ISSN : 0976-8505
Der Chemica Sinica
Biological Evaluation of Newly Synthesized Isoxazole Derivatives
Chandrashekara Raje Urs HR1, Sharath N1* and Jyothi K2
1 Department of P.G. Studies and Research in Analytical Chemistry, Alva’s College, Moodbidre, Dakshina Kannada, Karnataka, India
2 Department of Studies of Chemistry, St. Joseph Engineering College, Vamanjoor, Mangalore Karnataka, India
Abstract
A new bis-isoxazole derivative were synthesized and characterized by the spectral and analytical techniques. The compounds were prepared by the cyclo-addition reaction and comparatively studied by antimicrobial screening with different strains of gram positive and gram negative bacteria showed considerable against bacterial growth. The plasmid DNA interaction with compounds revealed the complete cleavage in 1c and 1d compounds, whereas in other compounds cleaved partially from supercoiled in to linear stranded DNA under UV-visible spectroscopic window. The differential DNA damage beside with H2O2 and light, compound exhibited cleavage in light.
Keywords
Antimicrobial cleavage; Screening; Supercoiled; UV-visible
Introduction
The heterocyclic compounds results in enormous significance in the field of drug discovery process [1]. 1,3-Dipolar cycloaddition is a valuable route for the construction of pharmacological active five-membered isoxazoles. A good yields of isoxazoles is achieved by 1,3-Dipolar cyclisation of nitrile oxides with alkynes were reported [2-4]. Substituted oxazole, pyrazole, isoxazole and their analogues have been used as precursors for synthesis of various biologically dynamic molecules [5,6].
The heterocyclic moieties were found in the most of the natural products with more than one nitrogen, oxygen and sulphur atoms which are fused with unsaturated cyclic compounds. These compounds made the interest in the synthetic and medicinal fields [7]. The natural products having isoxazole rings have property to cure malaria, inflammatory and microbial activity [8,9], some isoxazole compounds cured diseases like viral, allergies and cholesterol level [10-12].
The intercalation of compounds with DNA leads to breaking of nucleotide bonds by the enzymatic activity. The results of cleaving and releasing DNA due to topoisomerase II were found evident in the anticancer activity [13]. In earlier 1980’s the chemical interaction activities with heterocyclic compounds were reported [14-16]. In the recent year synthetic compounds were intended and used for studies as therapeutic moieties in the basic level for the DNA cleavage activity [17]. The interaction of isoxazoles with DNA constitute a significant area of research which has attract considerable attention from biochemists, since studies has shown that they are related to the development of new DNA reagents for biotechnology and medicine [18].
Recently, the effort have been made with continuation of previous work to synthesis the biologically significant isoxazole derivatives [19,20], we demonstrate a new strategy to employing isoxazole rings to the novel precursors without using high dilution conditions. In support of the literature survey, antibacterial activity, DNA binding and DNA photonuclease activity was screened. Further to reveal and understand the mode of action against this biological activity with our newly synthesized bis-isoxazole compounds. We further evaluated the in-vitro antitumor activity of these synthesized compounds.
Materials and Methods
The reagents and solvents were purchased commercially of AR grade. All the solvents were purified by rota evaporation before use. The uncorrected method is followed to evaluate the melting point by using capillary method. The characterization of the compounds has been done by using FT-IR spectrophotometer, proton NMR and ESMS by mass spectrum. The elemental analysis is done by CHNOS analyzer. The thin layer chromatography is done by precoated silica plates. The bacterial strains are procured from national laboratories, Pune, India.
The different substituted aldehyde (1equiv) was dissolved in butanol water system in ratio (3:1) in round bottom flask. Then NH2OH.HCl (1.25 equiv) were added to form aldoxime then TsNCl.3H2O (1.25equiv) were added slowly followed by metal salt and metal turnings(copper sulphate (0.4equiv) and copper metal). The above mixture was stirred for 30 min, later HC2-OH(1.25 equiv) was added. The complete reaction was stirred in room temperature for 4 hours. The completed reaction was poured in to ice after tartan by TLC. Then ice cold mixture is treated with ammonium hydroxide to confiscate the excess metal salts. Then compound was obtained by buckner filtration and dried under vaccum. The purification of compound done by solvent extraction using suitable solvent to obtain 3-derevatives-1, 2-isoxazol-5-yl) methanol [m(1-6)].
The 2 ratio of m (1-6) was dissolved in 20mL of dried DMF in a round bottom flask. The solution is stirred with 60ºC and dichlorobenzene was added drop-wise in one ratio in presence of activated K2CO3 as catalyst. Then mixture was heated for 3-4 hours, after confirmation of TLC reaction mixture was quenched on ice. Then it was filtered, dried and recrystallised using ethyl acetate s(1-6).
Compound 1s: 1-(5-{[3-(1,2-isoxazol-5-ylmethyl)phenoxy]methyl}-2(1,2-isoxazol-3-yl)-benzene): Green in colour, solid, yield-81%, melting point (220-222ºC). FT-IR (KBr) 3100(=C-H), 2854(C-H str ), 1612(C=N), 1578, 1542, 1474(Ar-C=C str), 1350(C-O), 1150(C-O-C)cm-1. 1H-NMR(DMSO-d6), δ 3.5(s, 4H, -CH2), 6.2(s, 2H, Ar-H), 6.3(s, 2H, Ar-H), 6.9(d, 2H, Ar-H), 7.0(t, 2H, Ar-H), 7.2(t, 2H, Ar-H), 7.6(d, 2H, Ar-H), 7.4(s, 1H, Ar-H), 7.5(d, 2H, Ar-H), 7.7(t, 1H, Ar-H). Elemental analysis; Calculated (Found); C26H20N2O4; C,73.57(73.55); H,4.75(4.72); N,6.60(6.56), O,15.08(15.11). Mass ES-MS m/z(%) [M]+; [425]+.
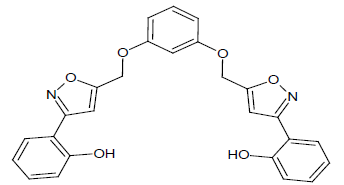
Compound 2s: 1-(5-{[3-(1,2-isoxazol-5-ylmethyl)phenoxy]methyl}-2(1,2-isoxazol-3-yl)phenol): Pale Green in colour, solid, yield-77%, melting point(236-238ºC). FT-IR (KBr) 3400(Ar-OH), 3104(=C-H), 2852 (C-H str), 1609(C=N), 1576, 1544, 1470(Ar-C=C str), 1352(C-O), 1152(C-O-C) cm-1. 1H-NMR(DMSO-d6), δ 3.4(t, 2H, C-H), 4.8(bs, 2H, Ar-OH), 6.2(d, 2H, Ar-H), 6.6(d, 4H, Ar-H), 6.9(d, 4H, Ar-H), 7.3(s, 2H, Ar-H), 7.5(s, 4H, -CH2),. Elemental analysis; calculated (Found); C26H20N2O6; C,68.42 (68.44); H,4.42(4.44); N,6.14(6.11), O,21.03(21.08). Mass ES-MS m/z(%) [M]+; [457]+.
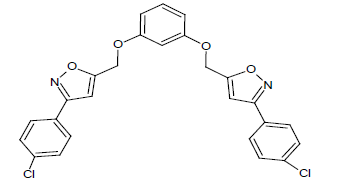
Compound 3s: 1-(5-{[3-(1,2-isoxazol-5-ylmethyl)phenoxy]methyl}-2(1,2-isoxazol-3-yl)-chloro-benzene): Brown in colour, solid, yield-74%, melting point(198-200ºC)FT-IR (KBr) 3104(=C-H), 2850(C-H str ), 1611(C=N), 1580, 1540, 1470(Ar-C=C str), 1354(C-O), 1153(C-O-C) 750(C-Cl) cm-1. 1H-NMR(DMSO-d6), δ 3.3(s, 4H, -CH2), 6.2(s, 1H, Ar-H), 6.9(t, 2H, Ar-H), 7.0(t, 2H, Ar-H), 7.2(d, 2H, Ar-H), 7.4(t, 1H, Ar-H).7.8(s, 4H, Ar-H), 7.9(d, 2H, Ar-H), Elemental analysis; calculated(Found); C26H18N2O4Cl2; C,63.30 (63.32); H,3.68(3.72); N,5.68(5.64), O,12.97(12.94). Mass ES-MS m/z(%) [M]+; 35Cl [494]+ and 37Cl [496]+
Compound 4s: 1-(5-{[3-(1,2-isoxazol-5-ylmethyl)phenoxy]methyl}-2(1,2-isoxazol-3-yl)naphthalen -2-ol): Dark Green in colour, solid, yield-70%, melting point(208-210ºC)FT-IR (KBr) 3400(Ar-H), 3100(=C-H), 2856(C-H str ), 1614(C=N), 1578, 1542, 1474(Ar-C=C str), 1350(C-O), 1150(C-O-C)cm-1. 1H-NMR(DMSO-d6), δ 3.4(s, 4H, Ar-H), 5.1(s, 2H, Ar-OH), 6.0(d, 2H, Ar-H), 6.8(d, 2H, Ar-H), 7.0(d, 2H, Ar-H), 7.1(t, 2H, Ar-H), 7.3(d, 2H, Ar-H), 7.4(s, 2H, Ar-H), 7.5(d, 2H, Ar-H), 7.6(t, 2H, Ar-H), 7.7(s, 1H, Ar-H), 7.9(t, 1H, Ar-H). Elemental analysis; Calculated (Found); C34H24N2O6; C,73.37 (73.35); H,4.35(4.32); N,5.03(5.02), O,17.25(17.22). Mass ES-MS m/z(%) [M]+; [557]+.
Compound 5s: 1-(5-{[3-(1,2-isoxazol-5-ylmethyl)phenoxy]methyl}-2(1,2-isoxazol-3-yl)-3-hydoxy-4-methoxybenzene): Pale brown in colour, solid, yield-69%, melting point(250-252ºC)FT-IR (KBr) 3420(Ar-OH), 3102(=CH), 2850(C-H str ), 1609 (C=N), 1576, 1546, 1476(Ar-C=C str), 1354(C-O), 1254(O-CH3), 1152(C-O-C) cm-1. 1H-NMR(DMSO-d6), δ 3.6(s, 4H, -CH2), 4.1(s, 6H, Ar-O-CH3), 5.0(s, 2H, Ar-OH), 6.7(s, 2H, Ar-H), 6.9(d, 2H, Ar-H), 7.4(s, 2H, Ar-H), 7.5(d, 2H, Ar-H),7.6(d, 2H, Ar-H), 7.7(t, 2H, Ar-H). Elemental analysis; calculated(Found); C28H24N2O8; C,65.11 (65.13); H,4.68(4.71); N,5.42(5.40), O,24.78(24.76). Mass ES-MS m/z(%) [M]+; [517]+.
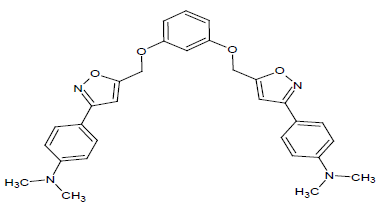
Compound 6s: 1-(5-{[3-(1,2-isoxazol-5-ylmethyl)phenoxy]methyl}-2(1,2-isoxazol-3-yl)-4,4-dimethyl aminobenzene): Yellowish Brown in colour, solid, yield-84%, melting point(254-256ºC)FT-IR (KBr) 3100(=C-H), 2854(CH str) ,1720(-O-CH3) 1612(C=N), 1578, 1542, 1474(Ar-C=C str), 1348(C-O), 1152(C-O-C)cm-1. 1H-NMR(DMSO-d6), δ 2.5(s, 12H, N-CH3), 3.4(s, 4H, CH2), 6.2(s, 1H, Ar-H), 6.9(d, 2H, Ar-H), 7.0(s, 4H, Ar-H), 7.2(s, 4H, Ar-H), 7.4(t,1H, Ar-H)7.6(s, 2H, Ar-H),. Elemental analysis; Calculated (Found); C30H30N4O4; C,70.57 (70.55); H,5.92(5.58); N,10.97(10.95), O,12.53(12.49). Mass ES-MS m/z(%) [M]+; [511]+.
Antibacterial activity
Both the gram +ve and -ve pathogenic bacterial strains were maintained at 30ºC in the infusion mixture. The testing bacterial sample were cultured 24 hours before testing, the inocula was prepared depending on its turbidity were made(w/v) dilutions which matches the standards [21].
The cup-plate methods were adopted for the antibacterial assay. The agar is used as substrate for the bacterial growth with peptone and beef extract [22]. The bacterial strains were inoculated and incubated for 24 hours in 90 mm diameter petriplates were used with appropriate ingredients in media. The final concentration of bacteria of each strain was used with different concentrations of compounds (10 mg/mL) and reference drug (ciproflaxin). After incubation at 37ºC for 24 h inhibition zones are measured the diameter in millimeters.
MIC evaluation
The micro dilution technique is used for evaluating MICs of the newly synthesized compounds s (1-6). As per USA guidelines, the bacterial colonies were diluted for 1:10 ratio. The diluted colonies were incubated in microtiter plates for 24 hours. The minimum inhibitory concentration of bacteria with compound reveals the potent antibacterial activity with standard drug.
DNA Photocleavage studies
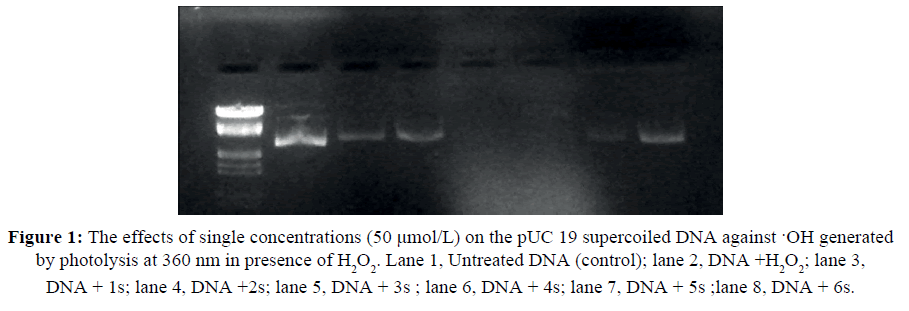
The scope of DNA cleavage studies of pUC19 DNA (0.5 ml, 0.5 mg) was resolute by agarose gel electrophoresis. The Tris-HCl buffer is used as electrolyte for electrophoretic method. The DNA and compounds were irradiated in UVtransilluminator in room temperature for 1hour. The gel is prepared by addition of dyes and casted in electrophoretic boat [23]. The experimental setup was carried out at 50 V for 3 hours in Tris-borate EDTA (TBE) buffer. The DNA cleavage were visualized by UV light and photographed to determine the extent by gel doc system. The supercoiled is cleaved single stranded after nic form is also observed in 360 nm [24].
Results and Discussion
The synthesis of bis-isoxazole is two-step process. The different aldehydes were first converted to aldoxime by using click chemistry. The room temperature conditions were maintained due to presence of catalyst in first step and the oxidative coupling is avoided by using of slight excess of TsNCl.3H2O which was observed and reported. The presence of specific metal catalyst mediates the synthesis of isoxazole series of compounds m(1-6).
In the first step aldehyde (1-6) were reacted to form respective aldoxime by using NH2OH.HCl, then it is converted to nitrile oxide using TsNCl.3H2O. Then is cyclisation of nitrile oxide by using propagyl alcohol to form the derivatives of m (1-6). This procedure affords good yields by cyclization with short reaction times and simple purification. The second step 1:2 ratio of dichlorobenzene and 3-derevatives-1,2-isoxazol-5-yl)methanol[m(1-6)] were refluxed for 4 hrs in presence of activated K2CO3 as catalyst to obtain bis-isoxazole[s(1-6)]. The compounds were spectrophotometrically characterized by using FT-IR, ES-MS, 1H NMR. The different derevatives of bis-isoxazole are shown in Table 1.
| Comp.No | Aldehyde | Product | Yield(%) |
|---|---|---|---|
| 1s | 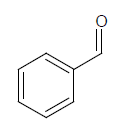 |
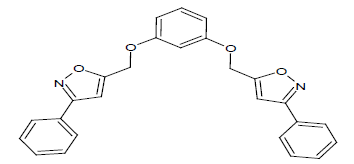 |
68 |
| 2s |  |
 |
72 |
| 3s | 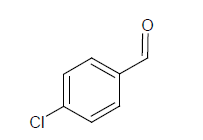 |
 |
69 |
| 4s | 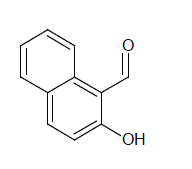 |
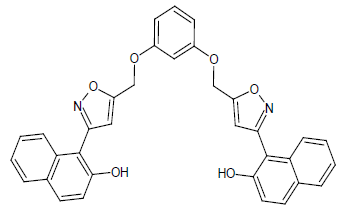 |
84 |
| 5s | 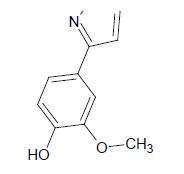 |
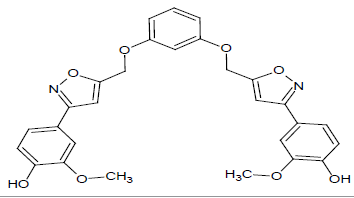 |
86 |
| 6s | 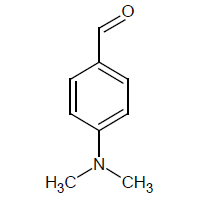 |
 |
74 |
Table 1: The different derivatives of isoxazole and its yield.
The synthesized derevatives of isoxazoles s(1-6), The IR bands of compounds showed at 2847-2850 cm-1 (C-H str) ,at 1612-1608 cm-1 (C=N), 1648-1652 cm-1 (C=O), 3388-3382 cm-1 (Ar-OH), 1724-1720 cm-1 (O-CH3) and 694-690 cm-1 (Ar-Cl). Whereas in 1H-NMR, the δ value showed the singlet peak at 3.5 for -CH2, the presence of -OH protons showed at 5.0, the CH group of isoxazole moeity showed singlet signal at 6.1-6.3, and aromatic protons signal at 6.9- 7.7 ppm.
In compound 2s, The FT-IR showed peak at 2854 cm-1 for C-H streching, the peaks at 1578, 1542, 1474 cm-1 showed for Ar-C=C, In proton NMR δ value showed broad singlet for aromatic -OH group, the signal at 6.9 showed doublet for Ar-H, The compounds 2s, 4s and 5s showed the broad singlet signals at 5.0-5.2 ppm corresponds to -OH proton. The aromatic protons showed the multiplet in 6.9-8.2ppm. The CHON analysis obtained which is having ± 0.5% vatiation to the calculated values was reported. The ES-mass spectroscopic results of compound s(1-6) in a positive ionization mode was observed with peak intensities and molecular weight had determined. The characteristic isotopic peaks found in the presence of halogen compounds. The molecular ion peak of compounds 1s; 425.2, 2s; 457.6, 3s; 35Cl: 494.0, 37Cl: 496.0, 4s; 557.2, 5s; 517.4. 6s; 511.9 m/z values were obtained respectively.
Antibacterial activity
The antibacterial activities of the synthesized compounds were screened with pathogenic gram+ve and gram-ve bacterial strains with reference drug. The zone of inhibition is calculated in term of diameter (mm). The compounds s (1-6) showed good results compared to that of ciproflaxin. The MIC values of compounds with bacteria were discussed in the Table 2 and Table 3.
| Zone of inhibition(mm) in diameter | ||||
|---|---|---|---|---|
| compounds | S. aureus | S.pyogenes | P.aeruginosa | B.subtilis |
| 1s 2s 3s 4s 5s 6s Ciproflaxin |
8.10 ± 0.10 9.80 ± 0.10 8.60 ± 0.10 9.50 ± 0.10 8.40 ± 0.10 8.00 ± 0.10 12.60 ± 0.10 |
7.20 ± 0.10 8.40 ± 0.10 7.10 ± 0.10 7.60 ± 0.10 7.60 ± 0.10 8.10 ± 0.10 13.50 ± 0.10 |
7.50 ± 0.10 8.20 ± 0.10 8.30 ± 0.10 8.20 ± 0.10 7.80 ± 0.10 8.20 ± 0.10 12.20 ± 0.10 |
7.60 ± 0.10 9.40 ± 0.10 8.60 ± 0.10 8.40 ± 0.10 7.40 ± 0.10 6.80 ± 0.10 11.80 ± 0.10 |
Table 2: The compound s(1-6) showing the bacterial inhibition.
| Minimal inhibitory concentration of synthesized compounds (mg/mL) | ||||
|---|---|---|---|---|
| compounds | S. aureus | S.pyogenes | P.aeruginosa | B. subtilis |
| 1s 2s 3s 4s 5s 6s Ciproflaxin |
6.2 7.5 6.1 6.4 6.3 6.5 3.55 |
5.8 8.9 8.7 5.8 5.9 5.2 3.65 |
6.3 6.5 6.5 6.8 7.1 5.9 3.35 |
5.2 6.1 6.2 6.6 6.4 7.0 3.85 |
Table 3: The table with different MIC values of compounds.
The considerable values of compound due to the presence of functional group like -OH, Cl and -O-CH3 played an important role in the breaking of peptidoglycan layer and the phospholipid layer of the bacteria [25,26].
The compound 2s, 3s, 4s and 5s showed potent inhibition zone and MIC values. Whereas the other moiety showed meager values compared to standard drug. The S. aureus and B. subtilis showed good MIC values such as 7.5 and 6.6 mg/mL where other compound for P. aeruginosa and S. pyogenes showed 8.9 and 6.1 mg/mL concentration in the compounds.
The highest zone inhibition activity of compounds shows better results in the minimum inhibitory concentration. The gram +ve and gram -ve pathogenic bacteria showed least MIC and more zone inhibition due to the breaking of permeable membrane. This effect showed good anti-bacterial activity.
DNA Photocleavage studies
The photo cleavage activity of DNA of compounds s (1-6) was studied under the UV-therapeutic window. The oxidative reagent and the UV light treatment were used for the comparison of activity with same concentration (50 μM/L) of compounds after incubation at 37ºC and irradiation at 360 nm. The UV light treatment showed better results in cleaving up off plasmid in to single stranded through NIC form. The compound 3s and 4s showed complete cleavage whereas the other compounds showed moderate cleavage from double stranded to single stranded DNA.
The cleavage of DNA reveals that the compound have anti-carcinogenic property by the results shown in Figure 1. The same concentration of compounds can stop the multiple division by the cell in cancer due to its DNA nuclease activity. Thus our synthesized compound s (1-6) has the property to inhibit the cancer cell growth.
Figure 1: The effects of single concentrations (50 μmol/L) on the pUC 19 supercoiled DNA against ·OH generated by photolysis at 360 nm in presence of H2O2. Lane 1, Untreated DNA (control); lane 2, DNA +H2O2; lane 3, DNA + 1s; lane 4, DNA +2s; lane 5, DNA + 3s ; lane 6, DNA + 4s; lane 7, DNA + 5s ;lane 8, DNA + 6s.
Conclusion
The result of investigation reports reveals the structures of synthesized compounds s (1-6) by analytical techniques. In view of importance of biological application of bis-isoxazole derivatives, the compounds showed inhibition in the bacterial growth with inhibitory concentration. The compounds by their interactions with DNA are of major biochemical and biological importance. The biological evaluation and the DNA interaction studies showed high potential as DNA photo cleaving agent. The DNA cleavage activity is evidence for the anti-carcinogenic activity. By this we can conclude that our newly synthesized compounds have importance in medicinal/ biological field.
References
- Katritzky AR (2010) H. B. Peptidomimetics via modifications of amino acids and peptide bonds Hetero. Chem. Elsevier Ltd. III Edn.
- Violette A, Averlant-Petit MC, Semetey V, Hemmerlin C, Casimir R, et al. (2005) N, N '-Linked oligoureas with proteinogenic side chains are peptide backbone mimetics belonging to the γ-peptide lineage. J Am Chem Soc 127: 2156-2164.
- Matthew P, Bourbeau, James TR (2006) Copper-Catalyzed Alkylation of Nitroalkanes with α-Bromonitriles: Synthesis of β-Cyanonitroalkanes. Org let 8: 3679-3680.
- Tornoe CW, Christensen C, Meldal. M (2002) Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67: 3057-3064.
- Gothelf KV, Jorgensen KA (1998) Asymmetric 1, 3-dipolar cycloaddition reactions. Chem Rev 98: 863-909.
- Jose A, Arno SM, Luis R, Domingo (2003) A DFT study for the regioselective 1,3- dipolar cycloadditions of nitrile N-oxides towardalkynylboronates. Tetrahedron 59: 9167-9171.
- Hill DJ, Mio MJ, Prince RB, Hughes TS, Moore JS (2001) A Field Guide to Foldamers. Chem Rev 101: 3893-4011.
- Dube D, Blouin M, Brideau C, Chan CC, Desmarais S, et al. (1998) Quinolines as potent 5-lipoxygenase inhibitors: Synthesis and biological profile of L-746,530. Bioorg Med Chem Lett 8: 1255-1260.
- Maguire MP, Sheets KR, McVety KT (1994) A New Series of PDGF Receptor Tyrosine Kinase Inhibitors: 3-Substituted Quinoline Derivatives. J Med Chem 37: 2129- 2137.
- Jiang JB, Isaacson D (1987) US Patent. 4, 656, 274.
- Graeve RE, Pociask JR, Stein RG (1971) US Patent 3, 600,393.
- Raghavendra M, Naik HSB, Sherigara BS (2006) One pot synthesis of some new 2-hydrazino-[1,3,4]thiadiazepino[7,6-b]quinolines under microwave irradiation conditions. Arkivoc15: 153-159.
- Gatto B, Capranico G, Palumbo M (1999) Drugs acting on DNA topoisomerases: recent advances and future perspectives. Curr Pharmacol Design 5: 195-215.
- Song YL, Li YT, Wu ZY (2015) Synthesis and Biological Studies of Some Lanthanide Complexes of Schiff Base. J Inorg Bio Chem 45: 1617-1626.
- Downey VM, Que BR, So AG (1980) Synthesis, characterization, theoretical study and biological activities of oxovanadium (IV) complexes with 2-thiophene carboxylic acid hydrazide. Biochem Biophys Res Comm 93: 264-270.
- Yang ZS, Wang YL, Zhao GC (2004) Electrocatalytic Oxidation of Dopamine and Ascorbic Acid at Poly (Eriochrome Black-T) Modified Carbon Paste Electrode. Anal Sci 20: 1127-1135.
- Mokle SS, Khansole SV, Patil RB, Vibhute YB (2010) Synthesis and antibacterial activity of some new chalcones and flavones having 2-chloro-8-methoxyquinolinyl moiety. Inter J of Pharma and Bio Sci 1: 1-7Med. Chem.
- Sigel H, Sigel A (2002) Metal ions in biological systems, Marcel Dekker, New York. 26-32.
- Kumar BV, Naik HSB, Girija D, Sharath N (2012) Synthesis and Biological Evaluation of New Tetra-aza Macrocyclic Scaffold Constrained Oxadiazole, Thiadiazole and Triazole Rings. Arch Pharm Chem Life Sci 345: 240-249.
- Sharath N, Naik HSB, Kumar VB, Hoskeri J (2011) Antibacterial, Molecular Docking, DNA Binding and Photocleavage Studies on Novel Heterocyclic Pyrazoles. British J Pharma Res 1: 46-65.
- Nair R, Kalariya T, Chanda S (2004) Antibacterial Activity of Some Selected Indian Medicinal Flora. Turk J Biol 29: 41-47.
- Bhat R, Xue Y, Berg S, Hellberg S, Ormö M, et al. (2003) Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem 278: 45937-45945.
- Naik P, Naik HRB, Lamani HS, Aravinda DS, VinayKumar T, et al. (2009) Benzo[h]quinoline based Macrocyclic Copper(II), Cobalt(II) Complexes: Synthesis, Characterization and Light induced DNA Cleavage Studies J Macromol Sci Part A Pure Appl Chem 46: 790-795.
- Sharath N, Naik HSB, et al. (2012) Synthesis, Antibacterial, Molecular Docking, DNA Binding and Photonuclease Activity of Quinoline Isoxazoles. Der Pharmacia Sinica 3: 254-265.
- Prabahkara MC, Naik HSB (2008) Co (III) and Ni(II) Complexes Containing Bioactive Ligands: Synthesis, DNA Binding, and Photocleavage Studies. Biometals 2: 675-684.
- Binkowski TA, Naghibzadeg, et al. (2003) CASTp:computed atlas of surface topography of proteins. Nucleic Acid Res 31: 3352-3355.
- Sharath N, Naik HSB, Kumar BV (2012) Synthesis, DNA binding and photonuclease activity of new tetraaza macrocyclic constrained isoxazole rings as sub unit in metal complexes. Nucleosides, Nucleotides and Nucleic Acids 31: 1-17.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences