Change in Formulary from Plain to Liposomal Bupivacaine: Retrospective Analysis of Impact on Postsurgical Pain
Jose Zeballos1#, Megan E Patton1#, David Sansonetti2, Marjan Sadegh2, Namrata Patil3, Mieke A Soens1 and Kristin L Schreiber1*
1Department of Anesthesiology Perioperative and Pain Medicine, Brigham and Women’s Hospital, Boston, MA, USA
2Department of Pharmacy, Brigham and Women’s Hospital, 75 Francis St., Boston, MA, USA
3Division of Thoracic Surgery, Brigham and Women’s Hospital, 75 Francis St., Boston, MA, USA
*Corresponding Author:
Kristin L Schreiber
Brigham and Women’s Hospital
75 Francis St., Boston, MA
USA
Tel: 612-205-0186
E-mail: klschreiber@bwh.harvard.edu
Received Date: January 10, 2019; Accepted Date: February 03, 2020; Published Date: February 10, 2020
Citation: Zeballos J, Patton ME, Sansonetti D, Sadegh M, Patil N, et al. (2020) Change in Formulary from Plain to Liposomal Bupivacaine: Retrospective Analysis of Impact on Postsurgical Pain. J Pharma Prac Edu Vol.3 No.1. DOI: 10.36648/pharmacypractice. 3.1.20
Copyright: © Zeballos J, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Excellent postsurgical pain management impacts the comfort and respiratory function of thoracic surgical patients in the time immediately following surgery, and is associated with lower rates of persistent postsurgical pain. Given the negative sequalae associated with perioperative opioid exposure, and the time pressure diminishing enthusiasm for regional anesthesia, interest in extended release formulations of local anesthetics that can be employed by surgeons has grown, and increasingly these drugs are being employed in clinical practice. However, few careful studies have estimated the pharmacodynamics of the dosages being employed in practical use.
Objective: To compare the effects of plain versus liposomal bupivacaine on postoperative pain and opioid requirements in adult surgical patients undergoing Video Assisted Thoracoscopic Surgery (VATS).
Methods: This was an IRB-approved, retrospective, matched cohort study at a large academic tertiary care hospital, which followed a change in the OR formulary when liposomal bupivacaine was made available, resulting in a rapid change in practice. Patients undergoing VATS (n=112), received surgeon-placed intercostal and wound infiltration with 0.25% bupivacaine or 0.25% bupivacaine+liposomal bupivacaine mixture. Average and highest pain scores and opioid consumption, as well as demographic, surgical, medical and anesthetic variables were extracted from the electronic medical record for distinct postoperative epochs (recovery and each postoperative day (POD) 0-3).
Results: Repeated measures ANOVA revealed no overall difference in postoperative opioid consumption between patients who received liposomal vs plain bupivacaine. A significant time by treatment interaction revealed possible higher consumption in patients receiving liposomal bupivacaine in PACU, but lower on POD0 and 1, and no difference at later time points. At the same time, however, these patients reported higher average and highest pain scores across the postoperative period.
Conclusion: The introduction of liposomal bupivacaine was not associated with reduced overall postoperative opioid consumption, but was associated with higher pain scores, raising questions about the appropriate dosing, timing, and efficacy of its use for postoperative analgesia in VATS patients.
Keywords
Liposomal bupivacaine; Postoperative pain; Opioids; Video assisted thoracoscopic surgery; Exparel
Introduction
Postoperative pain after thoracic surgery is challenging to manage [1]. The use of local anesthetics in regional anesthesia (RA), including epidural or paravertebral block, has often been used to reduce pain, and is associated with decreased cardiac and respiratory complications, as well as postoperative opioid administration [2], but requires time, resources, and carries some procedural risk. Because Video Assisted Thoracoscopic Surgery (VATS) involves a smaller incision than thoracotomy [3,4], patients undergoing VATS are often not offered RA. However, VATS patients still bear a considerable burden of postsurgical pain, with up to 60% developing chronic pain [5,6]. In the absence of RA, opioids may provide analgesia, but are associated with respiratory depression, sedation, nausea, constipation, opioidinduced hyperalgesia, and risk of long-term opioid dependence and misuse.
An alternative to RA and opioids is a longstanding practice of infiltration by surgeons of local anesthetics (LA) (most commonly bupivacaine) at the wound site. Since postoperative pain typically extends beyond 6-8 hours, there has been considerable interest in extending the duration of LA analgesia with adjuvants including epinephrine, dexamethasone, and clonidine.
Formulations of local anesthetics, such as liposomal bupivacaine (LipoB) (Exparel, Pacira Pharmaceuticals, Inc., Parsippany, NJ, USA), have promised a longer lasting block. When used for local infiltration, studies have shown plasma bupivacaine concentrations peaks at 1 and 12-36 hours, with detectable levels up to 72 hours after administration [7]. This detection of bupivacaine in blood at 72 hours has formed the common assumption of a prolonged duration of effect to this time, despite the fact that systemic detection may not correlate with efficacy at the same timepoints [8].
ERAS protocols, which often include use of LA, and increasingly, use of LipoB, have generally improved outcomes for many surgical patients [9], although protocol and adherence vary considerably between institutions and surgical services [10]. While these protocols often include LA or RA, it is difficult to assess the efficacy of individual ERAS elements, such as LipoB, as they are typically introduced simultaneously [11]. Ethical and practical considerations often preclude a more controlled and definitive comparison, which the randomization of patients to old and new regimens would afford. However, careful matching of patient groups immediately before and after addition of a single element to a protocol may allow meaningful investigation of that element.
Circumstances converged at our institution allowing for a pragmatic investigation of the effect of LipoB on postoperative outcomes in thoracoscopy patients. We compared postoperative pain scores and opioid utilization between matched groups of patients before and after a change in practice whereby the injectate used for VATS patients changed from plain bupivacaine to bupivacaine plus liposomal bupivacaine (LipoB) mixture, to test the hypothesis that patients treated with LipoB have decreased pain and opioid use in the early postoperative period (Post-Operative Day (POD) 0-3).
Materials and Methods
A retrospective chart review was performed at Brigham & Women’s Hospital, a 793-bed tertiary academic teaching hospital. After obtaining Institutional Review Board approval, 56 sequential patients were identified who had received LipoB during VATS between 3/2018 and 6/2018, immediately following its increased availability. An age- and sex-matched cohort of control patients who received 0.25% bupivacaine, were selected from an electronic medical record-generated list of patients who underwent VATS during the time period 12/2017 and 4/2018, immediately preceding and partially overlapping with the increased availability of LipoB. Precise sites of injection varied somewhat according to surgeons, but in all cases included a combination of segmental (intercostal) and wound infiltration sites, and injection technique did not change during this time period.
All patients received general anesthesia with neuromuscular blockade and mechanical ventilation with lung isolation. Anesthesia was maintained using desflurane, and intraoperative opioids were given to the majority of patients (Table 1). At the end of surgery, muscle relaxation was antagonized, the trachea was extubated and patients were taken to recovery. Per hospital policy, LipoB patients wore an identifying bracelet to prevent further administration of LA. Other perioperative analgesic use was recorded for comparison between groups.
| Patient characteristics | Bupi | LipoB | p |
|---|---|---|---|
| N | 56 (50%) | 56 (50%) | |
| Age | 54.5 (47.0-62.8) | 55.0 (46.0-62.0) | 0.998 |
| Sex | 0.849 | ||
| Male | 24 (42.9%) | 25 (44.6%) | |
| Female | 32 (57.1%) | 31 (55.4%) | |
| BMI normal | 27.8 (23.6-31.8) | 27.9 (24.1-30.3) | |
| ASA | 0.701 | ||
| II | 7 (12.5%) | 7 (12.5%) | |
| III | 47 (83.9%) | 45 (80.4) | |
| IV | 2 (3.6%) | 4 (7.1%) | |
| Estimated Blood Loss (mL) | 25.0 (16.3-65.0) | 30.0 (20.0-85.0) | 0.6 |
| Surgical Duration (min) | 92.0 (59.0-131.3) | 95.5 (59.5-148.0) | 0.513 |
| Length of Stay (hours) | 54.0 (33.0-58.0) | 52.8 (34.0-81.5) | 0.759 |
| Surgical Classification | 0.501 | ||
| Wedge resection | 34 (60.7%) | 26 (46.4%) | |
| Lobectomy | 5 (8.9%) | 13 (23.2%) | |
| Segmentectomy | 6 (10.7%) | 5 (8.9%) | |
| Lung Biopsy | 5 (8.9%) | 6 (10.7) | |
| Pleurodesis/Decortication | 2 (3.6%) | 1 (1.8%) | |
| Mediastinal Mass | 2 (3.6%) | 2 (3.6%) | |
| Other | 2 (3.6%) | 3 (5.4%) | |
| Anesthetic Variables | |||
| Local Anesthetic Vol. (mL) | 30.0 (20.0-35.8) | 40.0 (33.5-40.0) | *0.000 |
| mg of “free” bupivacaine in mixture | 75.0 (50.0-89.375) | 50.0 (41.875-50.0) | *0.000 |
| Total mg of bupivacaine | 75.0 [50.0-89.38] | 316.0 [264.65-316.0] | *0.000 |
| Intraoperative medications | |||
| Fentanyl | 52 (92.9%) | 38 (67.9%) | *0.001 |
| Dilaudid | 51 (91.1%) | 32 (57.1%) | *0.000 |
| Remifentanil | 4 (7.1%) | 4 (8.9%) | 0.728 |
| Ketamine | 1 (1.8%) | 0 (0%) | 0.315 |
| Midazolam | 47 (83.9%) | 35 (62.5%) | *0.010 |
| Decadron | 23 (41.1%) | 18 (32.1%) | 0.327 |
| Lidocaine | 0 (0%) | 12 (21.4%) | *0.000 |
| Morphine | 1 (1.8%) | 0 (0%) | 0.315 |
| Sufentanil | 5 (8.9%) | 24 (42.9%) | *0.000 |
| Precedex | 3 (5.4%) | 2 (3.6%) | 0.647 |
| Methadone | 1 (1.8%) | 0 (0%) | 0.315 |
| Postoperative Medications | |||
| Acetaminophen | 52 (92.9%) | 53 (94.6%) | 0.696 |
| Ibuprofen | 19 (33.9%) | 21 (37.5%) | 0.693 |
| Ketorolac | 36 (64.3%) | 35 (62.5%) | 0.844 |
| Tramadol | 3 (5.4%) | 6 (10.7%) | 0.297 |
Table 1: Patient Surgical and Anesthetic Characteristics.
Nursing-reported pain scores and opioid and non-opioid analgesic administration were extracted from the electronic medical record for the following postoperative epochs: end of procedure-Post Anesthesia Care Unit(PACU) discharge, PACU discharge-midnight POD0, midnight day of surgery until following midnight, or discharge (POD1), midnight (POD1) until following midnight, or discharge (POD2), midnight POD2 until following midnight, or discharge (POD3). The number of hours in each epoch and the number of pain scores reported was also recorded, and an average morphine mg equivalents/hour was calculated for each epoch, using standard conversion values (Table 2) (Appendix). Average and maximum pain scores were also determined for each epoch.
| Opioid | Oral MME Conversion Factor |
|---|---|
| IV Hydromorphone (mg) | 20 |
| IV Fentanyl (mcg) | 0.3 |
| IV Morphine (mg) | 3 |
| Oxycodone PO (mg) | 1.49 |
| Percocet PO (mg) | 1.49 |
| Oxycontin PO (mg) | 1.49 |
| Hydrocodone PO (mg) | 1 |
| Vicodin PO (mg) | 1 |
| Hydromorphone PO (mg) | 4 |
| Tramadol PO (mg) | 0.1 |
Table 2: Opioid conversions. Statistical analysis
Patient characteristics were summarized using frequencies and percentages for categorical variables, and median values with interquartile ranges (Q1–Q3) for continuous variables. Betweengroup comparisons were made for categorical variables using Chisquared tests. For group comparisons of continuous variables, Mann-Whitney U or independent samples t-tests were used, as appropriate. Repeated measures ANOVA was used to assess group differences in pain scores and opioid consumption over multiple time points. The level of significance was set at p<0.05. All statistical analyses were performed with SPSS Version 25 (IBM, Armonk, NY, USA). The sample size (n=112) was chosen based on a convenience sample of VATS patients who has been treated with LipoB at the time of data extraction, with an age- and sexmatched sample of 56 additional patients who received plain bupivacaine only.
Results
Patient demographics
There were no significant group differences in BMI, ASA class, or surgical characteristics, including type, duration, and estimated blood loss (Table 1) between age- and sex-matched patients undergoing VATS who received either plain bupivacaine (n=56) or LipoB (n=56). However, LipoB patients received a statistically significant overall higher volume of LA, as well as a higher total dose of bupivacaine (mg) (Table 1). Patients received either plain bupivacaine or LipoB mixed with bupivacaine.
Postoperative opioid consumption
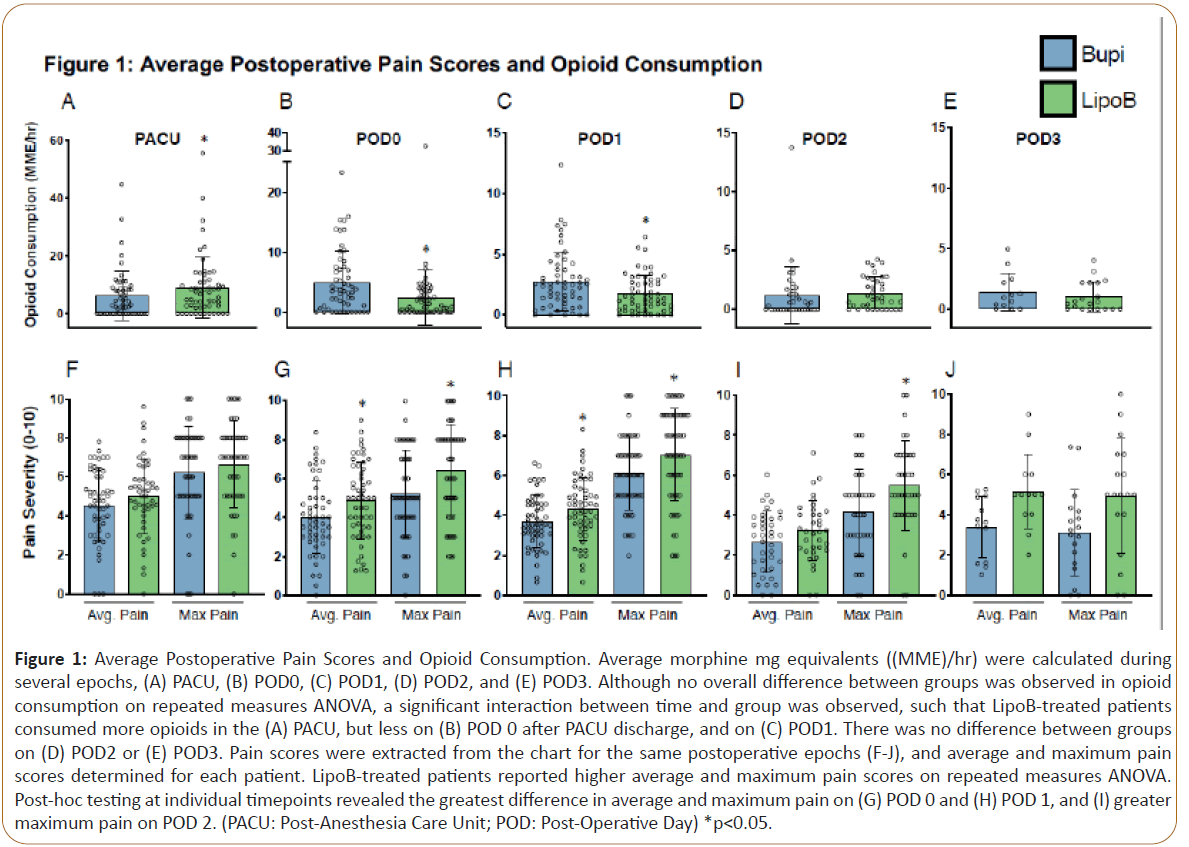
Average morphine mg equivalents ((MME)/hr) were calculated during several epochs (PACU, POD 0, POD1, POD2, POD3). To examine differences in opioid consumption between groups receiving 0.25% bupivacaine (plain bupivacaine) or 0.25% bupivacaine+ liposomal bupivacaine mixture (LipoB), a repeated measures ANOVA analysis was performed. This revealed a significant main effect for time, (F= 22.4, p<0.001), such that a higher MME/hr was administered closer to surgery (Figures 1A-E). However, there was no main effect for group, (F=0.073, p=0.778). A significant time by group interaction was observed, (F=5.7, p=0.007), with secondary analysis of group differences at each timepoint revealing that patients treated with LipoB received significantly greater MME/hr in the PACU than those treated with plain bupivacaine (Figure 1A). Conversely, in the period after PACU discharge on POD0 (Figure 1B), as well as on POD1 (Figure 1C), LipoB-treated patients received significantly lower MME/hr than plain bupivacaine-treated patients. 11. Importantly, no difference in MME/hr was observed on POD2 (Figure 1D) or POD3 between the groups (Figure 1E).
Figure 1: Average Postoperative Pain Scores and Opioid Consumption. Average morphine mg equivalents ((MME)/hr) were calculated during several epochs, (A) PACU, (B) POD0, (C) POD1, (D) POD2, and (E) POD3. Although no overall difference between groups was observed in opioid consumption on repeated measures ANOVA, a significant interaction between time and group was observed, such that LipoB-treated patients consumed more opioids in the (A) PACU, but less on (B) POD 0 after PACU discharge, and on (C) POD1. There was no difference between groups on (D) POD2 or (E) POD3. Pain scores were extracted from the chart for the same postoperative epochs (F-J), and average and maximum pain scores determined for each patient. LipoB-treated patients reported higher average and maximum pain scores on repeated measures ANOVA. Post-hoc testing at individual timepoints revealed the greatest difference in average and maximum pain on (G) POD 0 and (H) POD 1, and (I) greater maximum pain on POD 2. (PACU: Post-Anesthesia Care Unit; POD: Post-Operative Day) *p<0.05.
Postoperative pain
Pain scores were extracted from the chart for the same postoperative epochs (PACU, POD0, POD1, POD2, POD3), and average and maximum pain scores determined for each patient. To examine differences in postoperative pain scores between groups receiving plain bupivacaine and LipoB, a repeated measures ANOVA analysis was performed for both average and maximum pain scores. For average and maximum pain scores, a significant main effect of time was found (F= 8.7, p<0.001;F= 8.9, p=0.046), with higher pain scores being reported closer to surgery (Figures 1F-J). There was also a main effect for group, such that higher average pain scores (F=5.2, p=0.026) and higher maximal pain scores (F=7.5, p=0.007) were reported in the LipoB group. Secondary analysis investigating group differences at individual timepoints revealed no difference in PACU pain scores (Figure 1F). However, in the period after PACU discharge on POD0 (Figure 1G), as well as on POD1 (Figure 1H), LipoB-treated patients reported significantly higher average and maximum pain scores. On POD2, higher maximum pain was reported by LipoBtreated patients, but no significant difference in average pain was observed (Figure 1I). Average and maximum pain scores were not different between groups on POD3 (Figure 1J).
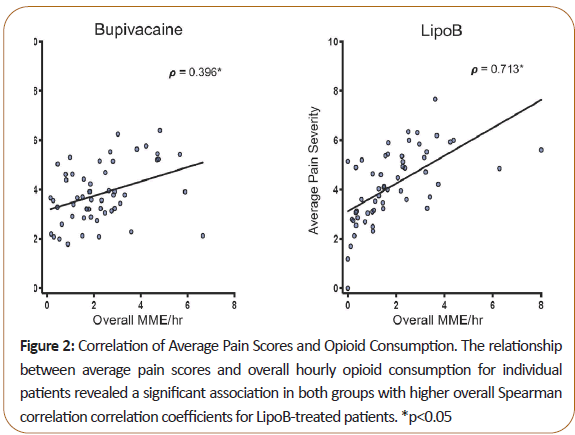
Both average and maximum pain scores positively correlated with opioid MME, indicating that overall, patients with higher pain scores received more PRN opioids. Spearman correlation coefficient was higher in the LipoB than the plain bupivacaine group (Figure 2).
Figure 2: Correlation of Average Pain Scores and Opioid Consumption. The relationship between average pain scores and overall hourly opioid consumption for individual patients revealed a significant association in both groups with higher overall Spearman correlation correlation coefficients for LipoB-treated patients. *p<0.05
Use of intraoperative fentanyl and hydromorphone was greater in patients receiving plain bupivacaine, while sufentanil, IV lidocaine, and midazolam use was greater in patients receiving LipoB. No difference in remifentanil use was observed between groups (Table 1). Other postoperative analgesic adjuvant medication use, including acetaminophen, ibuprofen, ketorolac, or tramadol, was not different between groups. There was no difference in length of stay between groups (54.0 vs. 52.8 hours) (Table 1).
Discussion
The findings of this study, in combination with the widespread use of LipoB, raise important questions about the pharmacodynamics and pharmacokinetics of the long acting LipoB. While previous pharmacokinetic analysis of LipoB has reported elevated plasma concentration at relatively later timepoints (48-72 hours), it is also important to consider whether this is associated with a pharmacodynamic effect (analgesia) at the intended site of action (intercostal nerves). This pragmatic retrospective observational study compared opioid consumption and pain scores before and after a switch to the use of liposomal bupivacaine (LipoB) in thoracic surgery patients. The impact of this change was mixed. While there was no overall difference in postoperative opioid consumption between groups, a significant time x treatment effect was found, revealing higher opioid consumption in the PACU for LipoB-treated patients, but lower opioid consumption in these same patients on POD 0 and 1. Interestingly, opioid consumption was not different on POD 2 or 3, timepoints where one would expect a greater benefit of LipoB based on its reported pharmacokinetics. Importantly, at the same time, patients receiving LipoB reported higher overall average and maximum pain scores compared to patients receiving bupivacaine alone. Secondary analysis at individual timepoints revealed that this effect was most pronounced on POD 0 and 1, the same timepoints when opioid consumption was decreased in the LipoB group. Taken together, these findings raise questions about the timing and efficacy of analgesia offered by LipoB, supporting an argument for further study (RCTs) before its wholesale adoption to ERAS protocols.
More severe acute postoperative pain is associated with a higher incidence of chronic post-thoracotomy pain [12]. This relationship has led to a significant focus on reducing acute pain. While VATS is a less invasive surgery, thought to be associated with less postoperative pain, it may still be associated with significant intercostal nerve injury due to manipulation of the scopes during the procedure and compression of the nerve against the adjacent rib [6-13], and often includes ongoing postoperative nociceptive stimulation in the form of a chest tube [14]. While a blinded RCT comparing VATS to anterolateral thoracotomy revealed that VATS was associated with less acute and chronic postoperative pain [15], others found no difference in persistent postsurgical pain at 3 and 6 months between patients undergoing VATS versus thoracotomy [16].
The gold standard for post-thoracotomy analgesia is thoracic epidural analgesia, although thoracic paravertebral blocks may provide comparable postoperative analgesia [12-17]. Analgesic efficacy of intercostal nerve blocks is likely more limited, with most studies finding pain scores and opioid consumption to be higher with intercostal, compared to epidural, nerve blocks [12-18]. With increased numbers of patients requiring anticoagulation, more distal blockade is favored in order to avoid the neuraxis. Intercostal nerve block and local wound infiltration by surgeons with bupivacaine is common, but duration of block at the intercostal site is shorter, as LA is absorbed faster at this more vascularized area.Thus understanding the extent to which formulations like LipoB may extend this, is a question that has great clinically relevance to postoperative analgesia.
While recent interest in the use of LipoB has led to its widespread use, no large randomized studies are available to confirm its purported benefit to a comparable treatment. The current study observed that LipoB was not associated with decreased overall opioid consumption, but was associated with greater overall pain, consistent with some previous reports, which show no improvement in LOS or pain scores, and relatively limited decreases in opioid use, usually confined to the first 24 hrs [19,20]. In contrast, one small retrospective study comparing LipoB infiltration to thoracic epidural observed no significant difference in pain and opioid consumption for VATS patients [21]. However, this and similar previous studies are limited by small sample size, poorly matched groups, or by lack of comparison to a clinically relevant control (i.e. comparing LipoB to saline or a very low dose of bupivacaine) [22-24].
The pharmacologic effects of LAs depends on the proportion of ionized to non-ionized form which is determined by the pKa of the LA and PH of environment, with potency and onset increasing with a non-ionized state, favoring membrane permeability. At physiological pH (7.4), bupivacaine, with pKa of 8.1, favors its ionized form. The lower pH of inflamed tissue at the surgical site further favors an ionized form, thus decreasing membrane diffusion and delaying onset and efficacy [25,26]. The ionized form also allows protein binding, further reducing the free fraction (5.4% preoperatively to 2.7% in one report) [27]. The LipoB formulation contains a novel excipient, Dieurrocoylphosphotidylcholine (DEPC), the erosion and/or reorganization of which results in bupivacaine release. However, the byproduct, phosphatidylcholine, is metabolized to fatty acids, which further lower pH, acidity; potentially further reducing non-ionized bupivacaine [25-28]. Another pharmacological consideration is that by mixing LipoB 1:1 with 0.25% bupivacaine (per manufacturer recommendations), the effective concentration of “free bupivacaine” is reduced to 0.125%, thus potentially resulting in less effective blockade at earlier timepoints.
There are several important limitations to this study related to its exploratory, pragmatic nature. There were differences between groups in the intraoperative opioids received (fentanyl and hydromorphone in plain bupivacaine vs sufentanil in LipoB group), which may have impacted pain in the early postoperative period. While this may explain why the LipoB group received significantly more opioids in PACU, it is unlikely to explain higher pain scores seen on POD 1 and 2. Second, the effective concentration of free bupivacaine was lower in the LipoB group (despite a higher total dose of bupivacaine), possibly accounting for higher pain scores seen in these patients at earlier timepoints, and raising a question of the common practice of mixing LipoB with 0.25% bupivacaine. Third, because of the lack of blinding (identifying bracelet worn for 5 days), systematic differences in the postoperative treatment of patients receiving LipoB must be considered as a source for decreased PRN opioid administration, and thus higher pain scores. Appropriately, there was a correlation between reported pain scores and opioid administration, but a somewhat stronger association in the LipoB group (Figure 2), perhaps suggesting that clinical providers were more stringent in their administration of opioids in this group, matching it more exactly to pain score.
Conclusion
In summary, this pragmatic study comparing plain bupivacaine to LipoB in VATS patients indicated very little benefit in terms of reduced opioid consumption (limited to a timeframe <24 hours after surgery), which was accompanied by an overall increase in average and maximal pain scores. This and other previous reports call into question the widespread use of LipoB as a superior modality to plain bupivacaine, or as an equivalent alternative to RA. Although this study compared matched samples of patients, a randomized, controlled study comparing LipoB to other analgesic modalities is needed to confirm these findings.
Acknowledgements
The authors would like to thank the surgical, anesthetic, and pharmacy teams for their cooperation in informing the design and conduct of this study. This research was not supported by any specific grant from funding agencies in the public, commercial or non-for-profit sectors.
Conflict of Interests
The authors have no conflicts of interest to disclose.
References
- Bayman EO, Brennan TJ (2014) Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: Meta-analysis. J Pain. 15:887-897.
- Popping DM, Elia N, Van Aken HK, Marret E, Schug SA, et al. (2014) Impact of epidural analgesia on mortality and morbidity after surgery: Systematic review and meta-analysis of randomized controlled trials. Ann Surg. 259:1056-1067.
- McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: Experience with 1,100 cases. The Annals of Thoracic Surgery 1;81:421-426.
- Nagahiro I, Andou A, Aoe M, Sano Y, Date H, et al. (2001) Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: A comparison of VATS and conventional procedure. Ann Thorac Surg. 72:362-365.
- Katz J, Jackson M, Kavanagh BP, Sandler AN (1996) Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 12:50-55.
- Wildgaard K, Ravn J, Kehlet H (2009) Chronic post-thoracotomy pain: A critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg. 36:170-180.
- Hu D, Onel E, Singla N, Kramer WG, Hadzic A (2013) Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 33:109-115.
- Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, et al. (2012) Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 5:107-116.
- Liu VX, Rosas E, Hwang J, Cain E, Foss-Durant A, et al. (2017) Enhanced Recovery After Surgery Program Implementation in 2 Surgical Populations in an Integrated Health Care Delivery System. JAMA Surg. 152: 171032.
- Kehlet H (2018) ERAS Implementation-Time To Move Forward. Ann Surg. 267:998-999.
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, et al. (2019) Guidelines for enhanced recovery after lung surgery: Recommendations of the Enhanced Recovery After Surgery (ERAS(R)) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 55:91-115.
- Joshi GP, Bonnet F, Shah R, Wilkinson RC, Camu F, et al. (2008) A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 107:1026-1040.
- Inada K, Shirakusa T, Yoshinaga Y, Yoneda S, Shiraishi T, et al. (2000) The role of video-assisted thoracic surgery for the treatment of lung cancer: Lung lobectomy by thoracoscopy versus the standard thoracotomy approach. Int Surg. 85:6-12.
- Gerner P (2008) Postthoracotomy pain management problems. Anesthesiol Clin. 26:355-367.
- Bendixen M, Jorgensen OD, Kronborg C, Andersen C, Licht PB (2016) Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 17:836-844.
- Bayman EO, Parekh KR, Keech J, Selte A, Brennan TJ (2017) A Prospective Study of Chronic Pain after Thoracic Surgery. Anesthesiology. 126:938-951.
- Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F (2016) Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2: 009121.
- Debreceni G, Molnar Z, Szelig L, Molnar TF (2003) Continuous epidural or intercostal analgesia following thoracotomy: A prospective randomized double-blind clinical trial. Acta Anaesthesiol Scand. 47:1091-1095.
- Dominguez DA, Ely S, Bach C, Lee T, Velotta JB (2018) Impact of intercostal nerve blocks using liposomal versus standard bupivacaine on length of stay in minimally invasive thoracic surgery patients. J Thorac Dis. 10:6873-6879.
- Kelley TM, Bailey DW, Sparks P, Rice R, Caddell E, et al. (2018) Intercostal Nerve Blockade with Exparel(R) Results in Lower Opioid Usage during the First 24 Hours after Video-Assisted Thorascopic Surgery. Am Surg. 84:1433-1438.
- Rice DC, Cata JP, Mena GE, Rodriguez-Restrepo A, Correa AM, et al. (2015) Posterior Intercostal Nerve Block With Liposomal Bupivacaine: An Alternative to Thoracic Epidural Analgesia. Ann Thorac Surg. 99:1953-1960.
- Hamilton TW, Athanassoglou V, Mellon S, Strickland LH, Trivella M, et al. (2017) Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database of Systematic Reviews.
- Ma TT, Wang YH, Jiang YF, Peng CB, Yan C, et al. (2017) Liposomal bupivacaine versus traditional bupivacaine for pain control after total hip arthroplasty: A meta-analysis. Medicine. 96:7190.
- Parascandola SA, Ibanez J, Keir G, Anderson J, Plankey M, et al. (2017) Liposomal bupivacaine versus bupivacaine/epinephrine after video-assisted thoracoscopic wedge resectiondagger. Interact Cardiovasc Thorac Surg. 24:925-930.
- Babst CR, Gilling BN. Bupivacaine: (1978) A review. Anesth Prog. 25:87-91.
- Becker DE, Reed KL (2006) Essentials of local anesthetic pharmacology. Anesth Prog. 53:98-109.
- Wulf H, Winckler K, Maier C, Heinzow B (1988) Pharmacokinetics and protein binding of bupivacaine in postoperative epidural analgesia. Acta Anaesthesiol Scand. 32:530-534.
- Wong JT, Chan M, Lee D, Jiang JY, Skrzypczak M, et al. (2000) Phosphatidylcholine metabolism in human endothelial cells: Modulation by phosphocholine. Mol Cell Biochem. 207:95-100.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences