Chronic Voluntary Exercise-Induced Bdnf and Rcan1 is Associated with Improved Cognition and Depressive Mode in Apoe-/- Mice with Obese
Tsugiyasu Kanda1*, Masayuki Mori1, Yuji Kasamaki1, Yasuhiro Kawasaki2 and Nobuo Kato3
1Department of Community Medicine, Kanazawa Medical University, Uchinada, Ishikawa, 920, Japan
2Department of Neuropsychiatry, Kanazawa Medical University, Ishikawa, Japan
3Department of Physiology, Kanazawa Medical University, Ishikawa, Japan
- *Corresponding Author:
- Tsugiyasu Kanda
1-1 Uchinada-machi, Kahoku-gun Ishikawa
920-0293, Japan
Tel: +81762862211
E-mail: kandat@kanazawa-med.ac.jp
Received Date: May 15, 2018 Accepted Date: May 26, 2018 Published Date: June 05, 2018
Citation: Kanda T, Mori M, Kasamaki Y, Kawasaki Y, Kato N (2018) Chronic Voluntary Exercise-Induced BDNF and RCAN1 is Associated with Improved Cognition and Depressive Mode in Apoe-/- Mice with Obese. J Physiother Res Vol. 2 No. 3:8
Abstract
Background: Obesity is associated with cognitive dysfunction and dementia, as well as a reduction in brain-derived neurotrophic factor (BDNF). The calcineurin 1 regulator (RCAN1) regulates neuronal plasticity and depressivefunction by regulating BNDF. Voluntary exercise may improve this condition.
Methods and findings: Voluntary exercise was loaded in apolipoprotein E (ApoE)-/- mice by a high-fat diet (HFE, n=15) over a period of three months. Nonexercising mice with a high-fat diet (HFD, n=15) became overweight. Mice in voluntary exercise group ran on a wheel 5 days/week on the same period, mice without a high-fat diet as a control (NOR, n=15). A Morris water maze, novel object recognition, and forced swimming were evaluated. Chronic exercise significantly reduced body weight and increased the right psoas muscle weight/body weight ratio in the HFE group compared with HFD. Both of BDNF and RCA1 in HFE were expressed to a significantly higher extent in the hippocampus than with HFD. The Morris water maze test, novel object recognition test and forced swimming test were significantly improved in HFE.
Conclusion: Chronic voluntary exercise improved cognitive dysfunction and depressive modes along with increased expressions of BDNF mRNA in the hippocampus and of RCAN1 mRNA in the skeletal muscle.
Obesity; Brain-derived neurotropic factor; Regulator of calcineurin 1; ApoE-/-; Voluntary exercise
Introduction
Obesity is associated with cognitive deficits, depression and dementia, with the reduction of brain-derived neurotrophic factor (BDNF) [1]. Calcineurin 1 regulator (RCAN1) controls neuronal plasticity and depressive states by regulating BNDF [2]. Similarly, RCAN1 expression is expressed in both the nervous system and in skeletal muscle [3].
As voluntary exercise has been suggested to improve neural and skeletal functions [1-4], we hypothesized that chronic voluntary exercise could regulate the BDNF and RCAN1 mRNA in the hippocampus and skeletal muscles, thereby affecting cognitive deficit, depressive mode and physiological adaptation.
Synaptosomal dysfunction is induced in ApoE-/- mice compared to control animals [5]. Moreover, ApoE gene polymorphism is a robust genetic factor for Alzheimer’s disease (AD). The BDNF have a wide distribution and highly presented in the central nervous system and its constitutive expression is particularly high in the hippocampus [6]. Moreover, brain RCAN1 in patients with Down’s syndrome and AD is overexpressed and may be related with the pathogenesis of neurodegeneration [7].
Although BDNF is mainly situated in the brain, recent studies have shown that it is also expressed in non-neurogenic tissues, such as skeletal muscle [8]. Moreover, it is known to have a bearing on memory function and has also been identified as a key component of body mass control and energy homeostasis [9]. Finally, BDNF appears to play a major role not in both central metabolic pathways and energy metabolism in skeletal muscle [8].
Physical exercise is known to improve both cognitive function and depressive mode, with some evidence suggesting that BDNF and RCAN1 may activate these functions [10]. However, it is not yet known whether the effects of chronic voluntary exercise could regulate BDNF and RCAN1 gene expression in hippocampus and skeletal muscle, thereby inducing improvements in cognitive and depressive states. In recent clinical meta-analyses, physical exercise leads to benefits in older people even without dementia, such as improving mood, reducing mortality, and improving function [11].
To assess whether chronic voluntary exercise improves cognitive dysfunction and depressive states in the murine model of AD by regulating the expression of the BDNF and RCAN1 gene in the hippocampus and skeletal muscles, we monitored voluntary movements on a wheel in obese ApoE-/-mice over a threemonths period, and then used the test of Morris water maze, novel object recognition test, and forced swimming test to investigate the effectiveness.
Methods
Animals
Thirty-six female ApoE-/- mice (Charles River, Wilmington, USA) were used. All mice were housed in environmentally controlled conditions. The room temperature was kept at 22°C under a 12 h dark: 12 h light cycle. Drinking water and food were available add libitum. All experiments were followed by the guidelines of Kanazawa Medical University. The high-fat diet comprised 56.7% fat and 25.5% protein, as described previously [12]. All mice were raised in accordance with Animal Care Committee Guidelines of Kanazawa Medical University under pathogen-free conditions.
Methods
Voluntary exercise was loaded in ApoE-/- mice on a high-fat diet (HFE, n=15) for a period of three months. Non-exercising mice with the high-fat diet (HFD, n=15) became overweight. All mice were fed from the age of 8 weeks to 20 weeks. Mice in the voluntary exercise ran over a wheel for 5 days/week over the same period. Non-exercising mice not on a high-fat diet (NOR, n=15) were used as a control. The test of Morris water maze was used to evaluate spatial memories, the novel object recognition test to evaluate recognition memory, and the forced swimming test to evaluate depressive mode. All mice were euthanized using pentobarbital followed by cervical dislocation. The procedures were approved by the Kanazawa Medical University Animal Care Committee Guidelines.
Histological analysis: At the termination, the brain and skeletal muscle were divided and fixed in 10% neutral-buffered formalin. The brains were gently removed and processed as previously described [13]. Histological analyses were performed in ApoE- /- mice of three groups. The morphological fixation process of hippocampus and skeletal muscles were described previously [13]. Hematoxylin and eosin staining was used for histological analyses.
Gene expression analysis: The hippocampus and skeletal muscle were immediately harvested for RNA until the gene expression analysis described previously [13]. These RNA samples of hippocampus and skeletal muscle were performed according to the manufacturer’s protocol (RNeasy Kit; Qiagen, Valencia, CA).
The BDNF and RCAN1 RT-PCR were prepared respectively as follows. PCR analysis was performed using Primers specific for RCAN1 isoform 1 and GAPDH (for normalization) are using a CFX96 Real Time PCR machine, all according to the manufacturer (BioRad, Hercules, CA, UAS). Primer sequences were described elsephere for BDNF, RCAN1 and GAPDH [14,15]. These analyses were examined by the ABI PRISM7700 Sequence Detection System.
Morris water maze test: The use of Morris water maze test is assessed in the learning and memory of the animals. The Morris water maze comprised a plastic cylindrical pool surrounded by the wall of 45 cm tall with a lucent plastic platform. Mice were subjected to four different objects situated above the edge of this pool. We examined 4 sessions of this trial on each five days. Mice were released from the randomly chosen points from 4 prefixed positions. The escape latency was measured as the time-duration consumed to reach the platform. Experiment was ended soon after the mouse founded the platform or the time after 60 s passed. The averages of each day were calculated for each mouse. At the end of the final experiment on the last 5th day, the mice were subjecting to a trial for 60 s with the removal of platform. The examining trajectory was analyzed using SMART as previously mentioned [16].
Novel object recognition test: The use of Novel Object Recognition Test has been assessed to investigate a novel object, spending more time exploring than the familiar one. A cubidshaped box of plastic (44 × 26 × 20 cm) was used. Mice were allowed to search for 3 min, and taken away from the area. After 5 minutes, two same objects were situated on the bottom 10 cm apart. Animals were situated again into the same area and allowed to search for 3 min. Murine movement was analyzed by Panlab SMART video tracking system. The consuming time was measured within 2 cm from the border of a box (A1 and A2). On the second day at a 1 day interval, mice sought the box for 3 min in the presence of a familiar and a novel different-shaped object. We measured the time spent within the 2 cm neighbour in these objects apart from the familiar object (A3) and novel one (A4). The ratio of discrimination index was defined as A4/(A3+A4). The wider discrimination index shows the better retrieval on familiar object experienced 1 day before. This index of 0.5 means no preference for either object. This test is based on the murine habits of spending more time with a novel object than a familiar one [17].
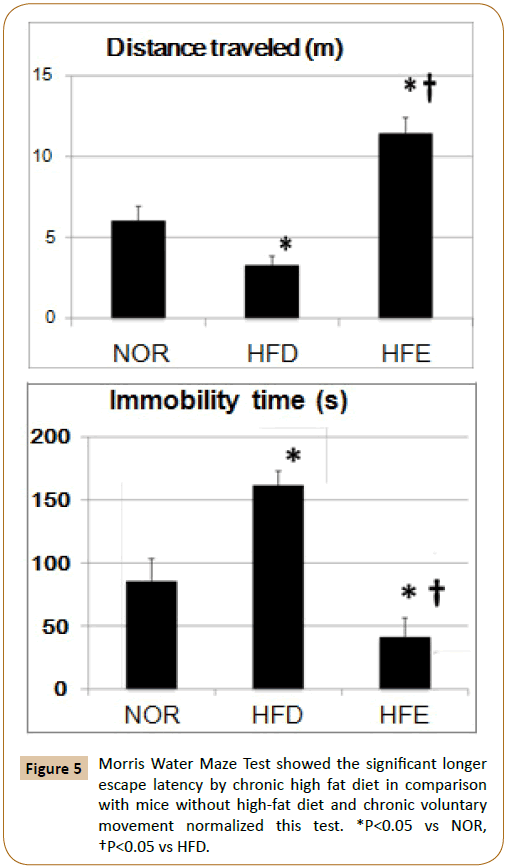
Forced swimming test: For forced swimming, we utilized the test paradigm uniquely designed to test rat behaviour rather than the five-day paradigm previously employed to induce depressive behaviour in mice. The time duration of forced swimming were 10 min daily for two con days in a transparent plastic cylinder (24 cm 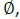 , 60 cm high) filled with water at 25 C in the depth of 25 cm. Data were analysed by ANY-Maze (Stoelting, Wood Dale, IL, USA) [17].
, 60 cm high) filled with water at 25 C in the depth of 25 cm. Data were analysed by ANY-Maze (Stoelting, Wood Dale, IL, USA) [17].
Statistical analysis: Data were recorded as mean ± SED. For comparative analysis, the significant differences in body weight, peripheral biochemical parameters, and gene expressions were calculated using student’s t-test. Comparisons among groups in Morris Water test, Novel object cognition, test of force swimming were performed using one-way ANOVA followed by Fisher's least significant difference analysis. A value of P<0.05 was decided to be statistically significant.
Results
Organ weight
Body weight (BW) of the HFD was significantly heavier than that in the NOR (Table 1). The BW in the HFE group was significantly normalized compared with that in the HFD group. The brain weight in the HFD was statistically lower than that in the NOR. The right psoas muscle weight/body weight ratio was significantly elevated in the HFE compared with the HFD. Liver weight in the HFD group was predominantly heavier than that in the NOR and normalized in the HFE.
| BW (g) | Brain weight (mg) | Heart weight (mg) | Right iliopsoas muscle weight (mg) | Muscle weight/BW | Liver weight (mg) | Liver weight /BW ratio (10- 3) | |
|---|---|---|---|---|---|---|---|
| NOR | 30.4 ± 1.6 | 431 ± 24 | 200 ± 30 | 264 ± 24 | 8.7 ± 0.6 | 1460 ± 330 | 4.8 ± 0.95 |
| HFD | 49.7 ± 2.7* | 409 ± 12* | 220 ± 30 | 244 ± 24 | 4.9 ± 0.8* | 3800 ± 990* | 7.65 ± 1.26* |
| HFE | 36.5 ± 4.7*† | 432 ± 31† | 230 ± 30 | 308 ± 30 | 8.4 ± 0.7† | 2200 ± 450† | 6.03 ± 1.02 |
Abbreviations: NOR, non-exercised mice without high-fat diet: HFD, ApoE-/- mice with high-fat diet: HFE, voluntary exercises were performed in ApoE-/- mice with high-fat diet. Statistically significant differences tested by student t. *P<0.05 vs NOR, †P<0.05 vs HFD.
Table 1: Effects of chronic voluntary movement on body weight (BW) and organ weights in high fat diet ApoE -/- mice.
Laboratory data
Blood sugar levels did not differ among the three groups. In contrast, AST and ALT levels in the HFD were significantly elevated than those in the NOR. The LDL cholesterol levels in the HFD and HFE group were higher than in the NOR group (Table 2).
| Blood (mg/dL) | Sugar AST (U/L) | ALT (U/L) | TG (mg/dL) | LDL-C (mg/dL) | |
|---|---|---|---|---|---|
| NOR | 151 ± 17 | 557 ± 103 | 92 ± 14 | 44 ± 13 | 78 ± 22 |
| HFD | 145 ± 20 | 898 ± 174* | 557 ± 144* | 66 ± 20 | 187 ± 18* |
| HFE | 159 ± 28 | 723 ± 135† | 164 ± 84† | 40 ± 11 | 150 ± 24* |
Abbreviations: NOR, non-exercised mice without high-fat diet:HFD, ApoE-/- mice with high-fat diet: HFE, voluntary exercises were performed in ApoE-/- mice with high-fat diet. AST, aspartate aminotransferase; ALT, alanine aminotransferase; TG: triglyceride; LDL-C: low density lipoprotein cholesterol. Statistically significant differences ww test by student t’test. *P<0.05 vs NOR, †P<0.05 vs HFD.
Table 2: Effects of chronic voluntary movement on Laboratory data in high fat diet ApoE -/- mice.
Histological features
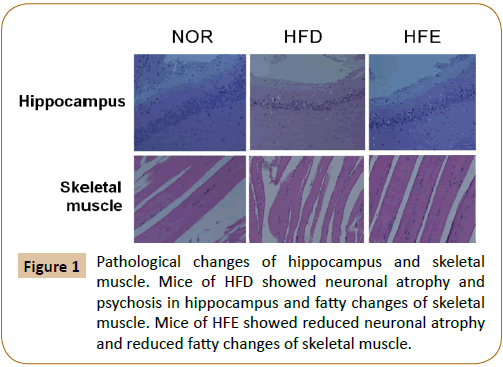
The staining by haematoxylin and eosin showed atrophy the pyknotic neurons in the hippocampus of HFD compared with NOR. Mice in HFE showed reduced pyknotic neurons compared with HFD (Figure 1). Histological examination of the skeletal muscle sections revealed the development of fatty changes in the HFD group, but fatty changes were reduced in HFE mice (Figure 1).
Gene expression
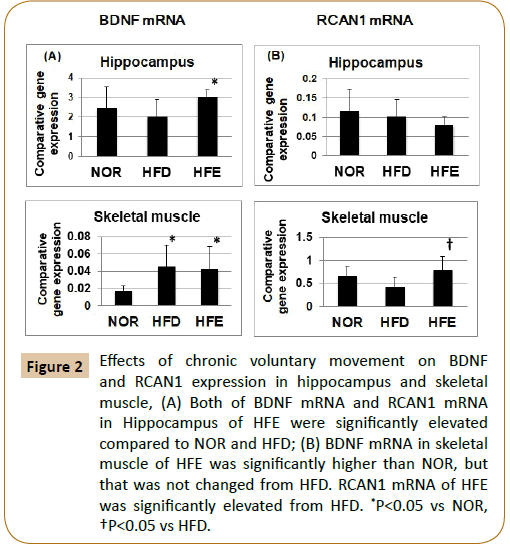
The enhanced expression of BDNF was revealed on the hippocampus and skeletal muscle in the HFE group compared with the HFD group. Similarly, BDNF expression in skeletal muscle was significantly higher in the HFD than in the NOR (Figure 2A). The expression of RCAN1 in the hippocampus did not differ significantly among the three groups, whereas RCAN1 expression in skeletal muscle of the HFE was statistically elevated than that of the HFD (Figure 2B).
Figure 2: Effects of chronic voluntary movement on BDNF and RCAN1 expression in hippocampus and skeletal muscle, (A) Both of BDNF mRNA and RCAN1 mRNA in Hippocampus of HFE were significantly elevated compared to NOR and HFD; (B) BDNF mRNA in skeletal muscle of HFE was significantly higher than NOR, but that was not changed from HFD. RCAN1 mRNA of HFE was significantly elevated from HFD. *P<0.05 vs NOR, †P<0.05 vs HFD.
Morris water maze test
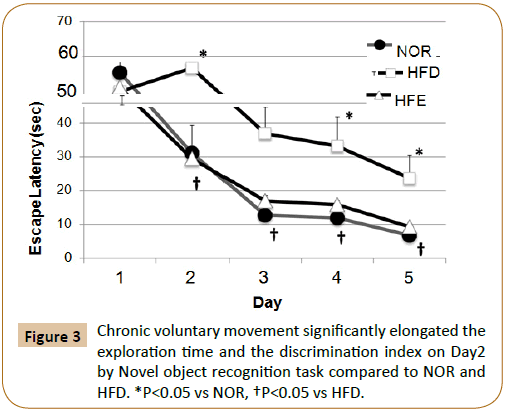
The escape latency for the HFD was statistically significantly decreased than that for the NOR group. Similarly, the results of the Morris water maze test were apparently better for the HFE group than for the HFD group (Figure 3).
Novel object recognition test
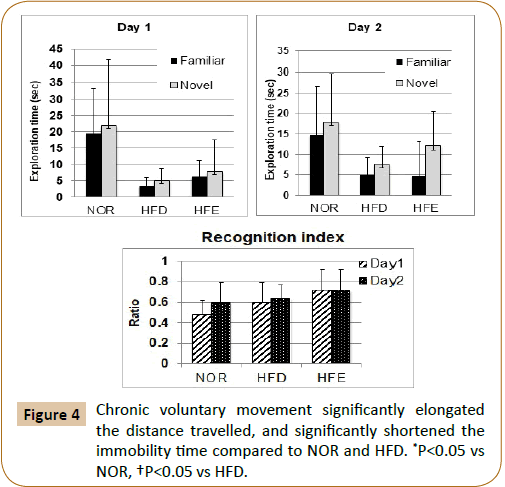
The exploration time for familiar and novel objects varied but did not differ significantly among the three groups on Days 1 and 2. However, the recognition index on Day 1 for the HFE group was significantly increased than that for the NOR group (Figure 4).
Forced swimming test
The distance travelled and immobility time in the HFD group were significantly impaired compared to those in the NOR group. Chronic voluntary movements induced the distance travelled and shortened the immobility time (Figure 5).
Discussion
Our findings suggest that chronic voluntary exercise improves cognitive dysfunction and depressive state, with induced the hippocampal expression of BDNF and RCAN1 mRNA, and increased RCAN1 mRNA expression in the skeletal muscle of obese ApoE-/- mice. They also showed that chronic voluntary exercise reduces body weight, liver weight, liver enzymes, and lipid levels while increasing brain weight and the muscle weight / body weight ratio compared with non-exercising obese mice.
The cells of central nervous system from ApoE-/- mice are more naive to excytotoxic and age-related synaptic loss [18], and synaptic dysfunction induced by Aβ in these mice is also enhanced in the contrast to control mice [5]. The gene dose of APOE ε 4 is a dominant risk factor for AD, with the association to age at onset [19], and cognitive decline [20]. Indeed, the APOE polymorphism is one of the strongest and most robust genetic risk factors forts the late-onset of AD.
Obesity reduces cognitive and motor function and suppresses brain plasticity [21]. The high-fat diet increases oxidative stress in both the brain and in other organs such as skeletal muscle and reduces BDNF, which is known to mediate the effect of obesity on cognition and behavior in the hippocampus [22]. We have shown that chronic voluntary movement reduces obesity and induces BDNF gene expression in the hippocampus and skeletal muscle. The BDNF gene expression induced in skeletal muscle of obese mice could not be explained clearly, although increased oxidative stress in muscle may induce the BDNF gene as an adaptive mechanism.
BDNF has been implied to neural growth and function, including neurogenesis, dendritic degeneration and long-term duration of neuronal function, since animal models provides the constant evidence for exercise-induced expression of BDNF mRNA. Therefore, physical exercise may be a successful strategy for promoting mood or cognition due to its ability to enhance BDNF activity.
Chronic exercise by high intensity alters the induction of BDNF mRNA via the muscle phenotype, reducing the protein levels of BDNF in fast muscles while increasing genetic expressions of BDNF in slow muscles [23]. BDNF is reported to act in an autocrine and paracrine fashion in skeletal muscle for fat oxidation. In the sedentary state, BDNF expression is affected by muscle phenotype, with high-intensity chronic exercise reducing BDNF protein levels in the fast muscles and increasing BDNF mRNA levels in the slow muscles [23]. Muscle-derived BDNF plays an important role in muscle repair, regeneration, and differentiation and our data show that exercise induces BDNF mRNA expression in both the hippocampus and in skeletal muscle, which could have a beneficial effect on recognitive function and anti-depression [24].
Elevated RCAN1 levels lasting just several hours can be neuroprotective under acute stress conditions, such as acute oxidative stress. Long-term overexpression of RCAN1 gene may help to understand the mechanism of neuro degeneration in diseases such as AD and Down’s syndrome [25]. RCAN1, which is reported to be a physiological modulator of oxidative stress, is regulated in the skeletal muscles after exhaustive physical exercise, which increases RCAN1 protein levels in the gastrocnemius. Indeed, protein oxidation, an index of oxidative stress, has been shown to be increased in the gastrocnemius. Induced expression of RCAN1 may regulate represent important components of the physiological adaptation to exercise-induced oxidative stress [4]. We showed increased expression of RCAN1 in the gastrocnemius skeletal muscle after chronic exercise but no change of RCAN1 gene expression in the hippocampus. Although the role of RCAN1 in the hippocampus after chronic exercise remains unclear, chronic exercise could play an important role in skeletal muscle adaptation in obese animals [26].
Both of Morris water maze test and novel object recognition test were improved by chronic exercise in obese ApoE-/- mice [27]. Similarly, chronic exercise was also beneficial in the forced swimming test, as an indicator of depressive condition. The improvement of cognition and mood is associated with BDNF activity. Moreover, chronic consumption of high-fat food and obesity induces plasticity-related changes in reward circuitry that are related with a depressive phenotype, with an alternation in BDNF activity beings implicated in depressive behaviour [5-7]. Chronic exercise in obesity may influence cognition and mood by inducing the expression of BDNF and RCAN1 gene in the hippocampus, and RCAN1 expression in skeletal muscle.
The limitations of this study were the three months period of chronic exercise and the examination of only one skeletal muscle [28]. A longer duration of exercise may alter the gene expression and recognition test results. Similarly, other skeletal muscles may need to be examined to clarify the different effects in slow and fast muscle. As such, further studies are required in this field.
Acknowledgements
This work was supported by the Grant for Strategic Research Project (H2015-16), Kanazawa Medical University and Ministry of Education, Culture, Sports, Science and Technology and Grant-in- Aid for Scientific Research (C), Japan (No. 17K09330).
References
- Pedersen BK, Febbraio MA (2012) Muscles exercise and obesity skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457-465.
- Hoeffer CA, Wong H, Cain P, Levenga J, Cowansage KK, et al. (2013) Regulator of calcineurin 1 modulates expression of innate anxiety and anxiogenic responses to selective serotonin reuptake inhibitor treatment. J Neurosci 33: 16930-16944.
- Emrani R, Rébillard A, Lefeuvre L, Gratas-Delamarche A, Davies KJ, et al. (2015) The calcineurin antagonist RCAN1-4 is induced by exhaustive exercise in rat skeletal muscle. Free Radic Biol Med 87: 290-299.
- Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, et al. (2004) Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience 123: 429-440.
- Hoeffer CA, Wong H, Cain P, Levenga J, Cowansage KK, et al. (2013) Regulator of calcineurin 1 modulates expression of innate anxiety and anxiogenic responses to selective serotonin reuptake inhibitor treatment. J Neurosci 43: 16930-16944.
- Patel A, Uamashita N, Ascano M (2015) RCAN1 links impaired neurotrophin trafficking to aberrant development of the sympathetic nervous system in Down syndrome. Nat Commun 8: 207-213.
- Emrani R, Rébillard A, Lefeuvre L, Gratas-Delamarche A, Davies KJ, et al. (2015) The calcineurin antagonist RCAN1-4 is induced by exhaustive exercise in rat skeletal muscle. Free Radic Biol Med 87: 290-299.
- Keller JN, Lauderback CM, Butterfield DA, Kindy MS, Yu J, et al. (2002) Amyloid beta-peptide effects on synaptosomes from apolipoprotein E-deficient mice. J Neurochem 74: 1579-1586.
- Paillard T, Rolland Y, de Souto Barreto P (2015) Protective Effects of Physical Exercise in Alzheimer's Disease and Parkinson's Disease. J Clin Neurol 11: 212-219.
- Wong H, Levenga J, Cain P, Rothermel B, Klann E, et al. (2015) RCAN1 overexpression promotes age-dependent mitochondrial dysregulation related to neurodegeneration in Alzheimer's disease. Acta Neuropatholgica 130: 829-843.
- Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, et al. (2009) Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 52: 1409-1418.
- Wisse BE, Schwartz MW (2003) The skinny on neurotrophins. Nat Neurosci 6: 655-656.
- Erickson KI, Miller DL, Roecklein KA (2011) The aging hippocampus: Interactions between exercise, depression, and BDNF. Neuroscientist 18: 82-97.
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, et al. (2017) Dementia prevention, intervention, and care. The Lancet Dec 390: 2673-2734.
- Winzell MS, Ahren B (2004) The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53: 215-219.
- Chen R, Moriya J, Yamakawa J, Takahashi T, Li Q, et al. (2008) Brain atrophy in a murine model of chronic fatigue syndrome and beneficial effect of Hochu-ekki-to (TJ-41). Neurochem Res 33: 1759-1767.
- Naumenko VS, Kulikov AV, Kondaurova EM, Tsybko AS, Kulikova EA, et al. (2015) Effect of actual long-term spaceflight on BDNF, TrkB, p75, BAX and BCL-XL genes expression in mouse brain regions. Neuroscience 284: 730-736.
- Zmijewski PA, Gao LY, Saxena AR, Chavannes NK, Hushmendy SF, et al. (2015) Fish oil improves gene targets of Down syndrome in C57BL and BALB/c mice. Nutr Res 35: 440-448.
- Shui T, Wang L, Luo X, Uchiumi O, Yamamoto R, et al. (2015) Homer1a disruption increases vulnerability to predictable subtle stress normally sub-threshold for behavioral changes. Brain Res 1605: 70-75.
- Wang M, Uchiumi O, Ogiso H, Shui Y, Zou J, et al. (2017) Stressful learning paradigm precludes manifestation of cognitive ability in sphingomyelin synthase-2 knockout mice. Behav Brain Res 319: 25-30.
- Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, et al. (1999) Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: isoform-specific effects on neurodegeneration. J Neurosci 19: 4867-4880.
- Chase G, Meyers D, Albert MS, Tanzi R, Harrell LE, et al. (1997) ApoE-4 and Age at onset of Alzheimer’s disease: the NIMH genetics initiative. Neuro 48: 139-147.
- Martins CA, Oulhaj A, De Jager CA, Williams JH (2005) APOE alleles predict the rate of cognitive decline in Alzheimer disease: A nonlinear model. Neuro 12: 1888-1893.
- Wang C, Chan J, Ren J, Yan JH (2016) Obesity Reduces Cognitive and Motor Functions across the Lifespan. Neural Plast 16: 2484-2498.
- Park HR, Park M, Choi J, Park KY, Lee J, et al. (2010) The high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett 482: 235-239.
- Maldonado JA, Cerna-Cortes J, Castro-Rodriguez EM, Montero SA, Muniz J, et al. (2016) Effects of moderate-and high-intensity chronic exercise on brain-derived neurotrophic factor expression in fast and slow muscles. Muscle Nerve 53: 446-451.
- Roh SG, Suzuki Y, Gotoh T, Tatsumi R, Katoh K (2016) Physiological Roles of Adipokines, Hepatokines, and Myokines in Ruminants. Asian-Australas J Anim Sci 1: 1-15.
- Ermak G, Davies KJ (2013) Chronic high levels of the RCAN1-1 protein may promote neurodegeneration and Alzheimer disease. Free Radic Bio Med 62: 47-51.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences