ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Bioequivalence Comparison of Two Formulations of Fixed Dose Combination Sitagliptin/Metformin (50/1000 mg) Immediate Release (IR) Tablets in Fed Condition
Evelyn Pena1*, Alfredo Inatti1, Jose Chacon2, Anyoli Taly3 and Xenon Serrano Marti3
1Department of Clinical Research, Industrias Biocontrolled C.A, Guarenas, Venezuela
2Department of Medicine, Megat Pharmaceutical Internal Medicine, Quito, Ecuador
3Department of Research and Development, Industrias Biocontrolled C.A, Guarenas, Venezuela
- *Corresponding Author:
- Evelyn Pena
Department of Clinical Research, Industrias Biocontrolled C.A, Guarenas, Venezuela
E-mail: martinpena24@gmail.com
Received date: October 22, 2024, Manuscript No. IPAPP-24-19825; Editor assigned date: October 25, 2024, PreQC No. IPAPP-24-19825 (PQ); Reviewed date: November 8, 2024, QC No. IPAPP-24-19825; Revised date: November 15, 2024, Manuscript No. IPAPP-24-19825 (R); Published date: November 22, 2024, DOI: 10.36648/2393-8862.11. S1.02
Citation: Pena E, Inatti A, Chacon J, Taly A, Marti SX (2024) Bioequivalence Comparison of Two Formulations of Fixed Dose Combination Sitagliptin/Metformin (50/1000 mg) Immediate Release (IR) Tablets in Fed Condition. Am J Pharmacol Pharmacother Vol.11 No. S1: 02.
Abstract
Background: This study aimed to evaluate the bioequivalence of the Fixed-Dose Combination (FDC) sitagliptin 50 mg/metformin 1000 mg tablets compared to Janumet® (sitagliptin 50 mg/metformin 1000 mg tablets) in healthy male volunteers under fed conditions.
Methods: This was a randomized, single-dose, open-label, two-period, twosequence, crossover and single-dose study to compare the Bioequivalence (BE) profile of two FDC of sitagliptin 50 mg/metformin 1000 mg Immediate Release (IR) in 26 adult healthy subjects. The Pharmacokinetic (PK) parameters Cmax and AUC0-t were calculated based on the plasma drug concentration-time profile measured by Liquid Chromatography-Mass Spectrometry (LC-MS/MS). The safety was assessed throughout the study. Bioequivalence was evaluated using 90% Confidence Intervals (CIs) for the ratio test/reference of log Area Under the Concentration- Time Curve (AUC) from time 0 to the last quantifiable concentration and log peak concentration. The two formulations Test (T) and Reference (R) were considered bioequivalent if 90% Confidence Interval (CI) were within BE acceptance range of 80.00%-125.00% for Cmax and AUC0-t.
Results: All 26 subjects completed both study periods. The 90% Confidence Intervals (CIs) of the test/reference ratio were Cmax: 110.46% (103.26%-118.15%) and AUC0-t 104.82% (99.81%-110.08%) of sitagliptin and Cmax: 99.85% (93.61%- 106.52%) AUC0-t: 102.51 (96.57%-108.82%) to metformin. PK parameters were within the accepted bioequivalence criteria. The results show that no significant differences were observed between the pharmacokinetic profiles of the T and R formulations. No serious adverse events were reported in this study.
Conclusion: The two formulations of sitagliptin 50 mg/metformin 1000 mg IR (FDC) were bioequivalent in healthy subjects under fed conditions. The geometric mean ratio and 90% CI for primary PK parameters, Cmax and AUC0-t, of T and R formulation were within the range 80% to 125%.
Keywords
Bioequivalence; Fixed dose combinations; Pharmacokinetic; Sitagliptin
Introduction
Sitagliptin and metformin HCl tablets contain two oral antihyperglycemic drugs used in the management of Type 2 Diabetes Mellitus (T2DM). Sitagliptin phosphate is a Dipeptidyl Peptidase-4 (DPP-4) inhibitor and metformin hydrochloride, is member of the biguanide class [1]. Sitagliptin phosphate with a molecular formula: C16H15F6N5O and weight of 523.32 g/mol, is an orally-active, potent and highly selective inhibitor of the Dipeptidyl Peptidase-4 (DPP-4) enzyme for the treatment of Type 2 Diabetes Mellitus (T2DM) [2]. The DPP-4 inhibitors are a class of agents which are incretin enhancers secretion from pancreatic alpha cells, leading to reduced hepatic glucose production. When blood glucose levels are low, insulin release is not enhanced and glucagon secretion is not suppressed. Sitagliptin is a potent and highly selective inhibitor of the enzyme DPP-4 and does not inhibit the closely-related enzymes DPP-8 or DPP-9 at therapeutic concentrations. By inhibiting the DPP-4 enzyme, sitagliptin increases the levels of two known active incretin hormones, Glucagon-Like Peptide-1 (GLP-1) and Glucose-dependent Insulinotropic Polypeptide (GIP). The incretins are part of an endogenous system involved in the physiologic regulation of glucose homeostasis. When blood glucose concentrations are normal or elevated, GLP-1 and GIP increase insulin synthesis and release from pancreatic beta cells. GLP-1 also lowers glucagon [2-4]. Following oral administration of a 100 mg dose in healthy subjects, sitagliptin was rapidly absorbed, with peak plasma concentrations (median Tmax) occurring 1 h to 4 h post-dose, the mean plasma AUC was 8.52 μM.h, Cmax was 950 nm. The absolute bioavailability of sitagliptin is approximately 87%.
Since co-administration of a high-fat meal with sitagliptin had no effect on the pharmacokinetic, sitagliptin may be administered with or without food. The mean volume of distribution at steady state is approximately 198 L and the fraction of sitagliptin reversibly bound to plasma proteins is low (38%) [2,3]. Sitagliptin is primarily eliminated unchanged in urine and metabolism is a minor pathway. Approximately 79% is excreted unchanged in the urine. Sitagliptin was eliminated in faeces (13%) or urine (87%) within one week of dosing. The apparent terminal T½ following a 100 mg oral dose of sitagliptin was approximately 12.4 h [2- 6]. In monotherapy, adverse events more frequently reported were headache, hypoglycaemia, constipation and dizziness [3,6]. Metformin is a biguanide with a molecular formula, C4H11N5 and molecular weight of 129.16 with anti-hyperglycaemic effects, lowering both basal and postprandial plasma glucose [7]. It does not stimulate insulin secretion and therefore does not produce hypoglycaemia [8]. Metformin may act via three mechanisms: 1) Reduction of hepatic glucose production inhibiting gluconeogenesis and glycogenolysis; 2) Modestly increasing insulin sensitivity, improving peripheral glucose uptake and 3) Utilization and delaying intestinal glucose absorption [9].
Metformin stimulates intracellular glycogen synthesis by acting on glycogen synthase. Metformin increases the transport capacity of specific types of membrane Glucose Transporters (GLUT-1 and GLUT-4). After an oral dose of metformin 500 mg, the Tmax was reached in 2.5 h and the bioavailability was 50%- 60% [8-10]. The non-absorbed fraction recovered in faeces was 20%-30%. The elimination half-life of metformin during multiple dosages in patients with good renal function is approximately 5 h. The blood peak is lower than the plasma peak concentration and appears at approximately the same time. Metformin is excreted unchanged in the urine. No metabolites have been identified in humans. Renal clearance of metformin is >400 mL/min, indicating that metformin is eliminated by glomerular filtration and tubular secretion. Following an oral dose, the apparent terminal elimination half-life is approximately 6.5 h.
Materials and Methods
The study was conducted ethically in accordance with the principles of the ICMR guidelines (2017) [16], New Drugs and Clinical Trials Rules 2019 India [17] and adhered to the ethical principles of the Declaration of Helsinki [18] the International Conference on Harmonization Good Clinical Practice Guidelines [19]. The study protocol (N°096-22) was approved by Aavishkar Ethics Committee, on March 06, 2023 (Version: 00, Dated 06 Dec 2022) and certified by CDSCO/DGHS to VerGo Clinical Research Pvt, Ltd.
Study design
This was an open-label, randomized, two-treatment, two-period, two-sequence, single oral dose and crossover Bioequivalence (BE) study under fed conditions comparing, two formulations of sitagliptin 50 mg/metformin 1000 mg tablets Immediate Release (IR), batch N°004, date of expiry 06/2024, of Laboratorios Leti S.A.V., as test formulation vs. Janumet® (sitagliptin 50 mg/ metformin 1000 mg) IR, batch N°W006655, date of expiry 11/2023, of Merck Sharp & Dohme Corp, as reference formulation [16-19].
The subjects were randomized, to one of the two sequences (T-R) or (R-T). The randomization schedule was generated using Statistical Analysis Software (SAS®, version 9.4, Institute. Inc., CARY, USA). One single dose was administered in each period. Subjects who received T product in period I were administered R product in period II and vice versa. Pre-screening period was 21 days. The total duration of the clinical phase of this study was of 11 days from the day of check-in of period I to last blood sample collection of period II (April 15, 2023-April 22, 2023) separated by a washout period of 7 days, considering the terminal half-life for sitagliptin is 12.4 h. [2,6] and metformin 6.5 h. [8,10,13]. This BE study in FDC met the principles described in bioequivalence European Union (EU) guideline [20] and the in fed condition selection was based on metformin immediate release and Summary of Product Characteristics (SmPC) recommendations [20,21]. Sitagliptin EU guidelines recommend to do BE with highest strength, is possible to use the lower strength with a linear PK and high solubility [22].
All volunteers underwent a screening procedure. A total of 26+2 (stand by) healthy, adults male volunteers who met the inclusion and exclusion criteria were enrolled, with a mean age of 32.30 years, mean weight 71.02 kg, mean height 1.67 cm and Body Mass Index (BMI) of 25.18 kg/m2 (Table 1).
| Age | Mean ± SD | 32 ± 4.87 | ||
| Years | Range | 25-43 | ||
| Age group | Male | % | Total | |
| 25-40 | 24 | 92.30% | 24/92.30% | |
| 41-43 | 2 | 7.70% | 2/7.70% | |
| Total | 26 | 26 | 100% | 26/ 100% |
| BMI (kg/m2) | Mean ± SD | 25.18 ± 2.7 | ||
| Range | (18.90-29.39) | |||
| Race | Asian | 26 | 100% | |
Table 1: Demographic profile of subjects completing the bioequivalence study (n=26).
A complete clinical history valid for 6 months before the start of the study, normal laboratory values as determined by medical history and physical examination at the time of screening, normal vital signs and physical examination, creatinine clearance of more than 50 mL/min, negative tests for hepatic transaminases, hepatitis B and C, human immunodeficiency virus and venereal diseases research laboratory and normal 12-lead Electrocardiogram (EKG) values, normal chest radiography and negative result in urine drug tests. Urine for drugs of abuse and urine test for alcohol consumption were performed on day of check in of each period. Random blood glucose test was performed in screening. Fasting blood glucose test was conducted prior to check-in of period I. In each period, subject´s blood glucose monitoring was performed before dosing and at 02.00, 04.00, 06.00 and 11.00 h ± (30 min) after dosing. Other key inclusion criterion was that subjects must be non-smokers or smokers who had not smoked at least 10 h before the start of the study. They all signed the informed consent. The exclusion criteria included volunteers incapable of understanding the informed consent, history of diabetes, tuberculosis and systemic hypertension. A history of hypersensitivity to the study medication or to any other medication belonging to the study group or cardiovascular, renal, hepatic, metabolic, gastrointestinal, neurological, endocrine, hematopoietic, psychiatric, or other organic abnormalities, under medication that interferes with the quantification, drugs that can potentially affect the hepatic metabolism of other drugs.
Drug administration
The subjects were admitted to the facility one night before study. Each subject received standard dinner on the day of check in after which they fasted for 10 h prior to consuming standard high-fat, high-calorie, non veg breakfast (800-1000 kcal) which was served 30 min before scheduled time of dosing. Being a fed study, subjects were served high-fat, high-calorie breakfast on the dosing day. Subjects were fasted for 4 h after dosing in each period. The subjects received standard meals at 04.00 (lunch), 08.00 (snacks) and 12.00 h (dinner) after dosing in each period. All meal plans were identical for each period of study.
A single oral dose (sitagliptin 50 mg/metformin 1000 mg tablets IR) either one T or R, following randomization schedule and was administered with 240 mL ± 2 mL of glucose solution in water, followed by 60 mL of 20% glucose solution in water administered every 15 min for up to 4 h after dosing at ambient temperature to each subject in sitting position [23,24].
A total of 24 × 5 ml of venous blood samples were collected through cannula from each subject during the two periods of the study, withdrawn at pre-dose (-02.00-00.00 h) and 00.50, 01.00, 01.33, 01.67, 02.00, 02.33, 02.67, 03.00, 03.33, 03.67, 04.00, 04.50, 05.00, 05.50, 06.00, 07.00, 08.00, 10.00, 12.00,16.00, 24.00, 36.00 and 48 h after dosing in each period. While 24.00, 36.00 and 48.00 h, post dose, blood samples were collected by direct venepuncture. The subjects received standard high-fat, high-calorie, non-veg breakfast (800-1000 kcal) 30 min before scheduled time of dosing and drinking water was provided ad libitum.
Analytical procedure
Venous samples were collected in pre-labelled K2 EDTA (ethylenediaminetetraacetic acid) vacutainers and were centrifuged at 3800 rpm for 10 min at 10°C within 45 min of sample collection. Plasma was separated, labeled and stored at -70°C ± 15°C before analysis. Subsequently, the plasma samples were processed, calibration curve of Internal Standards (ISTD) sitagliptin D4 and metformin D6, (Vivian Life Sciences Private Limited, Mumbai, India) and Quality Control (QC) samples were thawed and vortexed for preparation and analysis. Method of validation of sitagliptin and metformin was conducted with calibration 2.101 ng/mL to 599.208 ng/mL and 14.040 ng/mL to 4004.046 ng/mL respectively and validation results were reported in Method Validation Report (MVR) 191-20 version 00. The bio study was performed on API 4000 system coupled with LC using sitagliptin D4 and metformin D6 as internal standard. The interface used was turbo iron spray and positive ions were measured in MRM mode. The samples were extracted by a solid phase extraction chromatography column (StrataTM) × 33 μm coupled to a ZORBAX SB-C18, 46 × 75 mm, 3.5 μm column. Elution was performed at 25°C, with a mobile phase of methanol: 10 mM ammonium formate buffer (70:30/v/v). The lower limit of quantification was 2.102 ng/mL to sitagliptin and 14.083 ng/ mL for metformin whereas the upper limit of quantification was 597.549 ng/mL for sitagliptin and 4003.999 ng/mL for metformin. These data were acquired, integrated and quantified on AB Sciex systems Shimadzu, analyst version 1.7.3 software.
A Stock Solution (SS) for sitagliptin was prepared with 2.4483 mg to Calibration Curve (CC) standards and 2.3767 and for QC, SS was done by two different analysts (CC-ISTD 951511.373 ng/mL), (QC-ISTD 923684.630 ng/mL) to sitagliptin D4, the CC standard was 114820.061 ng/mL.
For metformin SS were prepared with 2.5319 mg for CC and 2.4417 mg for QC, the concentration of SS was 973735.138 ng/mL (CC) and 939045.415 ng/mL (QC). Metformin D6 (SS) was prepared with 1.0869 mg stock concentration of ISTD was 855789.324 ng/ mL were made using diluent (Methanol: Water in ratio 50:50 v/v) and stored at 2.0-8.0.
Metformin was prepared with 1.0869 mg (ISTD 855789.324 ng/ mL) and dissolved in 1000 mL of methanol. All solutions were prepared using diluent (Methanol: Water, 50:50 v/v) and stored at 2.0°C-8.0°C. The supernatant identifications were based on multiple reactions monitoring transitions, m/z 235.100-408.200 for sitagliptin and m/z 239.000-412.300 for the IS-sitagliptin D4. And m/z 71.200-130.100 for metformin and m/z 77.200-136.200 for the IS-metformin D6. The Inter-Batch Calibration Standard (IBCS) was 1.19% to 3.33%, accuracy 93.20% to 103.21% for sitagliptin and IBCS was 1.01% to 3.28%, accuracy 97.61% to 104.50% for metformin.
Statistical analysis
The sample size calculation for the study was based on intrasubject Coefficient of Variation (CV%) obtained of published data for sitagliptin (Cmax: 16.61% and AUC0-t 5.57%) [2,4,6,13] and metformin obtained from published literature (Cmax: 11,4% and AUC0-t 13.63%), with the expected CV% not exceeding 20% and the ratio within 80 and 125% [10,13,14].
The study required 26 evaluable subjects to demonstrate BE with a power of ≥ 90% at 5% level of significance. Based on a sample size, 26 subjects were sufficient to demonstrate BE between the two S/M formulations. Statistical analysis was conducted on all of the subjects who complete both periods of the study as per protocol, using SAS® (software version 9.4, Institute. Inc., CARY, USA).
The BE was determined using a PK sampling scheme suitable for the determination of the PK parameters of each individual component (sitagliptin/metformin), adhered to European Medicines Agency (EMA)-specific bioequivalence guide [21,22] and International Council for Harmonisation (ICH) M13A, guideline on bioequivalence for immediate release solid oral dosage forms [20].
The primary PK variables evaluated were maximum peak concentration (Cmax) and area under curve from time 0 to last measurable concentration (AUC0-t). Others secondary PK parameters evaluated were: Tmax (time to reach Cmax), time required for plasma concentration to decrease by 50% (T1/2), area under the plasma concentration-time curve from time 0 to infinity (AUC0-inf), AUC-%Extrap, Constant of Elimination (Kel) and the Number of points (Npoints) of the terminal log-linear phase used to estimate the terminal rate constant. The natural log transformed (i.e., Ln-transformed) values for the pharmacokinetic parameters Cmax and AUC0-t was analysed for statistical difference between test and reference formulations to each compound, sitagliptin and metformin Test and Reference (T & R) with ANOVA by a Generalized Linear Model (GLM) ANOVA using SAS®. Based on these parameters, the 90% Confidence Intervals (CIs) were constructed for the least square mean differences of logtransformed PK parameters Cmax and AUC0-t. The formulations, sitagliptin and metformin were regarded as bioequivalent when the 90% (CIs) of the T and R ratio of Cmax and AUC0-t, ranged from 80% to 125%. This is the BE standard accepted by the EMA guide for each individual compound [21,22].
Safety assessments
Safety assessments were performed during screening, during the study and the end of the study and the Adverse Events (AEs) were monitored throughout the study. Vital signs were measured during baseline screening and at the conclusion of the study. Twelve-lead electrocardiogram was recorded during screening. Random blood glucose test was performed in screening. Fasting Blood Glucose Test (FBGT) was conducted prior to check in of period I. In each period, blood glucose monitoring was performed before dosing and at 02.00, 04.00, 06.00 and 11.00 h (± 30 min) after dosing.
Results
All subjects (26) completed the study and were included in the PK and statistics evaluation. A non-compartmental analysis was applied for the estimation of PK parameters Cmax, AUC0-t, Tmax, Kel (h-1) and T½, of sitagliptin/metformin (S/M) in plasma concentration which are presented in Table 2, the AUC from 0 to last time point with measurable plasma concentrations was computed using linear trapezoidal-rule.
| PK parameters (Units) | Sitagliptin (Mean ± SD) | |
|---|---|---|
| Test (T) | Reference (R) | |
| Cmax (ng /mL) | 146.8720 ± 34.23664 | 134.7474 ± 36.22948 |
| AUC0-t (h*ng/mL) | 1601.8737 ± 227.43847 | 1534.1097 ± 246.13779 |
| AUC0-inf (h*ng /mL) | 1657.7560 ± 241.90543 | 1587.2834 ± 263.00806 |
| AUC%Extrap (%) | 3.317 ± 1.2643 | 3.285 ± 1.5428 |
| T1/2 (h) | 9.412 ± 1.3557 | 9.373 ± 1.2096 |
| Kel (1/h) | 0.07521 ± 0.011554 | 0.07505 ± 0.008946 |
| T1/2 (h) | 9.258 (6.25-12.00) | 8.982 (7.36-12.56) |
| PK parameters (Units) | Metformin (Mean ± SD) | |
| Test (T) | Reference (R) | |
| Cmax (ng/mL) | 1575.8543 ± 304.13060 | 1584.5823 ± 329.17890 |
| AUC0-t (hr*ng/mL) | 14422.9986 ± 2895.62014 | 14036.4871 ± 2560.61132 |
| AUC0-inf (hr*ng /mL) | 14643.7088 ± 2877.18270 | 14313.2584 ± 2538.94493 |
| AUC%Extrap (%) | 1.581 ± 0.8877 | 2.019 ± 1.0266 |
| T1/2 (hr) | 4.373 ± 0.7524 | 4.473 ± 1.0758 |
| Kel (1/hr) | 0.16277 ± 0.026117 | 0.16207 ± 0.031401 |
| T1/2 (hr) | 4.313 (3.38-6.04) | 3.999 (3.29-7.41) |
Note: Data presented as a mean ± standard deviation; Cmax: Maximum concentration; AUC0-t: Area under the plasma concentration-time curve from time 0 to the last measurable concentration; AUC0-∞: Area under the plasma concentration-time curve from time 0 to infinity, Tmax: Time to reach; Kel: Elimination constant. T1/2 time required for plasma concentration to decrease by 50%; Median (range).
Table 2: Pharmacokinetic parameters for sitagliptin after administration of Test product (T) and Reference product (R) N=26.
Analysis of variance analysis from Ln Cmax and AUC0-t, there were no statistically significant differences between the PK parameters of the two (S/M) formulations (p>0.05) (Tables 2 and 3).
| Sitagliptin | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters (units) | Least square means | Geometric least square means |
Ratio (%) (T vs. R) | 90% confidence intervals (%) | Intra subject | Power (T vs. R) | ||
| CV (%) | (%) | |||||||
| T | R | T | R | |||||
| Ln (Cmax) (ng/mL) | 4.965 | 4.865 | 143.275 | 129.712 | 110.46 | 103.26-118.15 | 14.27 | 99.97 |
| Ln (AUC0-t) (hr*ng/mL) | 7.369 | 7.322 | 1585.748 | 1512.792 | 104.82 | 99.81-110.08 | 10.34 | 100 |
| Metformin | ||||||||
| Parameters (units) | Least square means | Geometric least square means |
Ratio (%) (T vs. R) | 90% confidence intervals (%) | Intra subject | Power (T vs. R) | ||
| CV (%) | (%) | |||||||
| T | R | T | R | |||||
| Ln (Cmax) (ng/mL) | 7.3446 | 7.346 | 1547.783 | 1550.076 | 99.85 | 93.61-106.52 | 13.68 | 99.98 |
| Ln (AUC0-t) (hr*ng/mL) | 9.5572 | 9.532 | 14146.37 | 13799.83 | 102.51 | 96.57-108.82 | 12.63 | 100 |
Note: LSM: Least Square Mean; GLSM: Geometric Least Square Mean; Ratio; 90% Confidence Intervals (CI); ISCV: Intra-Subject Coefficient of Variation and power for the log transformed Cmax and AUC0-t. Sitagliptin 50 mg-metformin 1000 mg Immediate Release (IR) of Laboratorios Leti S.A.V. as test formulation and Janumet® (sitagliptin 50 mg-metformin 1000 mg IR) of Merck Sharp & Dohme Corp, as reference formulation.
Table 3: Bioequivalence assessment of a single dose of sitagliptin 50 mg test and reference products. Log transformed Cmax and AUC0-t (N=26).
The geometric mean ratios of the test and reference formulations for primary PK parameters Cmax and AUC0-t for sitagliptin, Cmax was 110.46 (90% IC 103.24-118.15) and AUC0-t was 104.82 (90% IC 99.81-110.08). For metformin, Cmax was 99.85 (90% IC 93.61- 106.52) and AUC0-t was 102.51 (90% IC96.57-108.82) (Table 3).
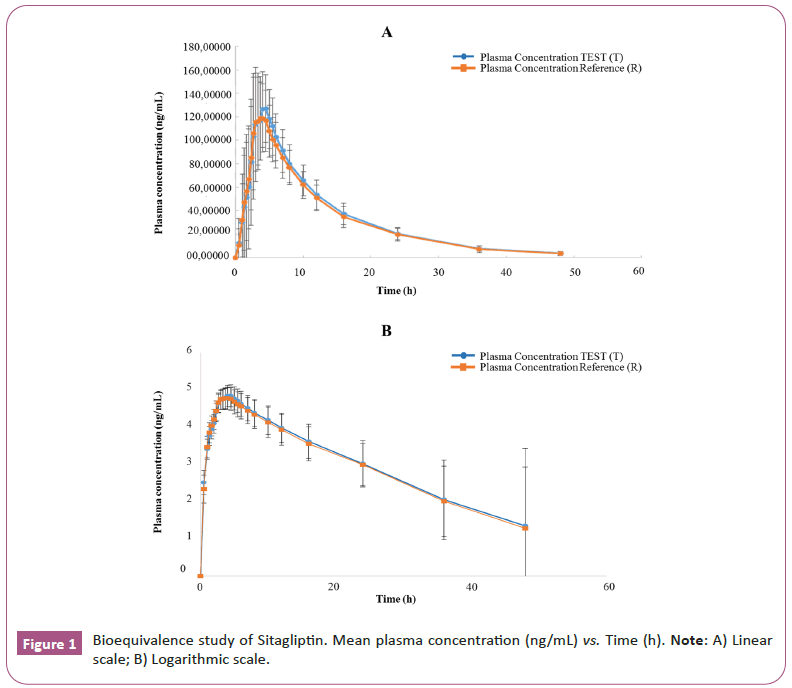
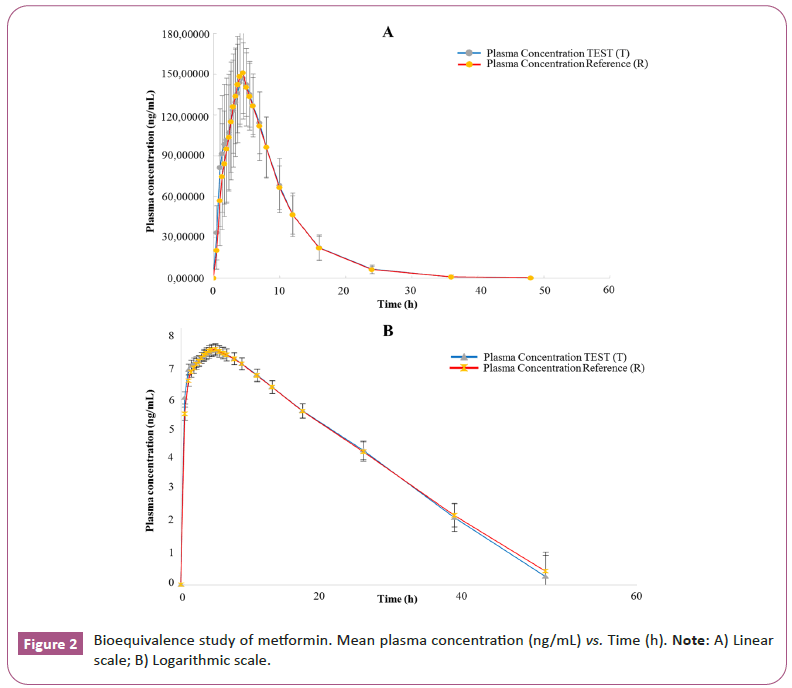
Cmax and AUC0-t intervals for test and reference formulations were within the acceptance limits (80.00%-125.00%) to establish bioequivalence [21,22]. The oral dosing of S/M for 48 h post-dose is represented on arithmetic and logarithm scales, test and reference formulations for sitagliptin 50 mg and or metformin 1000 mg Ir as shown in Figures 1 and 2, Tables 4 and 5.
| Arithmetic mean data | Logarithmic mean data | |||||||
|---|---|---|---|---|---|---|---|---|
| TIME (h) | Plasma concentration Test (T) (ng/mL) | Plasma concentration Reference (R) (ng/mL) | Standard Error (SE) (T) | Standard Error SE® | Log plasma concentration Test (T) | Log plasma concentration Reference (R) | 1/Ln SE (T) | 1/Ln SE (R) |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 12.338 | 10.311 | 21.078 | 14.512 | 2.675 | 0.984 | 0.374 | 1.016 |
| 1 | 30.235 | 31.956 | 40.903 | 31.149 | 3.439 | 1.235 | 0.291 | 0.81 |
| 1.33 | 42.9 | 47.029 | 50.422 | 40.322 | 3.697 | 1.307 | 0.27 | 0.765 |
| 1.67 | 51.364 | 56.569 | 53.365 | 41.837 | 3.734 | 1.317 | 0.268 | 0.759 |
| 2 | 59.935 | 67.046 | 52.189 | 42.539 | 3.75 | 1.322 | 0.267 | 0.757 |
| 2.32 | 81.252 | 84.759 | 53.779 | 44.148 | 3.788 | 1.332 | 0.264 | 0.751 |
| 2.67 | 103.346 | 105.637 | 53.166 | 47.91 | 3.869 | 1.353 | 0.258 | 0.739 |
| 3 | 113.517 | 115.725 | 48.628 | 41.776 | 3.732 | 1.317 | 0.268 | 0.759 |
| 3.33 | 116.14 | 116.169 | 41.341 | 37.377 | 3.621 | 1.287 | 0.276 | 0.777 |
| 3.67 | 116.455 | 118.793 | 33.227 | 35.48 | 3.569 | 1.272 | 0.28 | 0.786 |
| 4 | 126.199 | 118.726 | 31.978 | 28.961 | 3.366 | 1.214 | 0.297 | 0.824 |
| 4.5 | 126.717 | 116.584 | 28.759 | 26.135 | 3.263 | 1.183 | 0.306 | 0.846 |
| 5 | 118.2 | 107.691 | 24.964 | 22.028 | 3.092 | 1.129 | 0.323 | 0.886 |
| 5.5 | 111.693 | 100.663 | 24.203 | 19.041 | 2.947 | 1.081 | 0.339 | 0.925 |
| 6 | 102.664 | 95.782 | 19.906 | 19.312 | 2.961 | 1.085 | 0.338 | 0.921 |
| 7 | 91.107 | 84.989 | 18.148 | 17.121 | 2.84 | 1.044 | 0.352 | 0.958 |
| 8 | 79.923 | 76.92 | 15.994 | 14.444 | 2.67 | 0.982 | 0.374 | 1.018 |
| 10 | 65.926 | 62.091 | 13.259 | 11.384 | 2.432 | 0.889 | 0.411 | 1.125 |
| 12 | 53.654 | 50.947 | 12.486 | 10.927 | 2.391 | 0.872 | 0.418 | 1.147 |
| 16 | 37.263 | 34.813 | 8.895 | 8.881 | 2.184 | 0.781 | 0.458 | 1.28 |
| 24 | 20.39 | 19.867 | 4.99 | 5.788 | 1.756 | 0.563 | 0.57 | 1.776 |
| 36 | 7.77 | 7.436 | 2.55 | 2.876 | 1.057 | 0.055 | 0.947 | 18.189 |
| 48 | 3.856 | 3.595 | 1.618 | 1.832 | 0.605 | 0.01 | 1.078 | 0.089 |
Table 4: Sitagliptin plasma concentration over 48 h following a single dose of 50 mg oral tablet under fed condition. Test formulation (T) of laboratorios Leti S.A.V. and Reference formulation (R) of Merck Sharp & Dohme Corp.
| Arithmetic mean data | Logarithmic mean data | |||||||
|---|---|---|---|---|---|---|---|---|
| TIME (h) | Plasma concentration Test (T) (ng/mL) | Plasma concentration Reference (R) (ng/mL) | Standard Error (T) SE | Standard Error (R) SE | Log plasma concentration Test(T) | Log plasma concentration Reference (R) | 1/Ln SE (T) | 1/Ln SE (R) |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 334.45 | 203.329 | 199.496 | 137.241 | 4.922 | 1.594 | 0.203 | 0.627 |
| 1 | 812.513 | 569.003 | 432.465 | 329.984 | 5.799 | 1.758 | 0.172 | 0.569 |
| 1.33 | 914.384 | 745.679 | 428.923 | 390.2 | 5.967 | 1.786 | 0.168 | 0.56 |
| 1.67 | 984.099 | 840.5 | 439.012 | 387.091 | 5.959 | 1.785 | 0.168 | 0.56 |
| 2 | 1015.566 | 949.811 | 455.011 | 398.96 | 5.989 | 1.79 | 0.167 | 0.559 |
| 2.33 | 1070.384 | 1036.328 | 428.335 | 383.911 | 5.95 | 1.783 | 0.168 | 0.561 |
| 2.67 | 1156.16 | 1150.593 | 433.177 | 371.123 | 5.917 | 1.778 | 0.169 | 0.563 |
| 3 | 1232.704 | 1260.349 | 415.654 | 342.163 | 5.835 | 1.764 | 0.171 | 0.567 |
| 3.33 | 1293.315 | 1336.126 | 398.758 | 346.712 | 5.848 | 1.766 | 0.171 | 0.566 |
| 3.37 | 1357.507 | 1422.613 | 361.678 | 354.279 | 5.87 | 1.77 | 0.17 | 0.565 |
| 4 | 1436.457 | 1483.82 | 322.629 | 348.686 | 5.854 | 1.767 | 0.171 | 0.566 |
| 4.5 | 1470.615 | 1509.613 | 258.759 | 337.73 | 5.822 | 1.762 | 0.172 | 0.568 |
| 5 | 1424.16 | 1404.006 | 234.199 | 284.91 | 5.652 | 1.732 | 0.177 | 0.577 |
| 5.5 | 1352.682 | 1335.558 | 241.12 | 245.699 | 5.504 | 1.705 | 0.182 | 0.586 |
| 6 | 1270.261 | 1266.572 | 230.868 | 208.836 | 5.342 | 1.676 | 0.187 | 0.597 |
| 7 | 1141.93 | 1118.369 | 226.522 | 252.216 | 5.53 | 1.71 | 0.181 | 0.585 |
| 8 | 963.322 | 961.889 | 220.575 | 225.002 | 5.416 | 1.689 | 0.185 | 0.592 |
| 10 | 680.591 | 666.038 | 199.469 | 158.775 | 5.067 | 1.623 | 0.197 | 0.616 |
| 12 | 465.941 | 465.129 | 159.421 | 140.214 | 4.943 | 1.598 | 0.202 | 0.626 |
| 16 | 223.633 | 220.921 | 94.389 | 85.573 | 4.449 | 1.493 | 0.225 | 0.67 |
| 24 | 65.37 | 62.579 | 31.577 | 27.458 | 3.313 | 1.198 | 0.302 | 0.835 |
| 36 | 8.077 | 8.645 | 10.102 | 15.187 | 2.72 | 1.001 | 0.368 | 0.999 |
| 48 | 1.291 | 1.523 | 4.591 | 5.4002 | 1.686 | 0.523 | 0.593 | 0.342 |
Table 5: Metformin Plasma Concentration over 48 h following a single dose of 1000 mg IR oral tablet under fed condition. Test formulation of laboratorios Leti S.A.V. and Reference (R) formulation of Merck Sharp & Dohme Corp.
Tolerability and safety
All subjects (26) were included in the safety evaluation. A total of 3 adverse events were reported during the study, in the subjects dosed with test product. Two subjects (N14 and N26) experienced diarrhoea as an adverse event, however, both cases were not serious and resolved in <24 h. The subject N01 reported an AE of laboratory, increased potassium value, but it no was clinically significant. No clinically significant values were observed during the vital signs examination. There were no reports of death, serious or unexpected adverse events during the course of the study (Table 6). Hence the test product and reference product were found to be safe and well tolerated upon single dose administration in healthy male adults under fed conditions.
| Adverse Event (AE) | Mild | Moderate | Severe | Total | |||
| N=26 | |||||||
| R | NR | R | NR | R | NR | ||
| n (%) | n (%) | n (%) | |||||
| Diarrhoea | 2 (7.69)% | 2 (7.69)% | |||||
| Increase potassium value | 1 (3.87%) | 1 (3.87%) | |||||
| Total | 2 (7.69)% | 1 (3.87%) | 3 (11.54%) | ||||
Note: N: Total N° de subjects dosed with product T who had the Adverse Event.
1 and R: Probable/likely, possible/certain
2 and NR: No relation, unlike relation, conditional/unclassified, unassessable/unclassifiable.
Table 6: Intensity and causality of the adverse event for test product.
Discussion
This study was designed to evaluate the bioequivalence of two FDC formulations in a single-dose, two-period, crossover design involving healthy male subjects under fed conditions. The BE evaluation was based on 90% CIs ratios for sitagliptin and metformin and was assessed against bioequivalence standards of 80%-125% for Cmax and AUC0-t, as primary PK parameters, adhered to the EU guidelines [20-22]. The study included 26 male subjects covering the variability observed in others studies with a sufficient number of subjects to ensure statistical power to demonstrate the bioequivalence for both formulations [23,24]. Due to the metformin component and SmPC recommendations to this fixed dose combination of sitagliptin, 50 mg/metformin 1000 mg IR, this study was done under fed condition [21]. Comparative bioavailability of T and R formulations was demonstrated. The total amount of drug reaching the systemic circulation is proportional to the area under curve and fraction of drug absorbed is determined by comparing AUC0-t of the T and R formulations, no significant differences were demonstrated between the two formulations. The 90% confidence intervals for the ratios of sitagliptin and metformin, met the acceptance range of 80.00%-125.00% for primary PK parameter AUC0-t and Cmax [20-22]. The Geometric Mean Ratio ((GMR) T/R) for the primary PK parameters to sitagliptin was Cmax 110.46% (103.26%-118.15%) and AUC0-t 104.82% (99.81%-110.08%). The PK parameters for metformin were Cmax 99.85% (93.61%-106.52%) and AUC0-t 102.51 (96.57%- 108.82%). The mean plasma concentration-time curves for the sitagliptin 50 mg and metformin 1000 mg, IR tablets, were similar for both T and R formulations (Figure 1).
The Analysis of Variance (ANOVA) was used for crossover design in bioavailability testing. Was reported 3 adverse events no serious during test administration, clinically no significant, hence the T and R formulations were found to be safe and well tolerated in this study. T2DM is a complex, chronic illness characterized by persistent high blood glucose levels, with severe cardiovascular complications in patients uncontrolled, some patients can receive a simple drug or a combination of drugs, depending on glucose control levels. Many patients with T2DM, who receive monotherapy, are unable to maintain glucose levels with the progress of disease and combined therapy [25] have been indicated to achieve the better glycaemic control [1,13,15,25]. Metformin is the gold standard drug used for patients with T2DM, however several patients requires dual or triple therapy to achieve a glycaemic control due progressive deterioration of beta-cell function. Sitagliptin is an excellent option for combined with metformin because it has glucose-dependent action with lower risk of hypoglycaemia, as well as beneficial effects on betacell function and eventual protective action on beta-cell mass [1,3,5,7,14,25]. These components sitagliptin and metformin in FDC can enhance adherence to therapy resulting in improved glycaemic control and reduction of disease management costs [26]. This BE study of sitagliptin/metformin, FDC a generic product, demonstrated to be bioequivalent to reference product, Janumet®, in this dose-strength formulation (sitagliptin 50 mg/metformin 1000 mg IR) that permit dosing flexibility. A generic FDC formulation that is bioequivalent to the original product and available for patients with T2DM, could reduce pill burden, improve patients adherence to treatment, increase compliance and optimize cost-effectiveness [26-29]. This combination provides synergetic glucose control in patients with T2DM, in addition to providing patient adherence and offers potential cost advantages.
Conclusion
Two FDC formulations containing 50 mg of sitagliptin and 1000 mg of metformin IR were evaluated and found to be bioequivalent in healthy subjects under fed condition. The pharmacokinetic profiles of the test and reference formulations were similar, as demonstrated by the 90% Confidence Intervals (CIs) of Cmax and AUC0-t within the accepted EU-BE criteria of 80%-125%.
Limitations of the Study
This study analyzed PK parameters of sitagliptin/metformin under fed conditions, followed EMA BE guidelines. This study included healthy males only.
Acknowledgements
This study was conducted at the third party Pharmadesk Solutions Pvt., Ltd and VerGo Pharma Research Pvt. Ltd, (Division-VerGo Clinicals) India.
Conflict of Interest
All authors are employees of Industrias Biocontrolled C.A., (Leti Group Company) and may hold share and/or stock options in the company. The authors have no other potential conflicts of interest relevant to this study.
Author’s Contributions
Evelyn Pena, Alfredo Inatti, Jose Chacon, Anyoli Taly and Xenon Serrano Marti performed the statistical analysis, interpretation, writing, review of the manuscript.
Declaration of Patient Consent
All volunteers provided written informed consent after being well informed about the study before screening.
Financial Support and Sponsorship
This study was funded by Laboratorios Leti S.A.V.
References
- Williams-Herman D, Johnson J, Teng R, Golm G, Kaufman KD, et al. (2010) Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab 12: 442-451.
[Crossref] [Google Scholar] [Indexed]
- Bergman A, Ebel D, Liu F, Stone J, Wang A, et al. (2007) Absolute bioavailability of sitagliptin, an oral dipeptidyl peptidase-4 inhibitor, in healthy volunteers. Biopharm Drug Dispos 28: 315-322.
[Crossref] [Google Scholar] [Indexed]
- Lee M, Rhee MK (2015) Sitagliptin for Type 2 diabetes: A 2015 update. Expert Rev Cardiovasc Ther 13: 597-610.
[Crossref] [Google Scholar] [Indexed]
- Scott LJ (2017) Sitagliptin: A Review in Type 2 Diabetes. Drugs 77: 209-224.
[Crossref] [Google Scholar] [Indexed]
- Liu Z, Ma X, Ilyas I, Zheng X, Luo S, et al. (2021) impact of Sodium Glucose Cotransporter 2 (SGLT2) inhibitors on atherosclerosis: From pharmacology to pre-clinical and clinical therapeutics. Theranostics 11: 4502-4515.
[Crossref] [Google Scholar] [Indexed]
- (2016) Highlights of prescribing information. Merck Sharp and Dohme Corp.
- LaMoia TE, Shulman GI (2021) Cellular and molecular mechanisms of metformin action. Endocr Rev 42: 77-96.
[Crossref] [Google Scholar] [Indexed]
- Yendapally R, Sikazwe D, Kim SS, Ramsinghani S, Fraser-Spears R, et al. (2020) A review of phenformin, metformin and imeglimin. Drug Dev Res 81: 390-401.
[Crossref] [Google Scholar] [Indexed]
- MacDonald MJ, Ansari I, Logacre M, Stoker SW (2021) Metformin's therapeutic efficacy in the treatment of diabetes does not involve inhibition of mitochondrial glycerol phosphate dehydrogenase.Diabetes70:1575-1580.
[Crossref] [Google Scholar] [Indexed]
- Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes
- Foretz M, Guigas B, Viollet B (2019) Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol15: 569-589.
[Crossref] [Google Scholar] [Indexed]
- Nemeth DV, Lannelli L, Gangitano E, Andrea VD, Bellini MI (2024) Energy metabolism and metformin: Effects on ischemia-reperfusion injury in kidney transplantation. Biomedicines 12: 1534.
[Crossref] [Google Scholar] [Indexed]
- Scheen AJ (2010) Pharmacokinetic and pharmacodynamic evaluation of sitagliptin plus metformin. Expert Opin Drug Metab Toxicol 6: 1265-1276.
[Crossref] [Google Scholar] [Indexed]
- Reynolds JK, Neumiller JJ, Campbell RK (2008) Janumet: A combination product suitable for use in patients with Type 2 diabetes. Expert Opin Investig Drugs 17: 1559-1565.
[Crossref] [Google Scholar] [Indexed]
- Simonyi G, Ferenci T (2016) One-year persistence of metformin monotherapy and sitagliptin/metformin fixed combination. Orv Hetilap 157: 618-622.
- (2017) National ethical guidelines for biomedical and health research involving human participants. ICMR.
- Singh N, Madkaikar NJ, Gokhale PM, Parmar DV, (2020)New drugs and clinical trials rules 2019 India. Perspect Clin Res 11: 37-43.
[Crossref] [Google Scholar] [Indexed]
- World Medical Association (2001) World medical association declaration of helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ 79: 373-374.
[Crossref] [Google Scholar] [Indexed]
- (2002) ICH topic E6 (R1): Guideline for good clinical practice. European Medicines Agency
- (2024) ICH Guideline M13A on bioequivalence for immediate-release solid oral dosage forms: Scientific guideline. European Medicines Agency
- (2023) Metformin-immediate-release-film-coated-tablets-500, 850 and 1000 mg product specific bioequivalence guidance. European Medicines Agency
- (2016) Sitagliptin product-specific bioequivalence guidance, European Medicines Agency
- Nagadurga DH (2019) Bioavailability and bioequivalence studies. In: Pharmaceutical Formulation Design-Recent Practices. Intech Open United Kingdom.
- Migoya EM, Miller JL, Gutierrez M, Zheng W, Johnson-Levonas AO, et al. (2010) Bioequivalence of sitagliptin/metformin fixed-dose combination tablets and concomitant administration of sitagliptin and metformin in healthy adult subjects: A randomized, open-label, crossover study. Clin Drug Investig 30: 855-866.
[Crossref] [Google Scholar] [Indexed]
- Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, et al. (2022) Global, regional and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet Child Adolesc Health 6: 158-170.
[Crossref] [Google Scholar] [Indexed]
- Blonde L, San Juan ZT (2012) Fixed-dose combinations for treatment of type 2 diabetes mellitus. Adv Ther 29: 1-13.
[Crossref] [Google Scholar] [Indexed]
- Pena E, Inatti A, Taly A, Chacón JG, Serrano-Martin X (2023) Bioequivalence assessment of two formulations of empagliflozin in healthy adult subjects. Am J Pharmacother Pharm Sci 19: 1-7. [Crossref] [Google Scholar]
- Lingvay I, Beetz N, Sennewald R, Schuler-Metz A, Bertulis J, et al. (2020) Triple fixed-dose combination empagliflozin and metformin for patients with type 2 diabetes. Postgrad Med 132: 337-345.
[Crossref] [Google Scholar] [Indexed]
- Pena E, Inatti A, Taly A, Chacon J, Serrano-Martin X, et al. A bioequivalence study of empagliflozin/metformin fixed-dose combination in healthy subjects under fasting conditions. J Biosci Med 12: 235-250.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences