Cadmium Exposure Induces Early Catabolism In Male Wistar Rat Experiment
1 Department of Physiology, College of Medicine, Ambrose Alli University, Ekpoma, Nigeria
2 Department of Physiology, College of Medical Sciences, University of Benin, Benin City-Nigeria
- *Corresponding Author:
- Osifo CU
Department of Physiology, College of Medicine
Ambrose Alli University, Ekpoma, Nigeria.
E-mail: chunjiaosong@163.com
Received Date: August 22, 2018; Accepted Date: October 22, 2018; Published Date: November 06, 2018
Citation: Osifo CU, Iyawe VI (2018) Cadmium Exposure Induces Early Catabolism in Male Wistar Rat Experiment. J Mol Cell Biochem Vol.2 No.2:9
Abstract
This study investigates the effect of varying duration of cadmium exposure on anabolic-catabolic ratio. The study involves adult male Wistar rats divided into 8 groups of 5 rats each. Groups 1 and 2 served as control groups on to clean tap water for 1 and 6 weeks while groups 3, 4, 5, 6, 7 and 8 served as test groups exposed to Cadmium-contaminated drinking water (50 mg/L) for 1 through 6 weeks respectively. At the end of each treatment period, final body weight, and blood sample were obtained for the estimation of the variables herein presented. Appropriate statistical analysis (ANOVA) was conducted on the variables and presented as means ± SEM in charts and tables with p<0.05 (LSD) considered significant. The results showed that cadmium exposure stimulates significant increase in body weight lost, serum cortisol and protein levels and anabolic catabolic ration in acute state but improve by the 5th and 6th weeks. However, there was a significant duration dependent decrease in serum testosterone activity with cadmium exposure. These findings indicate that cadmium exposure induces an early catabolic damage. However, this may not be the case at the organ level and thus the need for further studies.
Keywords
Cadmium; Toxicity; Oxidative stress; Anabolism; Catabolism
Introduction
Heavy metal contamination has become a serious problem in crop and vegetable production. Of interest is Cadmium whose increasing environmental concentration has precipitated concern over the toxicological potential of heavy metal with which man has been brought more and more in contact during recent years [1]. Cadmium is a heavy metal pollutant which is toxic to plants, animals and human beings [2]. According to Muthukumar and Nachiappan [3], Cadmium is a prevalent non-essential, redoxinactive, highly toxic metal in the environment and causes a considerable threat to nature and human health. Cadmium is a by-product of the mining and smelting of lead and zinc and is used in nickel-cadmium batteries, dyes, plastics, electrochemistry and paint pigments and can be found in soils because insecticides, fungicides, sludge and commercial fertilizers containing cadmium are used in agriculture [4]. The main routes of human poisoning are either through occupational exposure or ingestion of contaminated food and water [5] has been reported to cause a wide range of biochemical and physiological dysfunctions in humans and laboratory animals [6]. Chronic Cadmium poisoning has been implicated in nephrotoxicity, osteoporosis, cardiovascular diseases, testicular necrosis, prostatic and testicular cancers, and renal failure and neurodegenerative conditions [7,8]. Osteomalacias, polynephritis, cancer of the nasopharynx and prostate have been reported in men professionally exposed to Cadmium [9-11].
Clinical stigmata of cadmium toxicity depend on route, quantity, and rate of exposure [12]. The exact mechanism by which Cd is accumulated in cells remains vague, but it is documented that deregulation of transition metal homeostasis and use of cellular transport systems dedicated to essential elements contributes to the cellular uptake mechanisms of Cd [13-15] reported Cd to possess a strong affinity to thiol groups of amino acids, especially cysteine and may affect the antioxidant barrier via inhibiting the functional thiol groups of antioxidant enzymes. Several studies proposed disturbance of pro-oxidant and antioxidant balance by generation of reactive oxygen species (ROS) as a possible mechanism of Cd toxicity [16]. Studies showed oxidative stress to occur as a result of an increase in Cd-induced peroxidation of membrane lipids in the organs where it accumulates [17,18].
It has been hypothesized that chronic stress shifts the balance of hormones to low levels of anabolic hormones (e.g., dehydroepiandrosterone [DHEA], growth hormone [GH], and insulin-like growth factor 1 [IGF]-1) and high levels of catabolic hormones (e.g., cortisol, adrenaline [epinephrine], and cytokines) [19]. The balance between anabolic and catabolic activities is represented by the ratio between testosterone and cortisol, which is known as testosterone/cortisol or free testosterone/ cortisol. Based on the premise that the testosterone has anabolic effects and the cortisol catabolic ones, the testosterone/cortisol ratio has been proposed as a great marker for stress [19]. This shift in hormonal balance, termed anabolic/catabolic imbalance, is thought to be linked to systemic inflammation [20] and oxidative stress [21]. Thus, suggesting an interactions between the endocrine system and metabolism which has been previously reported [22,23]. It is therefore the aim of this study to investigate the effect of varying duration of cadmium exposure on anaboliccatabolic ratio in adult male Wistar rats exposed to cadmium contamination in drinking water.
Materials and Methods
Cadmium chloride was purchased commercially from a chemical company in Nigeria. Adult male Wistar rats (N=40) of comparable weight (180 to 200 g) were obtained from the Department of Physiology animal holding unit of the Ambrose Alli University, Ekpoma, Nigeria and were transferred to the site of the experiments. They were housed in plastic cages (measuring 50 x 35 x 25) under standard environmental condition of 12/12hr light/dark cycle and were allowed to acclimatize for two weeks to the laboratory conditions before the experiment and fed ad libitum with rat chow and tap water.
The rats were divided into 8 groups of 5 rats each. Groups 1 and 2 served as the control groups which were exposed to clean tap water for 1 and 6 weeks. On the other hand, groups 3, 4, 5, 6, 7 and 8 served as the test groups that were exposed ad libitum to Cadmium-contaminated drinking water (50 mg/L) for 1 through 6 weeks respectively. The chosen cadmium dose was according [24].
At the end of the each treatment period, final body weight was determined and recorded and blood sample obtained for the estimation of serum cortisol, testosterone and protein. Serum cortisol and testosterone were determined using a commercial ELISA kit and the method for estimation were based on the procedure provided by the manufacturer. The protein concentration of the serum was determined by means of the Biuret method as described [25]. Anabolic-catabolic ratio was determined by the ratio of serum testosterone and cortisol.
The result was analyzed with appropriate statistical analysis (ANOVA) using SPSS 20 and p<0.05 (LSD) was considered statistically significant and then presented as means ± SEM in charts and tables.
Results
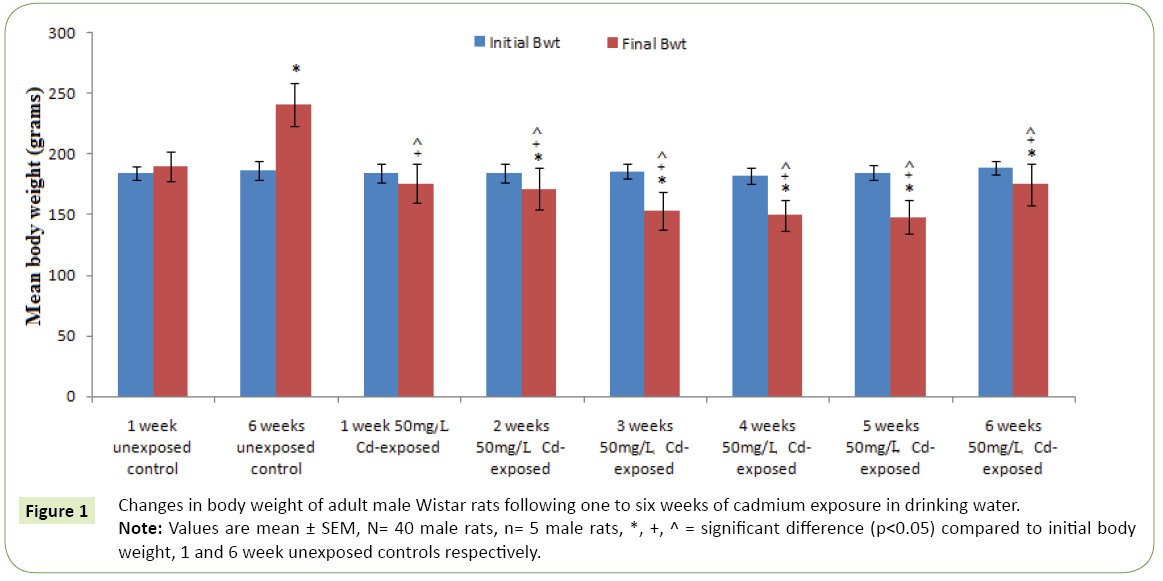
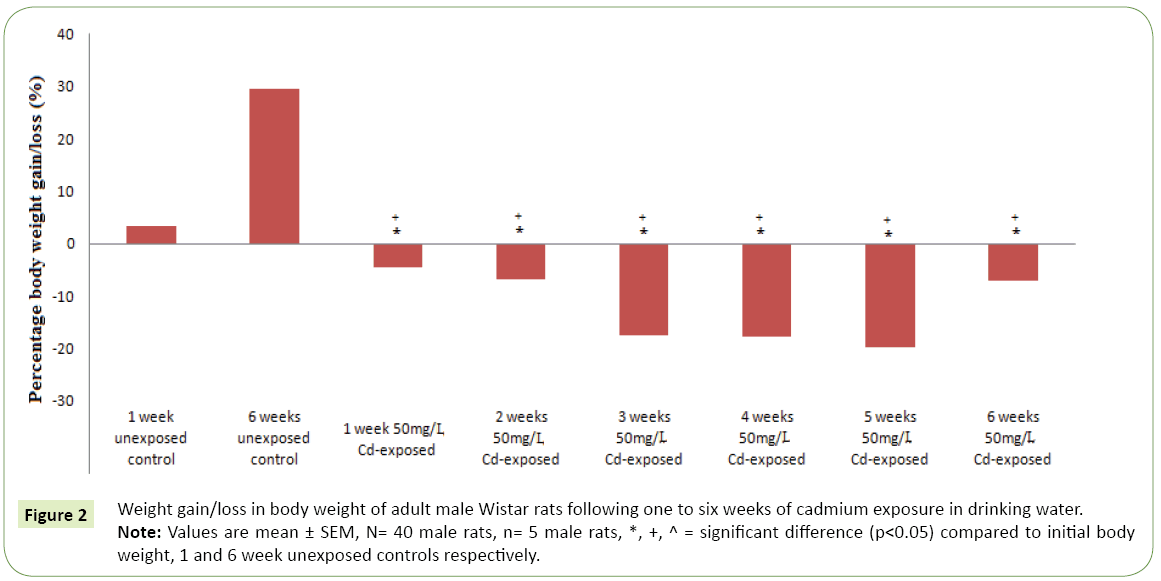
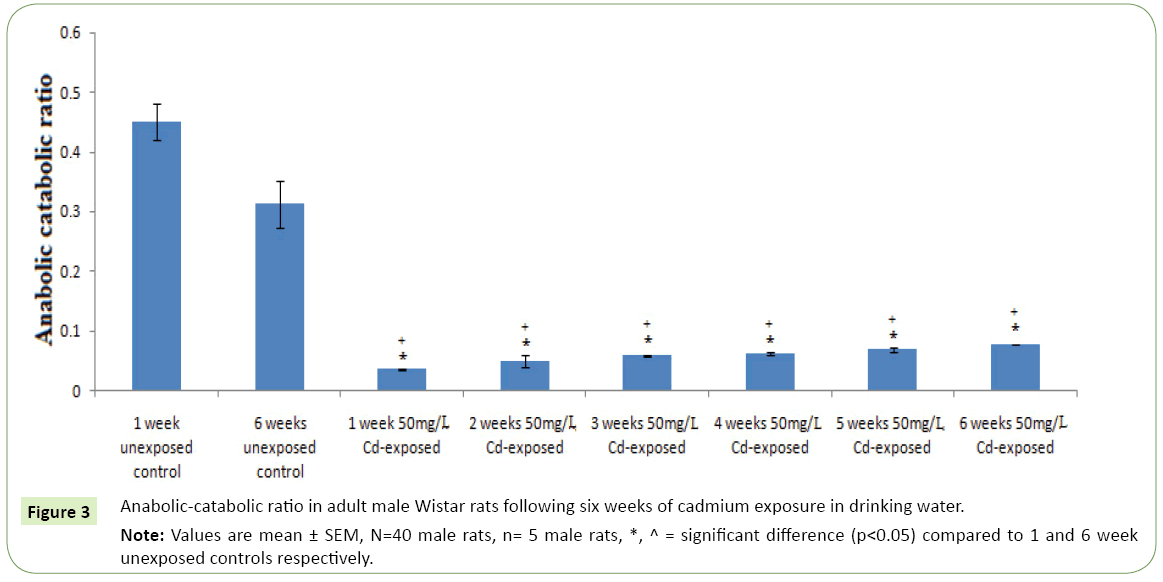
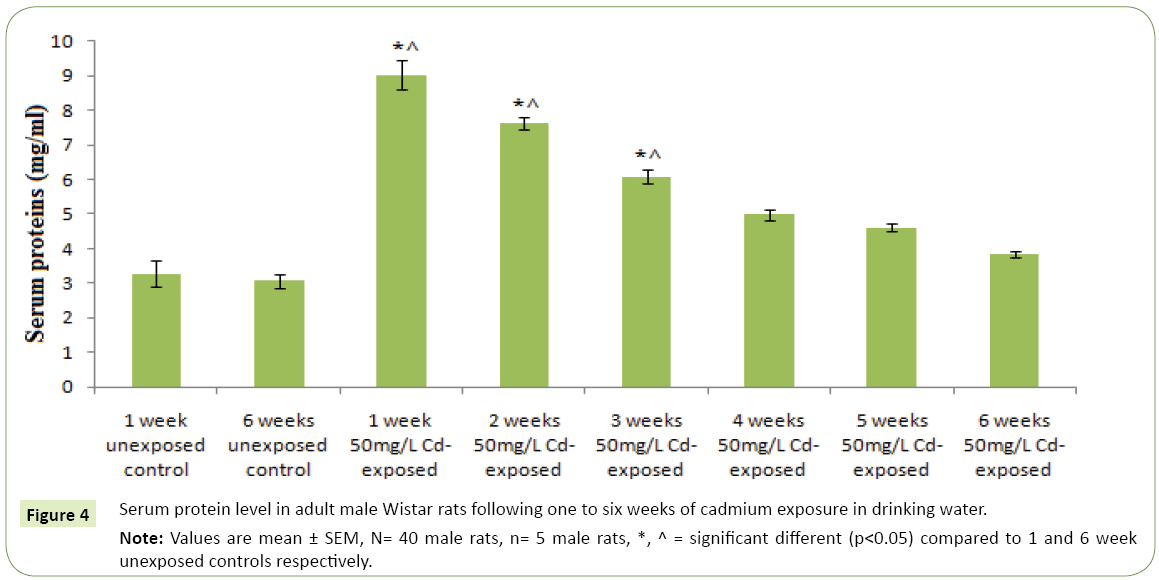
Figures 1 and 2 respectively represent the changes in body weight and weight gain/loss following six weeks exposure to cadmium in drinking water of adult Wistar rats. Except for the controls; 1 and 6 weeks unexposed control groups, where body weights gain were observed, cadmium exposure resulted in duration dependent significant body weight loss (p<0.05) compared to the controls and corresponding initial body weight. However, body weight was observed to kicks up at the sixth week of cadmium exposure compared to earlier exposure periods. The highest weight lost following cadmium exposure was observed in the 5th weeks followed by the 4th and 3rd weeks. Table 1 represents serum activities of cortisol and testosterone in adult Wistar rats following six weeks of cadmium exposure in drinking water. Serum cortisol activity decreases in a duration depended fashion following six week cadmium exposure. Compared to the controls there were significantly increase (p<0.05) in serum cortisol level in the 1st, 2nd and 3rd weeks cadmium exposure in drinking water of the rats. Similarly, serum testosterone activity following six week cadmium exposure was observed to decrease in a duration depended manner. Compared to the controls, there were significant decreases in serum testosterone level from the 1st week through the 6th week of cadmium exposures Figure 3 represents the anabolic-catabolic ratio in adult Wistar rats following six weeks of cadmium exposure in drinking water. Cadmium exposure in drinking water was observed to significantly decrease (p<0.05) the anabolic-catabolic ratio compared to the unexposed control groups. However, it was observed that the anabolic-catabolic ratio improves with increasing duration of cadmium exposure (Figure 4) represents serum protein level in adult male Wistar rats following six weeks of cadmium exposure in drinking water. There was a duration depended reduction in serum protein levels toward control level with cadmium exposure in drinking water of adult Wistar rats. Serum protein activity was significantly higher in the 1st, 2nd and 3rd weeks of cadmium exposure compared to control groups.
| Duration of exposure | Serum cortisol (ng/ml) | Serum testosterone (ng/ml) |
|---|---|---|
| 1 week unexposed control | 9.06 ± 1.05 | 4.08 ± 0.08 |
| 6 weeks unexposed control | 10.51 ± 2.13 | 3.28 ± 0.11 |
| 1 week 50 mg/L Cadmium-exposed | 41.10 ± 34.91*^ | 1.43 ± 0.07* |
| 2 weeks 50 mg/L Cadmium-exposed | 27.38 ± 4.34*^ | 1.33 ± 0.07* |
| 3 weeks 50 mg/L Cadmium-exposed | 21.09 ± 2.24*^ | 1.22 ± 0.09*^ |
| 4 weeks 50 mg/L Cadmium-exposed | 12.77 ± 5.65 | 0.79 ± 0.15*^ |
| 5 weeks 50 mg/L Cadmium-exposed | 10.93 ± 0.96 | 0.75 ± 0.21*^ |
| 6 weeks 50 mg/L Cadmium-exposed | 5.97 ± 2.84 | 0.46 ± 0.16*^ |
| Values are mean ± SEM, N= 40 male rats, n= 5 male rats, *, ^ = significant difference (p<0.05) compared to 1 and 6 week unexposed controls respectively. | ||
Table 1: Serum cortisol and testosterone activities in adult male Wistar rats following six weeks of cadmium exposure in drinking water.
Figure 1: Changes in body weight of adult male Wistar rats following one to six weeks of cadmium exposure in drinking water.
Note: Values are mean ± SEM, N= 40 male rats, n= 5 male rats, *, +, ^ = significant difference (p<0.05) compared to initial body weight, 1 and 6 week unexposed controls respectively.
Figure 2: Weight gain/loss in body weight of adult male Wistar rats following one to six weeks of cadmium exposure in drinking water.
Note: Values are mean ± SEM, N= 40 male rats, n= 5 male rats, *, +, ^ = significant difference (p < 0.05) compared to initial body weight, 1 and 6 week unexposed controls respectively.
Discussion
The results of this study showed that cadmium exposure induces body weight loss, stimulates serum cortisol and protein and inhibits serum testosterone and anabolic-catabolic ratio. The worse situation of this Cadmium induced effects take place in the 1st week. This suggests that cadmium induced oxidative damage might be more powerful in the first few weeks but reduces as the duration of exposure approaches 6th weeks. This assertion is in line with the report [26] that Cadmium promotes an early oxidative stress and afterward contributes to the development of serious pathological conditions because of its long retention in some tissues. Although the mechanism behind this correction of acute cadmium induced damage in the chronic state observed in this study is unclear, it may however be that the body develops adaptive mechanism to try to correct the increased oxidative damage in the long run.
Body weight, serum cortisol, protein and testosterone and anabolic-catabolic ratio were observed to be altered with Cadmium exposure in adult male Wistar rats following six weeks of cadmium exposure in drinking water. The observed Cadmiuminduced decrease in body weight in this study is in accordance with the study [27] who documented obviously significant decrease in body weight in Cadmium treated animals. Also, Amara and Zeng et al. [28,29] have previous reported reduction in body weight as a primary manifestation of Cadmium administration in rats.
As observed in this study, cadmium caused increase cortisol activity that weakens with duration of exposure. This finding suggests that cadmium exposure stimulate the stress response considering that the general adaptation response of the animal to stressors is the increase in cortisol secretion. Various studies had previously reported that the primary response to Cadmium induced stress is an indication of the elevation of serum cortisol level [30,31]. The effect of Cadmium on the adrenal gland physiology observed in this study correlates with the report [32] that Cadmium has a toxic effect on adrenal tissue and this causes an elevated ACTH and hence, an increased serum cortisol level.
In this study, cadmium exposure was observed to induce decrease serum testosterone activity in duration dependent fashion. This effect is not surprising considering that the testis has been documented as one of the major targets of Cadmium in many species of animals (rats, rabbits and dogs) [28,33]. Also Lafuente et al. [34] has documented that increased Cadmium accumulates in the hypothalamus, pituitary, and testis and cause decreased plasma levels of follicle stimulating hormone in rats and suggest a possible effect of Cadmium on the hypothalamic- pituitary-testicular axis. If this be the case therefore, our finding of decreased serum testosterone level in this study in Cadmiumexposure is in line. Cadmium-induced toxicity to the testis is probably the result of inter-digitizing complex interactions which involve the disruption of the blood-testis barrier via specific signal transduction pathways and signaling molecules [35].
The Cadmium-induced decrease in anabolic-catabolic ratio observed in this study indicates increased catabolism against anabolism or in other words, increased system metabolism and thus the muscle wasting. This observation is in line when one takes a look at the effect of endocrine parameters of stress here in investigated. The increased cortisol activity and decrease testosterone activity in Cadmium-exposed rats indicate increase catabolism/metabolism and decrease anabolism/body building. Consequently, it can be hypothesized that Cadmium exposure alters the anabolic-catabolic balance to favor catabolism and decreased anabolism considering the andrological effect of testosterone. In fact, it has been documented that animals living in Cadmium-contaminated environments experiences periods of high metabolic activity that could eventually lead to impaired cortisol secretion and cellular alterations [36]. Thus, the increase Cadmium-induced cortisol activity in this study and which is an indication of catabolism and might lead to reduced body weight and the decreased anabolic-catabolic ratio observed in this study.
To further support the toxic effect of Cadmium exposure, this study showed Cadmium-exposure to increase serum activity of protein. Note that assessment of serum total protein is one of the vital diagnostic tools for organ-system injury; especially the hepatic system. The significant increase in the activity of serum protein in Cadmium-exposed groups is a possibility of Cadmium-induced tissue damage. The observed increase serum protein activity by Cadmium-exposure in this study agrees with the reports [37,38] who reported increased total protein level in Cadmium-treated groups of rats. The increasing trend in protein concentration with exposure duration might be the result of Cadmium interrupting with cellular mechanisms as it accumulates in liver and conjugates with metallothionine [39].
Conclusion
In conclusion, this study showed that Cadmium exposure caused obvious and significant elevation in catabolic damage via stimulation of the endocrine system indicated by increase serum activity of cortisol and decrease serum activity of testosterone. Also, Cadmium induced oxidative damage might possibly be via induced tissue damage indicated by the increase serum level of protein. The toxic effect of Cadmium was more damaging in the acute state. Although this suggests the improved ability of the body system to reduce the damaging effect of cadmium in the chronic effect; thus indicating adaptation, however, this may not be the case in the organ level.
References
- Al-Motabagani MAH (2002) Effect of cadmium on the morphology of adrenal gland in mice. J Anat. Soc. India 51: 212-215.
- Nriagu JO (1980) Cadmium in the environment. Part I, Ecological Cycling. Wiley, NY: 12.
- Muthukumar K, Nachiappan V (2010) Cadmium-induced oxidative stress in Saccharomyces cerevisiae. Indian J. Biochem. Biophy 47: 338-387.
- Robards K, Worsfold P (1991) Cadmium: Toxicology and analysis. A review. Analyst 116: 549-568.
- Thorsen M, Perrone G, Kristianssa E, Traini M, Yel T, et al. (2009) Genetic basis of arsenite and cadmium tolerance in Saccharomyces cerevisiae. BMC Genomics 10: 105-120.
- Santos FW, Oro T, Zeni G, Rocha JBT, Nogueira CW, et al. (2004) Cadmium induced testicular damage and its response to administration of succimer and diphenyl diselenide in mice. Toxicol Lett 152: 255-263.
- Yu HN, Shen SR, Yin JJ (2007) Effects of metal ions, catechins, and their interactions on prostate cancer. Crit. Rev. Food Sci. Nutr 47: 711-719.
- Diana PH (2008) Effect of green tea polyphenols on cadmium toxicity in Coenorhabaditis elegons. NCDR, Poster session, No 2: 2008.
- Kjellstrom T (1979) Exposure and accumulation of cadmium in populations from Japan, the United States and Sweden. Environ. Health Perspect 28: 169-197.
- Lemen RA, Lee JS, Wagoner JR, Blejer HP (1976) Cancer mortality among cadmium production workers. Ann. N.Y. Acad. Sci 271: 273.
- Yosumura S, Vartsky D, Ellis KJ, Cohn SH (1980) Cadmium in Human Beings. In: Cadmium in the Environment, Part-I Ecological Cycling. Wiley, NY, USA. p: 12.
- Bernhoft AR (2013) Cadmium Toxicity and Treatment. The Scientific World Journal. Article ID 3946527.
- Moulis JM (2010) Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 23: 877-896.
- Vesey DA (2010) Transport pathways for cadmium in the intestine and kidney proximal tubule: Focus on the interaction with essential metals. Toxicol. Lett 198: 13-19.
- Gupta RS, Gupta ES, Dhakal BK, Thakur AR, Ahnn J (2004) Vitamin C and vitamin E protect the rat testes from cadmium – induced reactive oxygen species. Mol. Cell 17: 132-139.
- Kara H, Karatas F, Canatan H, Servi K (2005) Effects of exogenous metallothionein on acute cadmium toxicity in rats. Bio. Trace. Elem. Res 104: 223-232.
- Andersen HR, Andersen O (1988) Effect of cadmium chloride on hepatic lipid peroxidation in mice. Pharmacol. Toxicol 63: 173-177.
- Liu J, Liu Y, Michalska AE, Andy Choo KH, Klaassen CD (1996) Distribution and retention of cadmium in metallothionein I and II null mice. Toxicol. Appl. Pharmacol 136: 260-268.
- Vasunilashorn S, Cohen AA (2014) Stress Responsive Biochemical Anabolic/Catabolic Ratio and Telomere Length in Older Adults. Biodemography Soc Biol 60: 174-184.
- Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, et al. (2009) Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure. Impact of Oxidative Stress. Circ Heart Fail 2: 608-615.
- Davis RD (1984) Cadmium-A complex environmental problem: Cadmium in sludge used as fertilizer. Experientia 40: 117-126.
- Norris DO, Hobbs SL (2006) The HPA axis and functions of corticosteroids in fishes. In: Reinecke M, Zaccone G, Kapoor BG (Eds.) 721-766.
- Moore IT, Hopkins WA (2009) Interactions and trade-offs among physiological determinants of performance and reproductive success. Integr Comp Biol 49: 441-451.
- Caride A, Fernandez-Perez B, Cabaleiro T, Bernardez G, Lafuente A (2010) Cadmium chloride exposure modifies amino acid daily pattern in the mediobasal hypothalamus in adult male rat. J. Applied Toxicol 30: 84-90.
- Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biureto reaction. J. Biol. Chem 177: 751-766.
- Bagchi D, Bagchi M, Stohs SJ, Ray SD, Kuszynki CA, et al. (2000) Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology 148:187-197.
- Layachi N, Kechrid Z (2012) Combined protective effect of vitamins C and E on cadmium induced oxidative liver injury in rats. African Journal of Biotechnology 11: 16013-16020.
- Amara S, Abdelmelek H, Garrel C, Guiraud P, Douki T, et al. (2008) Preventive effect of zinc against cadmium-induced oxidative stress in rat testis. J Reprod Dev 54: 129-134.
- Zeng X, Jin T, Zhou Y, Nordberg GF (2003) Changes of serum sex hormone levels and MT mRNA expression in rats orally exposed to cadmium. J Toxicol 186: 109-118.
- Wu SM, Shih MJ, Ho YC (2007) Toxicological stress response and cadmium distribution in hybrid tilapia (Oreochromis sp.) upon cadmium exposure. Comp Biochem Physiol 145: 218-226.
- Chowhdury MJ, Mcdonald DG, Wood CM (2004) Gastrointestinal uptake and fate of cadmium in rainbow trout acclimated to sublethal dietary cadmium. Aquat Toxicol 69: 149-163.
- Yin S, Sheu J, Lin T (2000) Lipid peroxidation in rat adrenal glands after administration cadmium and role of essential metals. J Toxicol Env Heal 62: 47-56.
- Biswas NM, Sengupta R, Chatopadhyay GR, Choudhury A, Sarkar M (2001) Effect of ethanol on cadmium-induced testicular toxicity in male rats. Reproductive Toxicology 15: 699-704.
- Lafuente A, Marquez N, Perez-Lorenzo M, Pazo D, Esquifino AI (2000) Pubertal and postpubertal cadmium exposure differentially affects the hypothalamic-pituitary-testicular axis function in the rat. Food Chem Toxicol 38: 913-923.
- Siu ER, Mruk DD, Porto CS, Chang CY (2009) Cadmium-induced testicular injury. Toxicol Appl Pharm 28: 240-249.
- Sajjad S, Malik H, Farooq U, Rashid F, Nasim H, et al. (2014) Cadmium chloride toxicity revisited: Effect on certain andrological, endocrinological and biochemical parameters of adult male rabbits. Physiol. Res 63: 505-512.
- Sant’ Ana MG, Moraes R, Bernardi MM (2005) Toxicity of cadmium in Japanese quail: evaluation of body weight, renal function and cellular immune response. Environ Res 99: 273-277.
- Zalups RK, Ahmad S (2003) Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol 186: 163-188.
- Godt J, Scheidig F, Siestrup CG, Esche V, Brandenberg P, et al. (2006) The toxicity of cadmium and insulting hazards for the human health. J Occ Toxicol 1: 22-26.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences