Carbohydrate Metabolism Parameters and Incretin Levels in Morbidly Obese Patients, Including those who Underwent Biliopancreatic Diversion/Duodenal Switch
Natalya Valentinovna Mazurina1*, Ekaterina Anatolievna Troshina1, Natalya Anatolievna Ogneva1, Yury Ivanovich Yashkov2, Alexander Victorovich Ilyin1, Galina Aphanasievna Melnichenko1 and Ivan Ivanovich Dedov1
1Endocrinology Research Center, Dmitry Ulianov str. 11, Moscow, Russia
2Endosurgery and Lithotripsy Center, Shosse Enthuziastov 62, Moscow, Russia
- *Corresponding Author:
- Natalya Valentinovna Mazurina
Endocrinology Research Center
117036 Dmitry Ulianov str. 11
Moscow, Russia
Tel: +7499 612-77-40
E-mail: natalyamazurina@mail.ru
Received Date: 20 March 2017; Accepted Date: 16 May 2017; Published Date: 23 May 2017
Citation: Mazurina NV, Troshina EA, Ogneva NA, et al. Carbohydrate Metabolism Parameters and Incretin Levels in Morbidly Obese Patients, Including those who Underwent Biliopancreatic Diversion/Duodenal Switch. Endocrinol Res Metab. 2017, 1: 1.
Copyright: © 2017 Mazurina NV, et al. This is an open-access article distributed under the terms of the creative Commons attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Carbohydrate metabolism was studied in morbidly obese (MO) patients (n=22), in patients who underwent biliopancreatic diversion/duodenal switch (BPD/DS) (n=23) and in the control group (n=22). Blood glucose levels, IRI, GLP-1, GIP and glucagon during the OGTT (with 75 g glucose) at 0, 30, 60 and 120 min. were measured. In MO patients impaired glucose metabolism was revealed in 15 cases (68.2%); in the BPD group, postprandial blood glucose levels (120 min) tended to be lower compared to the other groups: 4 individuals (17.4%) even showed postprandial hypoglycemia (<2.8 mmol/l). Basal and peak GLP-1 levels were significantly higher after BPD/DS (р=0.037 and p=0.022, respectively). Morbidly obese patients had higher GIP values at all points. The basal glucagon concentrations were similar in the postsurgery and the control groups, while morbidly obese patients had higher initial levels of glucagon (p=0.013) and it was not suppressed during the OGTT (p=0.076). Impaired carbohydrate metabolism in MO patients is characterized by hyperglucagonemia, increased GIP levels and decreased GLP-1 secretion. Patients who underwent BPD/DS revealed a significantly higher IRI and GPP-1 secretion, which poses a high risk of postprandial hypoglycemia.
Keywords
Morbid obesity; Incretins; Glucagon-like peptide-1; Gastric inhibitory polypeptide glucagon; Biliopancreatic diversion; Hypoglycemia
Introduction
Morbid obesity (MO) is associated with a whole set of severe diseases, such as type 2 diabetes mellitus (T2DM), coronary heart disease, arterial hypertension, obstructive sleep apnea, and osteoarthrosis [1-4]. The strongest correlation is observed between BMI and the development of type 2 diabetes mellitus: the risk of diabetes onset increases by 20% if BMI grows by 1 kg/m2 [5-7]. In patients with a BMI>40, type 2 diabetes is detected in 20-30% of cases, and the incidence of borderline disorders of carbohydrate metabolism, such as impaired glucose tolerance (IGT) and impaired fasting glucose (IFG), reaches 25-50% [8-10].
The results of studies addressing the role of incretins in the regulation of carbohydrate metabolism conducted over the past 10 years serve as basis for the development of new drugs and new approaches to the treatment of type 2 diabetes mellitus [11,12]. It was found that glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) have the most pronounced effect on the postprandial glucose level [11,12].
At the same time, it was noted that bariatric surgery performed on morbidly obese patients often leads to a substantial improvement of carbohydrate metabolism parameters. Improvement or normalization of carbohydrate metabolism is observed in 80-100% of such patients, often as soon as a few days after a gastric bypass procedure (RYGB) or biliopancreatic diversion (BPD), that is, long before a significant weight loss is achieved [13,14].
In contrast to this, after restrictive procedures such as gastric banding (GB), the reduction of glycemic level is a direct result of weight loss [15]. A meta-analysis [16], which included a total of 135,000 patients who underwent bariatric intervention, demonstrated a complete remission of type 2 diabetes in 78.1% of patients, while another 8.5% of patients showed significant improvement in carbohydrate metabolism. It should be added that a more pronounced decline in glycaemia was observed after BPD and RYGB as compared to the patients who underwent GB.
The mechanism largely responsible for blood glucose normalization after bypass surgeries is the change of production of incretins, particularly glucagon-like peptide-1 and glucose-insulinotropic polypeptide, which modulate insulin response and produce multiple extrapancreatic effects [17].
The change of GLP-1 and GIP production after intestine repositioning may be due either to direct stimulation of L-cells by rapid delivery of food into the ileum, or to the exclusion of the duodenum from the digestion process. Until now, no studies on the production of incretins in morbid obesity, including the cases of postbariatric surgery, have been performed in the Russian Federation.
The purpose of this study was to evaluate the parameters of carbohydrate metabolism and production of incretins in patients suffering from morbid obesity and those following biliopancreatic diversion performed to treat MO.
Materials and Methods
The cross-sectional study included three groups of patients – a total of 67 men and women aged 25 to 65 with no history of diabetes. The Study Protocol was approved by Ethical Committee of the Endocrinology Research Center, and all participants signed informed consent before enrollment. All procedures were performed in accordance with ethical standards of the Endocrinology Research Center Research Committee and Helsinki Declaration (1964). The study was conducted in Moscow region.
Group 1 (MO) consisted of 22 patients with BMI ≥ 40 kg/m2, who suffered from morbid obesity and had a stable body mass during the last year. Patients were recruited from Endocrinology Research Center outpatient clinic. Exclusion criteria were the presence of diabetes mellitus in the patient’s history and/or targeted attempts to lose weight in the previous year.
Group 2 was composed of 23 patients with no history of diabetes mellitus who underwent biliopancreatic diversion/ duodenal switch (BPD/DS) for morbid obesity in The Center of Endosurgery and Lithotripsy. Postoperative follow-up period varied from 2.3 to 7.2 years, the median for the group being 4.7 years. Pre-operation BMI for the 2nd group patients was equal to 50.8 kg/m2 [46.5; 60.8] and corresponded with morbid obesity. The length of the alimentary limb was 248.4 ± 9.4 cm, and the common channel was 70.5 ± 2.3 cm.
Group 3 (control) included 22 healthy volunteers with no obesity or overweight (BMI 18.5-24.9 kg/m2). A brief description of the patients studied is presented in Table 1.
Table 1 Major characteristics of the patients involved in the study (median, 25th and 75th percentiles).
| Group 1 (ÃÂœÞ) | Group 2 (BPD) | Group 3 (control) | |
|---|---|---|---|
| Number of patients (n) | 22 | 23 | 22 |
| Sex | M – 6 | M – 6 | M – 6 |
| F – 16 | F – 17 | F – 16 | |
| Age (completed years) | 44.5 (40.0; 50.0) | 44.0 (40.0; 51.0) | 44.0 (42.0; 51.0) |
| BMI (kg/m2) | 50.8 (48.0; 56.0) | 32.8 (27.5; 38.7) | 22.3 (20.0; 23.5) |
| Waist circumference (cm) | 134.5(120.0; 140.0) | 106 (94.0; 122.0) | 71(68.0; 73.0) |
The three groups of patients included in the study did not differ by sex or age. BMI in the MO group was comparable to the preoperative BMI for the patients of group 2.
In each group, an oral glucose tolerance test with 75g of glucose (OGTT) was conducted to determine plasma levels of glucose, immunoreactive insulin (IRI), glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide and glucagon at 0, 30, 60 and 120 minutes.
The level of glucose in plasma was measured using a Hitachi 912 (Roche) biochemical analyzer by the hexokinase method in the Laboratory of biochemical endocrinology and hormonal analysis of the Endocrinology Research Center.
Immunoreactive insulin in blood plasma was measured by electrochemiluminescent immunoassay (ICLA) using an automatic analyzer Cobas 601 (Roche).
The Immunoassay method using commercially available kits was employed in the laboratory of Clinical Biochemistry of the Endocrinology Research Center to determine the plasma levels of GLP-1, GIP and glucagon (respectively, GLP-1 EIA kit, GIP (Total) EIA and Glucagon EIA by Peninsula Laboratories LLC, USA).
Disorders of carbohydrate metabolism were diagnosed in accordance with the criteria of WHO (1999-2006). Venous plasma glucose levels lower than 6.1 mmol/l fasting and lower than 7.8 mmol/l two hours after an oral glucose tolerance test (OGTT), respectively, were assessed as cases of no carbohydrate metabolism disorders. Impaired fasting glycaemia was diagnosed if the level of venous plasma glucose was more than or equal to 6.1 mmol/l but less than 7.0 mmol/l fasting and less than 7.8 mmol/l at the 120th minute of OGTT. The fasting glucose level lower than 7.0 mmol/l, with an increase to 7.8 mmol/l or higher but lower than 11.1 mmol/l at the 120th minute of OGTT was regarded as impaired glucose tolerance. Plasma glucose concentration below 2.8 mmol/l was viewed as hypoglycemia.
The level of insulin resistance was assessed using a mathematical model based on the values of IRI and fasting plasma glucose (FPG) with the calculation of the HOMA-IR (homeostasis model assessment of insulin resistance) index:
HOMA-IR = IRI (units/l)*FPG(mmol/l)/22.5
The values of HOMA-IR <2.77 were considered as normal insulin sensitivity (i.e. absence of insulin resistance).
The areas under the curve (AUC) of glucose, IRI, GLP-1, GIP and glucagon were calculated by trapezoidal rule:
0.5*(y1+y2)*(х2-х1)+0.5*(у2+у3)*(х3-х2)+0.5*(у3+у4)*(х4- х3), where х is time (х1 through x4 are 0, 30, 60 and 120 minutes of OGTT, respectively) and y is the parameter calculated during OGTT (y1 through y4 are the values of this parameter at minutes 0, 30, 60 and 120, respectively).
Statistical analysis was performed using the software package STATISTICA 6.0. The data are presented as the median, the 25th and the 75th percentiles.
To assess the significance of differences between the groups, we used the Kruskal-Wallis test for continuous variables and χ2 criterion and Fisher's exact test to compare qualitative features. To test the association between two features, we used Spearman rank correlation. P-value <0.05 was taken as the critical level of significance.
Results
Glycemic status
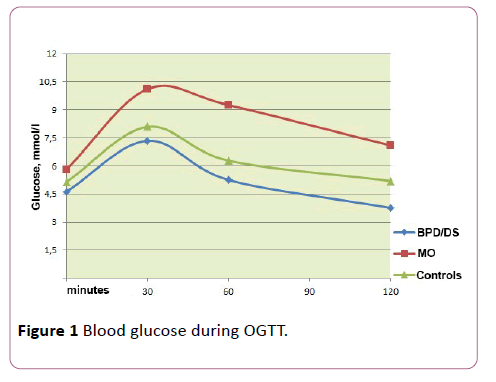
We evaluated glycaemia in the three groups of patients during the OGTT. Glucose level values are given in Table 2. In patients with MO, the fasting glucose was the highest (5.9 mmol/l vs. 4.8 and 5.1 in the other two groups, respectively), while 15 patients in this group (68.2%) were identified with impaired carbohydrate metabolism during the OGTT, which was regarded as IFG (4 patients) or IGT (11 patients).
Table 2 Glucose levels in the groups involved in the study (median, 25th and 75th percentiles).
| Glucose, mmol/l | Group 1 (ÃÂœÞ) | Group 2 (BPD) | Group 3 (control) |
|---|---|---|---|
| (0 min) | 5.9 (5.5; 6.2) | 4.8(4.4; 5.0) | 5.1(4.9; 5.4) |
| (30 min) | 10.1 (9.2; 11.7) | 7.4(6.7; 8.0) | 8.0(7.6; 8.4) |
| (60 min) | 9.5 (8.2; 10.3) | 5.2(4.7; 5.9) | 6.0 (5.8; 6.5) |
| (120 min) | 7.3 (5.9; 8.9) | 3.9(3.0; 4.3) | 5.2(4.8; 6.6) |
| AUC of glucose | 1015.4 (918.0; 1140.0) | 647.1 (576.0; 690.0) | 744(726.0; 780.0) |
Postprandial plasma glucose levels show no statistically significant difference between the groups (р=0.09). However, the BPD group revealed a tendency toward lower values; moreover, in 4 patients of this group, hypoglycemia was detected at the 120th minute of OGTT (respectively, 2.1, 2.2, 2.5 and 2.6 mmol/l), even though only one patient developed typical symptoms, primarily adrenergic ones (heart palpitations, tremor, pallor, anxiety).
AUC values for glucose were highest in people suffering from obesity and minimal in patients who underwent surgery, but the observed differences did not reach a statistically significant level (p=0.651 and p=0.9, respectively) (Figure 1).
IRI secretion
(Table 3 and Figure 2).
Table 3 IRI level indexes in the groups involved in the study (median, 25th and 75th percentiles).
| Index | Group 1 (ÃÂœÞ) | Group 2 (BPD) | Group 3 (control) |
|---|---|---|---|
| IRI (unit/l) 0 min | 44.9 (39.0; 50.0) | 5.2 (3.9; 6.0) | 5.7 (4.3; 6.0) |
| IRI (unit/l) 30 min | 100.0 (91.0; 130.0) | 98.1(87.0; 111.0) | 39.0 (35.0; 47.0) |
| IRI (unit/l) 60 min | 111.5 (100.0; 120.0) | 34.0 (27.0; 42.1) | 17.5 (15.0; 21.0) |
| IRI (unit/l) 120 min | 68.0 (59.0; 80.0) | 12.0 (9.0; 19.0) | 7.0 (6.0; 9.0) |
| AUC for IRI | 10783.5 (10170.0; 11985.0) | 4894.5 (4381.5; 5863.5) | 2298.7 (1987.5; 2565.0) |
| ÃÂÂÂÞÃÂÂœÃÂÂÂ-IR | 11.9 (9.5; 13.5) | 1.0 (0.8; 1.1) | 1.3 (1.0; 1.5) |
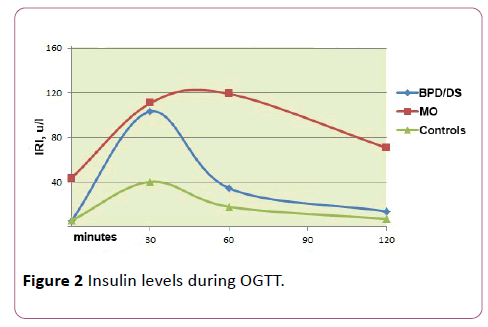
In MO patients, compared with the other two groups, basal IRI (44.9 units/l vs. 5.2 and 5.7 respectively) and the value of the HOMA-IR index (11.9 vs. 1.0 and 1.3, respectively) were the highest (p<0.001 in all cases).
In the BPD group and the control group, the peak concentration of IRI was achieved at the 30th minute of the OGTT being significantly higher in the group of operated patients (98.1 units/l vs. 39.0 units/l, respectively) (p=0.026). In the MO group, the IRI concentration curve was generally flatter, with the highest IRI values determined at the 60th minute of the OGTT (111.5 units/l) and not returning to the original level by the end of the test, whereas the remaining two groups showed a significant reduction of IRI by the 120th minute.
The comparison of AUC for IRI also demonstrated the difference between the groups (p<0.001), while the AUC for IRI in the MO group showed a statistically significant higher value (p=0. 008).
GLP-1 secretion
The evaluation of GPL-1 during OGTT revealed differences in the three groups under study (Table 4 and Figure 3).
Table 4 GLP-1 level indexes in the groups involved in the study (median, 25th and 75th percentiles).
| Index | Group 1 (ÃÂœÞ) | Group 2 (BPD/DS) | Group 3 (control) |
|---|---|---|---|
| GLP-1 (ng/ml) 0 min |
0.08 (0.05; 0.09) | 0.23 (0.22; 0.23) | 0.16 (0.15; 0.16) |
| GLP-1 (ng/ml) 30 min |
0.12 (0.10; 0.14) | 0.63 (0.61; 0.65) | 0.28 (0.28; 0.29) |
| GLP-1 (ng/ml) 60 min |
0.09 (0.07; 0.11) | 0.44 (0.42; 0.47) | 0.18 (0.17; 0.19) |
| GLP-1 (ng/ml) 120 min |
0.08 (0.06; 0.10) | 0.24 (0.22; 0.26) | 0.16 (0.15; 0.16) |
| AUC for GPL-1 | 11.17 (10.56; 11.83) | 49.56 (48.03; 51.37) | 23.33 (22.80; 23.76) |
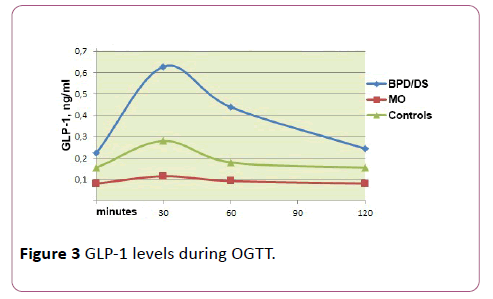
Patients with MO, as compared with healthy volunteers, showed a decrease in fasting GLP-1 level (0.08 ng/ml vs. 0.16 ng/ml) and no peak increase of GLP-1 in response to oral intake of glucose.
The basal GLP-1 levels (resp. 0.23 ng/ml vs. 0,08 ng/ml and 0.16 ng/ml) as well as GLP-1 levels stimulated at the 30th minute (resp. 0.63 ng/ml vs. 0.12 ng/ml and 0.28 ng/ml) were significantly higher in patients who had undergone BPD than in obese patients and healthy volunteers (p = 0.037 and p = 0.022 for the 0 and the 30th minute, respectively); besides, in the operated patients, the peak of GLP-1 secretion coincided with the peak of IRI secretion at the 30th minute of OGTT (r = 0.653, p = 0.013).
AUC of GLP-1 was significantly higher in the operated patients (p = 0.043). Also, a weak correlation was observed between the AUC for GLP-1 and the AUC for IRI (r = 0.215, p = 0.025). No significant correlation was found between the AUC of GLP-1 and the glycemic level; it is however worth noting that the total response (expressed in AUC) of GLP-1 and IRI to oral carbohydrate load was more pronounced in patients with documented hypoglycemia.
GIP secretion
During OGTT we measured GIP levels for the three groups (Table 5 and Figure 4).
Table 5 GIP level indexes in the groups involved in the study (median, 25th and 75th percentiles).
| Index | Group 1 (ÃÂœÞ) | Group 2 (BPD/DS) | Group 3 (control) |
|---|---|---|---|
| GIP (ng/ml) 0 min |
3.9 (3.7; 4.1) | 2.3 (2.1; 2.4) | 2.9 (2.8; 3.0) |
| GIP (ng/ml) 30 min |
4.9 (4.6; 5.2) | 3.2 (3.1; 3.3) | 3.5 (3.3; 3.6) |
| GIP (ng/ml) 60 min |
4.6 (4.4; 4.8) | 2.7 (2.6; 2.8) | 3.2 (3.0; 3.3) |
| GIP (ng/ml) 120 min |
4.4 (4.0; 4.6) | 2.3 (2.1; 2.4) | 2.9 (2.8; 3.0) |
| AUC for GIP | 548.6 (505.8; 571.1) | 318.0 (304.5; 334.5) | 374.1 (358.3; 393.4) |
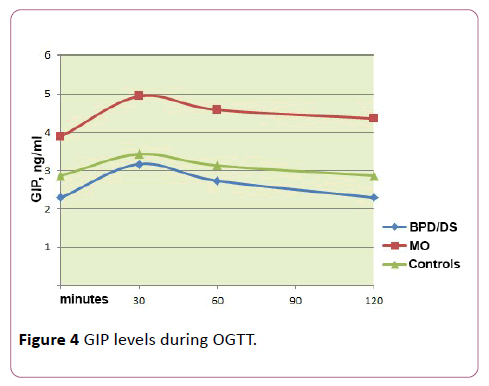
The basal GIP levels (for minute 0, 3.9 ng/ml) were significantly higher in the MO group than those for operated patients (2.3 ng/ml) and healthy volunteers (2.9 ng/ml) (р=0.027). In patients following BPD, fasting GIP values were minimal, however the differences did not achieve statistical significance (р=0.082).
In all groups, a peak increase of GIP level was observed at the 30th minute, which fell by the 120th minute of the test; in this case, the values of GIP for the MO group exceeded the respective levels for both other groups at all reference points. AUC for GIP also showed an increase in the group of patients with morbid obesity (р=0.041).
Glucagon secretion
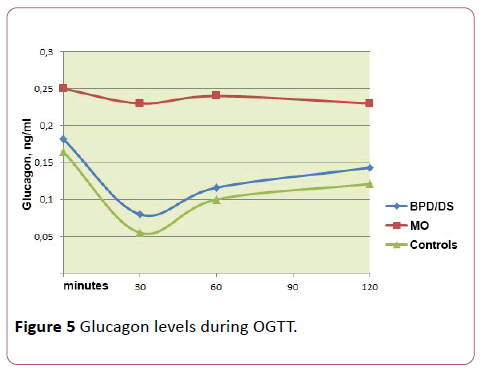
We also evaluated glucagon secretion during the test of oral glucose load in all three groups (Table 6 and Figure 5).
Table 6 Glucagon level indexes in the groups involved in the study (median, 25th and 75th percentiles).
| Index | Group 1 (ÃÂœÞ) | Group 2 (BPD/DS) | Group 3 (control) |
|---|---|---|---|
| Glucagon (ng/ml) 0 min |
0.25 (0.24; 0.27) | 0.18 (0.17; 0.19) | 0.17 (0.16; 0.17) |
| Glucagon (ng/ml) 30 min |
0.23 (0.21; 0.25) | 0.08 (0.08; 0.08) | 0.05 (0.051; 0.06) |
| Glucagon (ng/ml) 60 min |
0.23(0.22; 0.25) | 0.12 (0.11; 0.12) | 0.10 (0.10; 0.12) |
| Glucagon (ng/ml) 120 min |
0.23 (0.21; 0.25) | 0.15 (0.14; 0.15) | 0.12 (0.12; 0.13) |
| AUC for Glucagon | 28.05 (26.10; 30.01) | 14.82 (14.34; 15.18) | 12.49 (11.97; 13.09) |
The basal glucagon levels in the postsurgical and the control groups showed no difference (respectively, 0.18 ng/ml and 0.17 ng/ml), while MO patients originally had higher glucagon levels (0.25 ng/ml) (р=0.013). It is worth noting that hyperglucagonemia (as well as the increased GIP level and reduced GLP-1 secretion) were most pronounced in people with IGT and IFG.
During the OGTT in patients who had undergone bariatric treatment and in the control group the suppression of glucagon was noted at the 30th minute after oral glucose intake (respectively, 0.08 and 0.05 ng/ml), which was followed by a gradual increase in its level by the end of the test (respectively, 0.15 and 0.12 ng/ml). In contrast, the MO group of patients showed a virtually unchanged level of glucagon during the whole observation period (р= 0.076). The AUC of glucagon in the MO group was also significantly higher (p = 0.003).
We have been unable to trace any correlation between the levels of glucagon, IRI, GIP, or BMI in this study.
Discussion
Our study revealed a high frequency of carbohydrate metabolism disorders in the group of patients suffering from MO: IFG and IGT were detected in 68.2% of patients. It should be noted that the presence of diabetes mellitus in the patient’s history was a criterion of exclusion from this group.
Cohort studies showed that between 7.9 and 34.1% of adults in the general population have abnormal glucose metabolism [18-20]; among obese people, the prevalence of IGT and/or IFG reaches 20-34% [10,21] and among those suffering from MO it amounts to 50% [10,22,23]. To give an example, a study conducted in Norway, which included 1,253 patients with MO aged 34 to 50 (with BMI of 40 to 48), revealed the presence of type 2 diabetes in 31% of patients involved, while another 24% showed borderline carbohydrate metabolism (8% had IFG and 16% had IGT) [24].
A group of Italian scientists [10] followed 938 patients with a BMI of 30.0 to 84.2 kg/m2 (mean age 40.2 ± 13.4 years), and found a significantly higher incidence of changes in glucose metabolism in obesity. All patients included in the study were distributed into groups depending on body mass index: moderate obesity (BMI 30-39.9 kg/m2), morbid obesity (BMI 40-49.9 kg/m2), and super-obesity (IMT ≥ 50 kg/m2). The incidence of carbohydrate metabolism disorders and insulin resistance increased progressively with the increase of BMI. The newly diagnosed type 2 diabetes was found in 6.5% of patients with moderate obesity, in 15.5% of patients in the MO group, and 20.5% of super-obese people (p<0.0001). The prevalence of IFG and/or IGT increased similarly: 34.3%, 41.2% and 50.0%, respectively (p<0.0001).
The high prevalence of carbohydrate metabolism disorders in obesity is due to insulin resistance and impaired insulin secretion. In addition to genetic factors and direct lipotoxicity, impaired insulin secretion, in its turn, is due to impaired incretin response.
GLP-1 and GIP are the main incretins that make the largest contribution to the stimulation of postprandial insulin secretion. In obesity, a reduction of GPL-1 level is observed [25-28]. Besides, GLP-1 concentration is found to fall in nonobese people if their glucose tolerance is impaired.
For example, Rask et al. [29] followed 35 men with different body mass (from normal BMI to obesity) and different sensitivity to insulin (measured by clamp technique) revealed a correlation between the level of GLP-1 and insulin resistance (r=0.47, p<0.01). Multiple linear regression showed that resistance to insulin, rather than obesity, was an independent predictor of reduced GLP-1.
Muscelli et al. [26] evaluated the secretion of GLP-1 in response to intravenous and oral administration of glucose in 51 patients with a BMI of 20 to 61 kg/m2 (of which 17 people had IGT and 10 other suffered from type 2 diabetes mellitus). As a result, GLP-1 concentration proved to be more reduced in patients with type 2 diabetes as compared to individuals with normal carbohydrate metabolism or impaired glucose tolerance (p ≤ 0.05). Similar results were obtained if the subjects were distributed depending on BMI (p ≤ 0.05). On the whole, GPL-1 secretion reduction was inversely proportional to both the glucose tolerance and to BMI (r=0.27-0.59, p ≤ 0.05).
Thus, obesity and disorders of carbohydrate metabolism have an independent and negative effect on the production of GLP-1.
Our study also revealed reduced GPL-1 levels in the MO group, both basal and stimulated by oral glucose intake, which is probably not only due to pronounced obesity, with BMI median being 50.8 but also to the fact that most patients had disorders of carbohydrate metabolism (50% of MO patients had IGT and another 18.2% had IFG).
According to our findings, MO patients, as compared with the other two groups), show an increase of basal and postprandial GIP, which is fully consistent with the literature sources.
For example, T. Vilsbøll et al. [30] studied the production of incretins in two meal tests (260 kcal and 520 kcal) on patients with type 1 and type 2 diabetes mellitus, obese patients with normal carbohydrate tolerance and the control group of healthy individuals with normal body mass. As a result, obese individuals had a higher basal level of GIP and a higher level of stimulated GIP compared to the control group and patients with diabetes mellitus. Carr et al. [25] also noted a significant increase in postprandial levels of GIP in obesity.
It is worth noting that, in obesity, in addition to the development of insulin resistance and changes of incretin response, production of glucagon is impaired. It is well known that all forms of diabetes are characterized by increased levels of glucagon, however, obese subjects with normal carbohydrate tolerance also develop hyper-glucagonemia (primarily in fasting), which is regarded as an early predictor of carbohydrate metabolism disorder [31,32].
So, Knop et al. [32] performed an oral and an intravenous glucose tests of patients with different BMI values (from normal body weight to obesity) and different tolerance to carbohydrates (from normal to type 2 diabetes). They found that patients with normal body weight who had no carbohydrate metabolism disorders had the lowest level of glucagon, whereas the group of patients with type 2 diabetes and obese patients with normal carbohydrate tolerance showed an increased basal glucagon level and a noticeable elevation thereof during OGTT.So, Knop et al. [32] performed an oral and an intravenous glucose tests of patients with different BMI values (from normal body weight to obesity) and different tolerance to carbohydrates (from normal to type 2 diabetes). They found that patients with normal body weight who had no carbohydrate metabolism disorders had the lowest level of glucagon, whereas the group of patients with type 2 diabetes and obese patients with normal carbohydrate tolerance showed an increased basal glucagon level and a noticeable elevation thereof during OGTT.
In our study, too, the MO group of patients demonstrated fasting hyper-glucagonemia and no reduction in the level of glucagon after oral glucose intake.
Postoperative changes in the secretion of GLP-1 are explained by anatomical changes in the gastrointestinal tract as a result of bariatric surgery. After BPD and RYGB, an early (starting from the 2 day), significant (up to 20-fold) and stable (up to 10 years) increase of GLP-1 secretion was noted of [33-38]. According to our findings, patients who underwent BPD also showed higher basal and postprandial levels of GLP-1.
As a result of a more rapid delivery of nutrients to L-cells of the ileum and additional stimulation of enteroendocrine cells by bile discharged into the distal intestine during RYGB and BPD, increased secretion of GLP-1 is observed, which, in its turn, potentiates the postprandial insulin secretion, inhibits the secretion of glucagon, slows down gastric motility, has anorexigenic effect, thereby contributing to the improvement of the glycemic status in patients with type 2 diabetes and borderline disorders of carbohydrate metabolism [33,34].
The data relating to changes in the secretion of GIP after bariatric interventions are rather contradictory. According to some authors, there were no changes of GIP levels for 12 weeks after gastric bypass [39,40], whereas other researchers report a reduction in the concentration of GIP in the postoperative period [23,41], and still others [17,42] note an increase in GIP secretion after bypass procedures.
Our findings are that GIP levels in the postsurgical group were comparable with the control group (i.e. they were much lower than in the MO group).
Change in GIP concentration after bypass interventions is quite natural and is also explained by the exclusion of the proximal small intestine – the main site of GIP synthesis from digestion. However, a paper by Rudnicki et al. [43], which reports a study on rats, states that the presence of bile is a mandatory factor of GIP secretion stimulation. Thus, the discharge of bile into the terminal portion of the small intestine in BPD can be considered as an independent factor of reducing the secretion of GIP in the postoperative period.
Such a change in the production of incretins, which occurs after bypass procedures, changes the insulin response; namely, a significant increase in the secretion of postprandial IRI is observed and a pronounced early peak appears. Yet, the total area under the curve for IRI mostly remains unchanged (i.e. it corresponds to the pre-operative level), or even falls [33,37]. Our findings demonstrate a fasting hyperinsulinemia and a weak shifted peak of IRI secretion (by the 60th minute) in response to oral ingestion of glucose in MO patients and normal basal insulin values with a powerful peak increase in the BPD group at the 30th minute.
Bariatric intervention also influences the secretion of glucagon. It can be assumed that the recovery of sensitivity of α-cells of the pancreas due to body weight reduction, as well as improved sensitivity to insulin and the change of incretin response which lead to the normalization of carbohydrate metabolism, may be contributing to hyper-glucagonemia leveling [34,44]. These are exactly the results obtained in our study: glucagon secretion in the BPD/DS group was no different from the control group of individuals with normal body mass.
It should be noted that the restoration of insulin sensitivity combined with hypersecretion of GLP-1 and IRI, observed after bypass procedures, may in some cases promote postprandial hypoglycemic conditions [45,46]. Goldfine [47] demonstrated a significant increase in GLP-1 level in patients after GBP as compared with the control group of patients with obesity. In contrast to that, patients with symptoms of neuroglucopenia who underwent GBP showed much higher levels of GLP-1, insulin, and C-peptide.
In our study, 4 out of 23 patients were identified with hypoglycemia (of less than 2.8 mmol/l) at the 120th minute of OGTT, which in only one case was accompanied by adrenergic symptoms. Other cases of hypoglycemia remained asymptomatic. It should be added that all patients with postprandial hypoglycemia displayed hypersecretion of GLP-1 and IRI, as well as a reduced level of glucagon. Asymptomatic hypoglycemia in patients following GBP was also confirmed by other researchers, the incidence of asymptomatic hypoglycemia in this category of patients varied from 12 to 33% [38,47,48].
It can thus be concluded that the results of our study confirm the change in incretin response (first of all, the reduction of GLP-1 secretion) and elevated glucagon levels in MO patients, which may probably be regarded as early predictors of carbohydrate metabolism disorders.
Improvement in carbohydrate metabolism after bariatric bypass surgery is not only due to body mass loss, but also to a change in the production of incretins, which in some cases can provoke the development of a hypoglycemic condition.
Based on these data we conclude that all patients who had undergone bariatric surgery require lifelong postoperative monitoring not only for the prevention and treatment of metabolic disorders associated with malabsorption syndrome, but also for the timely diagnosis and correction of possible hypoglycemia.
Conclusions
1) Morbid obesity is characterized by a high incidence of carbohydrate metabolism disorders: among patients who do not have type 2 diabetes, IGT and IFG are identified in 68.2% of cases.
2) Impaired regulation of carbohydrate metabolism in morbid obesity is characterized by basal hyperinsulinemia hyper-glucagonemia, increased levels of GIP and reduced secretion of GLP-1.
3) In patients who underwent BPD, the secretion of GLP-1 and IRI in response to the oral intake of glucose is significantly increased, which is the cause of the high risk of postprandial hypoglycemias.
Acknowledgements
This study was supported by the Russian Foundation for Basic Research (Grant No. 11-04-00946). The authors declare that they have no conflict of interest.
References
- Freedman D, Ron E, Ballard-Barbash R, Doody MM, Linet MS (2006) Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond) 30: 822-829.
- Kanell W, D'Agostino R, Cobb J (1996) Effect of weight on cardiovascular disease. American Journal of Clinical Nutrition 63 (Suppl): 419S-422S.
- Manson JE, Willett WC, Stamfer MJ, Colditz GA, Hunter DJ, et al. (1995) Body weight and mortality among women. New England Journal of Medicine 333: 677-85.
- World Health Organization (1997) Obesity: Preventing and managing the global epidemic. WHO, Geneva.
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, et al. (2009) The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9: 88.
- Lenz M, Richter T, Muhlhauser I (2009) The morbidity and mortality associated with overweight and obesity in adulthood: A systematic review. Dtsch Arztebl Int 106: 641-648.
- Vazquez G, Duval S, Jacobs DRJ, Silventoinen K (2007) Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev 29: 115-128.
- Jankovic D, Wolf P, Anderwald CH, Winhofer Y, Promintzer SM, et al. (2012) Prevalence of endocrine disorders in morbidly obese patients and the effect of bariatric surgery on endocrine and metabolic parameters. Obes Surg 22: 62-69.
- Schinner S, Kempf K, Overmann H (2008) Association of impaired glucose metabolism in morbid obesity with hypoadiponectinaemia. Exp Clin Endocrinol Diabetes 116: S64-S69.
- Vinciguerra F, Baratta R, Farina M, Tita P, Vigneri R, et al. (2013) Very severely obese patients have a high prevalence of type 2 diabetes mellitus and cardiovascular disease Acta Diabetol 50: 443-449.
- Kim W, Egan Jm (2008) The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60:470-512.
- Murphy KG, Dhillo WS, Bloom SR (2006) Gut peptides in the regulation of food intake and energy homeostasis. Endocrine Reviews 27: 719-727.
- Cummings D, Overduin J, Foster-Schubert K, Carlson MJ (2007) Role of the bypassed proximal intestine in the antidiabetic effects of bariatric surgery. Surg Obes Relat Dis 3: 109-115.
- Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, et al. (2004) The early effect of Roux-en-Y gastric bypass on hormones is involved in body weight regulation and glucose metabolism. Ann Surg 240: 236-242.
- Dixon J, O’Brien PE, Playfair J, Chapman L, Schachter LM, et al. (2008) Adjustable gastric banding and conventional therapy for type 2 diabetes: A randomized controlled trial. JAMA 299: 316-323.
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, et al. (2009) Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122: 248-256.
- Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, et al. (2008) Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93: 2479-2485.
- Anjana R, Pradeepa R, Deepa M, Datta M, Sudha V, õt al. (2011) Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research– INdia DIABetes (ICMR–INDIAB) study. Diabetologia 54: 3022-3027.
- Diabetes atlas (4thedn) Brussels (Belgium): International Diabetes Federation; 2009. Available at: https:// www.diabetesatlas.org/. Accessed October 9, 2010.
- Satman I, Omer B, Tutuncu Y, Kalaca S, Gedik S, et al. (2013) Twelve-year trends in the prevalence and risk factors of diabetes and prediabetes in Turkish adults. Eur J Epidemiol 28: 169-180.
- Cosson E, Hamo-Tchatchouang E, Banu I, Nguyen MT, Chiheb S, et al. (2010) A large proportion of prediabetes and diabetes goes undiagnosed when only fasting plasma glucose and/ orHbA1c are measured in overweight or obese patients. Diabetes Metab 36: 312-318.
- Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, et al. (2009) Full accounting of diabetes and pre-diabetes in the U.S. Population in 1988–1994 and 2005–2006. Diabetes Care 32: 287-294.
- The DECODE study group (2003) Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care 26: 61-69.
- Hofsø D, Jenssen T, Hager H, Roislien J, Hjelmesaeth J (2010) Fasting plasma glucose in the screening for type 2 diabetes in morbidly obese subjects. Obes Surg 20: 302-307.
- Carr R, Larsen MO, Jelic K, Lindqren O, Vikman J, et al. (2010) Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 95: 872-878.
- Muscelli E, Mari A, Casolaro A, Camastra S, Seqhiei G, et al. (2008) Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 57: 1340-1348.
- Neary MT, Batterham RL (2009) Gut hormones: Implications for the treatment of obesity. Pharmacology & Therapeutics 124: 44-56.
- Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, et al. (2001) The role of postprandial releases of insulin and incretin hormones in meal-induced satiety - effect of obesity and weight reduction. Int J Obes Relat Metab Disord 25: 1206-1214.
- Rask E, Olsson T, Soderberg S, Holst Ji Ji, Tura A, et al. (2004) Insulin secretion and incretin hormones after oral glucose in non-obese subjects with impaired glucose tolerance. Metabolism 53: 624 -631.
- Vilsbøll T, Krarup T, Sonne J, Madsbad S, Volund A, et al. (2003) Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 88: 2706-2713.
- Ferrannini E, Muscelli E, Natali A, Gabreil R, Mitrkou A, et al. (2007) Association of fasting lucagon and proinsulin concentrations with insulin resistance. Diabetologia 50: 2342-2327.
- Knop F, Aaboe K, Vilsbøll T, Volund A, Holst JJ, et al. (2012) Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes Metab 14: 500-510.
- Falkén Y, Hellstrom P, Holst JJ, Naslund E (2011) Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab 96: 2227-2235.
- Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ (2007) Exaggerated glucagon-like peptide-1 and blunted glucosedependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 3: 597-601.
- Promintzer-Schifferl M, Prager G, Anderwald C, Mandl M, Esterbauer H, et al. (2011) Effects of gastric bypass surgery on insulin resistance and insulin secretion in nondiabetic obese patients. Obesity (Silver Spring) 19: 1420-1426.
- Reed M, Pories W, Chapman W, Pender J, Bowden R, et al. (2011) Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab 96: 2525-2531.
- Umeda L, Silva EA, Carneiro G, Arasaki CH, Geloneze B, et al. (2011) Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg 21: 896-901.
- Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, et al. (2009) Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab 94: 884-891.
- Campos G, Rabl C, Peeva S, Ciovica R, Rao M, et al. (2010) Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg 14: 15-23.
- Isbell J, Tamboli RA, Hansen EN, Saliba J, Dunn JP, et al. (2010) The importance of caloric estriction in the early improvements in insulin sensitivityafter Roux-en-Y gastric bypass surgery. Diabetes Care 33: 1438-1442.
- Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, et al. (2010) Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell func-tion and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes 34: 462-471.
- Laferrere B, Heshka S, Wang K, Khan Y, Mc Ginty J, et al. (2007) Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30: 1709-1716.
- Rudnicki M, Patel D, McFadden D, Balasubramaniam A, Nussbaum MS, et al. (1990) Proximal jejunal and biliary effects on the enteroinsular axis. Surgery 107: 455-460.
- Swarbrick M, Stanhope KL, Austrheim-Smith IT, Van Loan MD, Ali MR, et al. (2008) Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia 51: 1901-1911.
- Patti M, McMahon G, Mun EC, Bitton A, Holst JJ, et al. (2005) Severe hypoglycaemia postgastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia 48: 2236-2240.
- Service GJ, Thompson GB, Service FJ, Andrews JC, Lloyd RV, et al. (2005) Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 353: 249-254.
- Goldfine A, Mun EC, Devine E, Bernier R, Baz-Hecht M, et al. (2007) Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab 92: 4678-4685.
- Scavini M, Pontiroli A, Folli F (2005) Asymptomatic hyperinsulinemic hypoglycemia after gastric banding. N Engl J Med 353: 2822-2823.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences