years (20.3%) with age range between 18 to 75 years.
55.3% were male, 73.3% married, 76% from rural background; most patients had monthly earning in the 5 k-15 k range (54.8%) (Table 1).
60.5% without any addiction history, 27.8% smoker, 8.5% alcoholic, 3.3% tobacco chewer. 18.3% were illiterate; 27.8% students and 23.5% were housewives;
62.7% had community acquired and 37.3% had hospital acquired AKI.
| Characteristic | Number 400. (%) |
|---|---|
| Age (year) (mean ± SD ) | 42.3 ± 16.7 |
| Female gender | 179 (44.7) |
| Unmarried | 107 (26.7) |
| Rural | 304 (76) |
| No addiction | 242 (60.5) |
| Community acquired AKI | 250 (62.5) |
| Surgical cause AKI | 19 (4.8) |
| Oligo-anuria | 355 (88.8) |
| Advanced CKD after AKI risk score | N=148 |
| 1-8 | 11 (7.4%) |
| 9-14 | 48 (32.4) |
| 15-17 | 26 (17.6) |
| 18-19 | 23 (15.5) |
| >20 | 40 (27) |
| Comorbidity | |
| DM | 5 (1.3) |
| HTN | 19 (4.8) |
| CAD | 56 (14) |
| CLD | 13 (3.3) |
| COPD | 27 (6.8) |
| Rheumatic disease | 19 (4.8) |
| none | 261 (65.3) |
| Illiterate | 73 (18.3) |
| Type of AKI | |
| Persistent AKI | 57 (14.3) |
| AKD | 343 (85.8) |
| KDIGO stage | |
| 1 | 0.30% |
| 2 | 2.70% |
| 3 | 97% |
| Urine cast score | |
| 1 | 4.50% |
| 2 | 56.80% |
| 3 | 34.30% |
| 4 | 4.50% |
| Number of dialysis sessions mean ± SD) | 4.13 ± 3.8 |
| Creatinine (mg/dl) (mean ± SD ) | |
| At presentation | 2.3 ± 1.7 |
| Maximum creatinine | 6.05 ± 3.3 |
| Cause of AKI | |
| Snake bite | 139 (34.8) |
| Malaria/Dengue/Leptospirosis | 61 (15.3) |
| Acute gastroenteritis | 17 (4.3) |
| Sepsis | 56 (14) |
| Cardiac cause | 33 (8.3) |
| Liver cause | 14 (3.5) |
| Lung cause | 14 (3.5) |
| Drug induced | 21 (5.3) |
| others | 45 (11.3) |
| Urine ACR at (% of patients) | |
| Discharge | 6 |
| 1 month | 7.3 |
| 3 months | 9.3 |
| 6 months | 7.8 |
| Recovery at (% of patients) | |
| Discharge | 16.3 |
| 1 month | 47.8 |
| 3 months | 58.8 |
| 6 months | 60 |
| New onset hypertension at 6 months (%) | 15 |
Table 1: Demographics and clinical characteristics at baseline.
Clinical characteristic
KDIGO stage at presentation was 1,2,3 in 0.3%, 2.8%, 97% respectively; 88.8% had oligo-anuria at presentation, 95.3% had medical cause of AKI, amongst which Top 3 causes of aki were snake bite (34.8%), tropical infection (15.3%) and sepsis (14%). 65.3% patients had no co-morbidity, among those with co-morbidity CAD most common (14%), COPD (6.8%), HTN (4.8%), Rheumatic ds (4.8%), CLD (3.3%), DM (1.3%). Mean baseline, presentation, maximum, discharge creatinine was 1.08 mg/dl ± 0.13 mg/dl, 2.3 mg/dl ± 1.7 mg/dl, 6.05 mg/ dl ± 3.3 mg/dl, 1.8 mg/dl ± 0.53 mg/dl respectively [6].
In 148 cases baseline creatinine was available and in them “Advanced CKD after AKI risk index score” was calculated with majority had score in the range of 9-14 (32.4%) followed by >20 (27%). Urine cast score index was 1, 2, 3, 4 in 4.5%, 56.8%, 34.3%, 4.5% respectively. Mean no. of dialysis session given were 4.13% ± 3.8%. Urine ACR were present in 6%, 7.3%, 9.3% and 7.8% of patients at discharge, 1 month, 3 months, 6 months respectively. 85.8% progressed to AKD. Patient recovery at discharge, 1 m, 3 m and 6 m were 16.3%, 47.8%, 58.8%, 60% respectively. 6.5% patients developed new HTN at 3 m and 6 m each total of 15% developed new HTN at 6 m follow up visit. Significance of variables with recovery at 3 months and 6 months were shown in Table 2.
| Significant variables | Not significant variables |
|---|---|
| Age (p<0.0001) | Gender (p=0.1093) |
| Occupation (p<0.0001) | Income (p=0.5525) |
| Cause of AKI (p<0.0001) | Education (p=0.0769) |
| Baseline (p<0.0001)/peak (p=0.0073)/discharge | Rural vs. urban (p=0.9242) |
| ( p<0.0001) Creatinine | Medical vs. Surgical (p=0.1754) |
| Presence of oligo-anuria (p=0.0002) | Social habit (p=0.3164) |
| Setting of AKI (CA/HA) (p=0.0401) | Presentation creatinine (p=0.7046) |
| Higher number of dialysis sessions (5.2 ± 3.5 vs. 3.3 ± 3.8, p<0.0001) | |
| High Urine CSI (p<0.0001) | |
| High Advanced CKD after AKI risk index score | |
| (p<0.0001) | |
| Any Co-morbidity (p<0.0001) | |
| High Urine ACR at DX, 1 m, 3 m (p<0.0001) |
Table 2: Significance of variables with recovery at 3 months.
Regarding cause of AKI with recovery, only 72 (51.8%) patients of snake bite recovered at 3 month follow up ,while 53 (86.9%) and 16 (94.1%) patients of tropical infection and gastroenteritis recovered at 3 month follow up respectively [7]. In multivariate regression analysis peak creatinine (p=0.0359) and urine score (p=0.0004) was found to have significance with recovery at 3 months (Table 3), while only urine score (p=0.0025) was found to have significance with recovery at 6 months (Table 4).
| Odds ratio | 95% C.I. | Coefficient | S. E. | Z-statistic | P-value | |
|---|---|---|---|---|---|---|
| Age | 1.1946 | 1.1204-2.2988 | 0.786 | 0.343 | 2.2914 | 0.4219 |
| Cause of AKI | 1.7339 | 0.7933-3.7894 | 0.5503 | 0.3989 | 1.3796 | 0.1677 |
| Urine Score | 2.7103 | 2.8085-3.6179 | 0.5367 | 0.3823 | 1.4038 | 0.0004 |
| Max Creatinine | 2.9497 | 1.5615-5.5721 | 1.0817 | 0.3245 | 3.3331 | 0.0359 |
| Advanced CKD after AKI risk index score | 1.2178 | 0.6417-2.3110 | 0.197 | 0.3269 | 0.6028 | 0.5467 |
Table 3: Multivariate logistic regression variables with Recovery at 3 months.
| Odds ratio | 95% C.I. | Coefficient | S. E. | Z-statistic | P-value | |
|---|---|---|---|---|---|---|
| Age | 1.1241 | 1.0204- 2.1890 | 0.686 | 0.443 | 2.3021 | 0.3142 |
| Cause of AKI | 1.0125 | 0.8217- 2.3708 | 0.245 | 0.4869 | 0.5808 | 0.4067 |
| Urine Score | 2.5208 | 2.1057- 3.0142 | 0.5902 | 0.3682 | 1.3502 | 0.0025 |
| Max Creatinine | 1.0278 | 0.6047- 2.3110 | 0.197 | 0.3269 | 0.6028 | 0.5467 |
| Advanced CKD after AKI risk index score | 1.345 | 0.5407- 2.2140 | 0.1254 | 0.3805 | 0.5407 | 0.5102 |
Table 4: Multivariate logistic regression variables with recovery at 6 months.
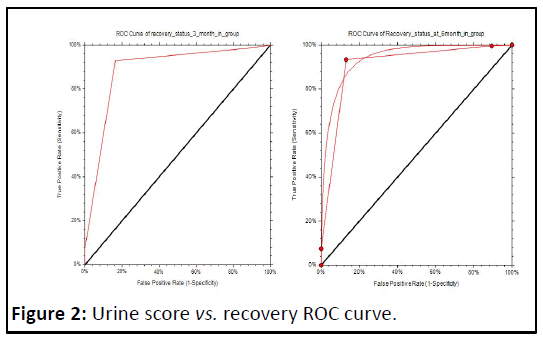
Urine score of ≤ 2 vs. recovery at 3 months has the maximum TPR+TNR score of 1.76 in ROC. Curve analysis with sensitivity of 0.9277 (95% confidence limits 0.8867-0.9573) and specificity of 0.8364 (95% confidence limits 0.7709-0.8893) with AUC of 0.8862 (95% confidence limits 0.8495-0.9144); urine score of ≤ 2 vs. recovery at 6 months has the maximum. TPR+TNR score of 1.80 in ROC curve analysis with sensitivity of 0.9333 (95% confidence limits 0.8940-0.9614) and specificity of 0.8688 (95%confidence limits 0.8064-0.9169) with AUC of 0.9077 (95%confidence limits 0.8739-0.9382) (Figure 2).
Discussion
This study evaluated the long-term risks of de novo CKD following reversible AKI ,we found that despite fairly rapid recovery of renal function, such episodes involving largely modest increases in serum creatinine were associated with an increased risk of developing CKD during longitudinal follow-up [8]. In our study 85.8% progressed to AKD; Patient recovery at discharge, 1 m, 3 m and 6 m were 16.3%, 47.8%, 58.8%, 60% respectively; while none of our patient was dialysis dependant at 3 months and 6 months of follow up, this is possibly because of the different cause of AKI studied, predominantly snake bite in our cohort and exclusion of patients who did not survive in the initial one month of follow up [9]. Among previously reported risk factors for CKD, we found variables such as older age, occupation of the patient, presence of comorbidity, discharge and peak in hospital creatinine to be significantly associated with non-renal recovery at 3 month and 6 month.
Previously, other investigators have assessed the diagnostic and predictive value of urine sediment analysis. In a study of 51 patients with AKI, Marcussen, et al. found that cytodiagnostic urinalysis may be valuable in establishing a diagnosis and predicting the severity of AKI. Schentag, et al. 22owed that the number of urinary casts were able to identify early aminoglycoside nephrotoxicity. we used AKI Cast Scoring Index (CSI) to grade the level of RTE casts and granular casts in urine sediment so that urine sediment could be evaluated in a uniform fashion, first studied and validated , who modeled a system with 4 different categories. In their study, the inter observer index was 99.8%, signifying good agreement. In study 8-18 patients with ATN urinary sediment was assessed for outcome, baseline serum creatinine was 1.1 mg/dl ± 0.5 mg/dl. The rate of nonrenal recovery was 61.1% and the mean AKI CSI was 2.2. They assessed the capacity of this score to predict nonrenal recovery [10]. The ROC area under the curve was 0.79, which is comparable to urinary KIM1 (0.61) and NAG (0.71). In our study ROC AUC was 0.8862, using urine CSI cut off value of ≤ 2, we can predict renal recovery at 3 month with sensitivity of 0.9277 (95%confidence limits 0.8867-0.9573) and specificity of 0.8364 (95%CL 0.7709-0.8893) and recovery at 6 month with sensitivity of 0.9333 (95% CL 0.8940-0.9614) and specificity of 0.8688 (95% CL 0.8064-0.9169).
We also calculated clinical risk scoring for at baseline for prediction of renal recovery at 3 months and 6 months using concept proposed 21 in his “derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury” showed that the 6- variable model (model 1) had the highest C statistic (0.86; 95% CI, 0.84-0.89) and lowest Akaike information criterion (1967.0) [11]. 6-variable model and corresponding risk index (including age, gender, baseline serum creatinine value, albuminuria, acute kidney injury severity and discharge serum creatinine value) showed the best performance for predicting whether patients who had AKI during their hospital stay would later develop advanced CKD in internal and external validation cohorts, with better performance than reduced models based on age, gender and discharge serum creatinine alone or based on age, gender or acute kidney injury severity alone. This work has integrated these variables into multivariable risk models and a practical risk index, so that these variables can be readily used to obtain individualized estimates of advanced chronic kidney disease for patients with acute kidney injury encountered in clinical practice. These models performed well in the geographically distinct Ontario cohort, despite some expected loss of discriminative performance (model 1 C statistic, 0.81; 95% CI, 0.75-0.86).
In our study in 148 patients baseline creatinine was available so that we can calculate the score majority had score in the range of 9-14 (32.4%) followed by >20 (27%) and lower baseline score vs. renal recovery at 3 month and 6 month was found statistically significant, but we could not demonstrate significance (p=0.54) in multivariate regression analysis [12-15]. This model were derived and validated in cohorts from Canada and generalizability to patients from other regions was not examined, so our study could be seen as a validation cohort for this model in Indian population, though sample size need to be more robust to come to a definite conclusion.
Potential limitations of this study being observational nature of our study, we cannot exclude the possibility that unmeasured factors related to both the exposure and outcomes resulted in biased estimates of the impact of AKI [16,17]. This study may have implications for patients, clinicians and policy makers. Clinicians in the community are not aware of acute kidney injury episodes that occur during hospitalization, lack awareness of the prognostic implications of these events or encounter barriers to timely access to chronic kidney disease care in the community [18-20]. This highlights the common clinical challenge of providing continuity of care between the hospital and community. These risk prediction tools/variables provide an accurate but simple strategy that could be used to stratify patients into clinically meaningful risk groups at the time of hospital discharge and guide further management in the community [21].
Conclusion
Urinary grading system is simple and the sample preparation is easily reproducible in any hospital where urine sediment is part of the clinical work-up of kidney disease. A point scoring system that incorporates the AKI CSI may improve current and/or future AKI diagnostic measures. If validated, an AKI CSI could be used in conjunction with serum and urinary biomarkers to help improve the diagnosis of AKI. Our data endorse the notion that the AKI CSI and ‘advanced CKD after AKI RISK Index’ may be useful in predicting renal outcomes. The acceptance of a unifying definition of AKI has increased awareness of the syndrome and generated much knowledge on morbidity and outcome. Less attention has been given to recovery from AKI. After an episode of AKI serial follow-up measurements of serum creatinine and proteinuria are warranted to diagnose progressive renal impairment given the large absolute numbers of hospitalized patients experiencing AKI worldwide, the impact and utility of management strategies for primary CKD prevention among those patients without preexisting CKD who experience AKI need further investigation and should be the research priority in the coming days.
References
- Lewington AJ, Cerda J, Mehta RL (2013) Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int 84:457-467
[Crossref] [Google Scholar] [PubMed]
- Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, et al. (2015) Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41:1411-1423
[Crossref] [Google Scholar] [PubMed]
- Tao Li PK, Burdmann EA, Mehta RL (2013) Acute kidney injury: Global health alert. J Nephropathol 2:90-97
[Crossref] [Google Scholar] [PubMed]
- Chawla LS, Eggers PW, Star RA, Kimmel PL (2014) Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371:8-66
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, et al. (2005) Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67:2089-2100
[Crossref] [Google Scholar] [PubMed]
- Bagshaw SM, Goldstein SL, Ronco C, Kellum JA, Group AC (2016) Acute kidney injury in the era of big data: The 15th Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Can J Kidney Health Dis 3:5
[Crossref] [Google Scholar] [PubMed]
- Chawla LS, Dommu A, Berger A, Shih S, Patel SS (2008) Urinary sediment cast scoring index for acute kidney injury: A pilot study. Nephron Clin Pract 110:145-150
[Crossref] [Google Scholar] [PubMed]
- Rimes-Stigare C, Frumento P, Bottai M, Martensson J, Martling CR, et al. (2015) Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care 19:221
[Crossref] [Google Scholar] [PubMed]
- Coca SG, Singanamala S, Parikh CR (2012) Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81:442-448
[Crossref] [Google Scholar] [PubMed]
- Schiffl H, Lang SM, Fischer R (2012) Long-term outcomes of survivors of ICU acute kidney injury requiring renal replacement therapy: A 10-yearS prospective cohort study. Clin Kidney J 5:297-302
[Crossref] [Google Scholar] [PubMed]
- Lai CF, Wu VC, Huang TM, Yeh YC, Wang KC, et al. (2012) Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care 16:1-10
- Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, et al. (2009) Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20:223-228
[Crossref] [Google Scholar] [PubMed]
- Coca SG, Singanamala S, Parikh CR (2012) Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81:442-448
[Crossref] [Google Scholar] [PubMed]
- Gautam SC, Brooks CH, Balogun RA, Xin Wb, Ma JZb, et al. Predictors and outcomes of post-hospitalization dialysis dependent acute kidney injury. Nephron 131:185-190
[Crossref] [Google Scholar] [PubMed]
- Pannu N, James M, Hemmelgarn B, Klarenbach S (2013) Alberta Kidney Disease Network. Association between AKI, recovery of renal function and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8:194-202
[Crossref] [Google Scholar] [PubMed]
- Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS (2017) Recovery after acute kidney injury. Am J Respir Crit Care Med 195:784-791
[Crossref] [Google Scholar] [PubMed]
- Bagshaw SM (2006) Epidemiology of renal recovery after acute renal failure. Curr Opin Crit Care 12:544-550
[Crossref] [Google Scholar] [PubMed]
- Jay L Koyner, et al (2015) Furosemide stress test and biomarkers for the prediction of AKI severity. J Am Soc Nephrol 26:2023-2031
[Crossref] [Google Scholar] [PubMed]
- Marcussen N, Schumann J, Campbell P, Kjellstrand C (1995) Cytodiagnostic urinalysis is very useful in the differential diagnosis of acute renal failure and can predict the severity. Ren Fail 17:721-729
[Crossref] [Google Scholar] [PubMed]
- James MT, Pannu N, Hemmelgarn BR, Austin PC, Tan Z, et al. (2017) Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA 18:1787-1797
[Crossref] [Google Scholar] [PubMed]
- Schentag JJ, Gengo FM, Plaut ME, Danner D, Mangione A, et al. (1979) Urinary casts as an indicator of renal tubular damage in patients receiving aminoglycosides. Antimicrob Agents Chemother 16:468-474
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences