Biocontrol potential of indigenous antagonist fungal species for the coffee leaf rust Hemileia vastatrix in Ethiopia
Kifle Belachew*, Girma Adugna and Weyesa Garedew
Department of Horticulture and Plant Sciences, College of Agriculture and Veterinary Medicine, Jimma University, P.O. Box 307, Jimma, Ethiopia
- *Corresponding Author:
- Kifle Belachew
Department of Horticulture and Plant Sciences,
College of Agricultureand Veterinary Medicine,
Jimma University,
Tel: 251911067034,
E-mail: kiflekef@gmail.com
Received Date: March 08, 2021;Accepted Date: March 22, 2021;Published Date: March 29, 2021
Citation: Belachew K, Adugna G, Garedew W (2021) Biocontrol Potential of Indigenous Antagonist Fungal Species for the Coffee Leaf Rust Hemileia vastatrix in Ethiopia. Plant Pathology Vol.4 No.3: 04
Abstract
Coffee Leaf Rust (CLR), caused by Hemileia vastatrix is the worst disease of coffee plantations worldwide. The successful strategy of escaping the disease by highland planting used became uneffective, possibly owing to climate change. Development of varieties with durable resistance to CLR is lengthy and rendered difficult because of the pathogen variability. Chemical control is broadly used but is too costly or inadequate for the organic or low pesticide residue markets. Its intensive use also provokes worries with environmental impact. Therefore, biological control of CLR is becoming an attractive alternative worth pursuing. A survey performed at major coffee-growing areas of Ethiopia aimed at collecting potential antagonists to the CLR fungus. This study yielded many mycological novelties as well as new records of known fungal species that were previously unknown to be potential antagonists for H. vastatrix. Based on the result the highest germination inhibition and efficacy percent (reducing the disease severity index) on coffee leaf discs were recorded with mycoparasitic antagonistic fugal isolates of Digitopodium sp. (ET568), Digitopodium sp. (ET567), Pleurodesmospora sp. (ET544), Lecanicillium sp. (ET651), Lecanicillium sp. (ET669), Phoma sp. (ET622), Lecanicillium sp. (ET600), Lecanicillium sp. (ET665), Fusarium sp. (ET642), Fusarium sp. (ET645), Cladosporium sp. (ET566), Cladosporium sp. (ET564), Lecanicillium sp. (ET627) Simplicillium sp. (ET553) and Alternaria sp. (ET614). With this study the best result being obtained with the inoculation of those isolates applied simultaneously, 24hs and 72 hs before H. vastatrix inoculation. This isolates deserves being further evaluated for use as biological control of H. vastatrix in greenhouse and field condition.
Keywords
Biocontrol; Coffee disease; Digitopodium; Mycoparasites; Phylogeny
Introduction
Coffee species are native from tropical Africa, and the most economically significant agriculture commodity in world trade [1]. Coffea arabica and C. canephora are the two main cultivated species. These represent, respectively 66% and 34%, of commercially planted coffee [2]. Ethiopia is the main coffee producer and exporter 5th in the world and first in Africa [3]. Coffee production has been challenged worldwide by several biotic and abiotic factors, especially draught and fungal diseases, both of which can drastically decrease yields [4,5]. Coffee leaf rust (CLR) is the main disease affecting coffee worldwide. It is caused by Hemileia vastatrix (Pucciniaceae) which is a host-specific obligate parasite [6].It is the main disease in coffee plantations worldwide, causing yield losses of one to two billion US dollars annually, limiting production in many coffee growing regions in tropical and subtropical countries [7]. Although, CLR is the main coffee plant pathogen worldwide, attacks the leaves reducing the photosynthetic area and leading to defoliation and occasionally causing branch dieback and even to plant death. High incidences of CLR can cause the loss of up to 50percent of the foliage and up to 70percent of berries leading to yield reductions of 30percent to 50% [8-10].
CLR control is based on the use of resistant varieties, fungicide applications [11] and on escaping the disease by establishing highland plantations [12]. However, there are limitations for each of these approaches. High pathogen variability and the emergence of new races of the pathogen, as well as the occurrence of a complex of races challenges the quest for obtaining durable resistant for coffee [13,14]. The use fungicides, although effective for rust control in plantation situations may be too costly and unpractical for smallholders and in upland plantations [15]. It may also exclude, particularly in the case of systemic fungicides, the product from the high value organic market. Additionally, although recent studies suggested that the use of triazol and strobilurin products do not cause negative physiological alterations to coffee plants [10], doubts still remain as to issues such as diffusional limitations of CO2 and H2O vapor for coffee leaves sprayed with fungicides. The continuous use of systemic fungicides can also lead to the selection of fungicide resistant populations whereas the use of broad spectrum fungicides may harm the population of beneficial organisms (including the, now revealed to be broad, diversity of fungal antagonists of H. vastatrix as well as contribute to environmental pollution [16]. In addition, chemical control may have unintended consequences on nontarget beneficial microbial community, which may play a role in plant defense against pathogens [17]. Finally, with the growing demand for pesticide residue-free coffee (organics or not) the search for novel non-chemical approaches to CLR management has been encouraged [18]. Among these is biological control with fungal antagonists, particularly mycoparasites [19].
Recent research results on non-chemical management of CLR include promising results obtained from cultural methods involving the combination of shade cultivation, proper nitrogen fertilization and the use of resistant varieties [20] as well as the use of bacterial biofungicides [21]. Although several authors recognized the potential of biological control for CLR management, published results of biological control studies aimed at CLR control are still few and mostly concentrated on the use of antagonistic bacteria such as Bacillus thuringiensis, B. subtilis and Pseudomonas putida [22,23]. One rare example of assessment of the diversity of antagonistic fungi which might be deployed against H. vastatrix is the work of Carrión and Rico- Gray [24]. These authors surveyed H. vastatrix mycoparasites in the state of Veracruz (Mexico). More recently [25] used singlemolecule DNA sequencing for evaluating the diversity of fungal communities associated with CLR lesions collected in Mexico and Puerto Rico.
No result of systematic surveys for fungal antagonists of H. vastatrix in Ethiopia has ever been conducted and published. Here survey conducted in 2017/2018 at major coffee producing areas of Ethiopia, 110 isolates of mycoparasites associated to coffee leaf rust were obtained. The present paper describes the preliminary evaluation on biocontrol potential of selected indigenous fungal isolates was performed.
Materials and Methods
Biocontrol agent’s collection, isolation and inoculum preparation
Coffee leaves samples bearing rust pustules seemingly hyperparasitized which used in this study were collected during survey conducted between 2016 and 2017, from a range of situations where coffee occurs. The survey included varieties of coffee production systems as plantation, garden, semi-forest and forest where coffee plants grow spontaneously as naturalized species. Plants bearing coffee leaf rust symptoms were collected at every locality, where high attention being given to leaves showing signs of rust pustules being overgrown by other fungi. Samples were dried in a plant press and later placed in paper envelopes. Upon arrival at the lab, samples were carefully screened. Pustules of H. vastatrix bearing sporulating mycoparasites were selected and spores or other fungal structures were transferred aseptically to plates containing potato dextrose agar (PDA Sigma- Aldrich, Merck KGaA, Darmstadt, Germany) supplemented with antibiotic streptomycin sulphate with the help of a sterile fine pointed needle. The plates were placed at 22 ± 2oC for 7 days under a 12 h daily light regime (light provided by two daylight fluorescent lamps and one near-UV lamp 200W, placed 35 cm above the plates). Pure cultures were isolated, identified and then transferred to appropriate media based on the fugal genera preference for abundant sporulation. When the sporulation was abundant, a suspension was prepared by flooding each plate with sterile distilled water and scraping the surface of the colony with a rubber spatula and inoculum concentration was adjusted with the help of haemocytometer to 1 × 105 mL-.Moreover, All isolates were kept in tubes containing potato carrot agar (PCA) slant tubes and cryo-tubes containing 10percent glycerol stock solution under -80oC. These were also preserved in silica-gel, as described in Dhingra and Sinclair [26]. Representative samples were deposited in the herbarium of the Universidade Federal de Viçosa (Herbarium VIC) and representative strains were maintained in the culture collection of the Universidade Federal de Viçosa “Coleção Octavio de Almeida Drummond” (COAD).
Cultural features and morphology
For culture characteristics colonies were cultivated on Malt Extract Agar 2 percent (MEA), Potato Dextrose Agar (PDA), and Oat meal Agar (OA) for 7 d at 25 ± 2°C with 12 h light regime. One representative isolate of each phylogenetic species was used for morphological characterization. Morphological descriptions were made for isolates grown on synthetic nutrient-poor agar plates (SNA), with a 5 mm diameter of mycelial plug placed onto the medium surface. Colony colours were described following Rayner’s terminology [27]. To study conidial development and branching patterns of conidial chains isolates were grown on microculture with synthetic nutrient-poor agar (SNA) for 14 d at 25°C with 12 h light regime and conidia were collected and mounted in lactophenol or lactofuchsin on microscope glass slides and observed under a light microscope. The conidial morphology was studied and 30 measurements at × 1000 magnification were made for all fungal structure with an Olympus BX51 light microscope (Olympus, Tokyo, Japan) with differential interference contrast (DIC) illumination and equipped with camera Olympus Q-color 3TM. Images were taken with a light microscope fitted with differential interference contrast (DIC) illumination (Olympus B X 53) and connected to an Olympus Q-color 5TM camera. Their shape, colour and presence of septation, were recorded to study genus of isolates.
Hemileia vastatrixinoculum production and preparation
To multiply H.vastatrix twenty three-month-old healthy coffee seedlings (cv. Caturra) were selected and placed in a growth chamber at a relative humidity of 85 ± 5percent, with in temperature of 22°C and a 12 h daily light regime (light provided by cool white fluorescent tubes) prior to inoculation. H. vastatrix (race II) inoculum was produced as described in [28] and summarized here. The abaxial surface of the expanded leaves of coffee seedlings was inoculated by spraying a urediniospore suspension (1 × 105 mL-1suspended in with Tween 20) with an atomizer. The seedlings were then kept in dew chamber, for 48 h in the dark at 25 ± 2°C. Subsequently, the plants were returned to the growth chamber. After 30-45 days, mature uredinospores were collected in microtubes, next the microtubes were put inside a desiccator for 48-h and finally stored at -80°C. Preservation at -80°C was never longer than 90 days. Before use in experiments H. vastatrix urediniospore were submitted to vitro germination test. Only urediniospore batches with a viability greater than 30percent were used in the experiments (Capucho et al.) Later, urediniospore suspensions were prepared in a 0.05 percent Tween 20 solution. Concentration was adjusted to 1 × 105 urediniospores mL-1 with the help of a hemocytometer.
Inhibition of uredioniospore germination test
Glass microscope slides fresh from the box were disinfected with 70 percent alcohol and then placed at room temperature until the residue evaporated. Two slides were placed inside each of several 11 × 11 × 35 cm poliestirene boxes (gerbox). These were previously prepared by careful washing, drying and internally cleaned with 70% ethanol and then lined with sterilized and moist towel paper. A 15 μL aliquot of H. vastatrix urediniospore suspension was transferred to the center of each slide followed by the transfer of a 15 μL aliquot of the potential antagonist suspension. The two drops were mixed with the micropipette tip on each slide. After that procedure the boxes were covered with their lids and sealed with PVC film in order to avoid evaporation of the water. Boxes were left in a bench in a controlled temperature room at 22 ± 1°C in the dark for six hours. Control consisted on equivalent apparatus treated identically but with slides supporting aliquots of H. vastatrix urediniospores as before but without antagonist addition. After six hours period, germination was interrupted by adding a drop of lactophenol over each drop of urediniospore or urediniospore/antagonist suspension either pure or mixed with possible antagonists. The urenidiospores were considered to have germinated when the germ tube size was longer than the urediniospore diam. Germination inhibition was calculated by the following equation:
Germination inhibition percent=(c-x/c) × 100
Where, c is the germinated urediniospores in the control and x is the germinated urediniospores exposed to possible antagonist [29].
Two sets of experiments were performed independently for germination inhibition using 30 antagonists each with total 60 mycoparasitic isolates. Each experiment had its own set of control.
Experimental design and statistical analysis
The experiments 1 and 2, aimed at determining the biocontrol potential of antagonist mycoparasites against CLR in terms of germination inhibition and reducing disease severity on leaf disc samples. The urediniospore germination inhibition potential experiments were arranged in completely randomized designs (CRD) with thirty treatments each (as set one and two) with four replications and we used sixty treatments in total. The leaf disc experiments were performed twice using a completely randomized design (CRD) with three replications, four discs with in the gearbox was used as one experimental unites (Figure 1). The F test was used for analyzing the data obtained from experiments 1 and 2 and to determine the degree of variance homogeneity between the replicates of each of the experiments. Data from all variables and parameters obtained from experiments were tested for Analysis of variance (ANOVA) assumptions and means from the treatments were compared using means separation test of least significant difference (LSD) with P ≤ 0.05 probability level, using SAS (version 9.2) software package [33].
Data collection
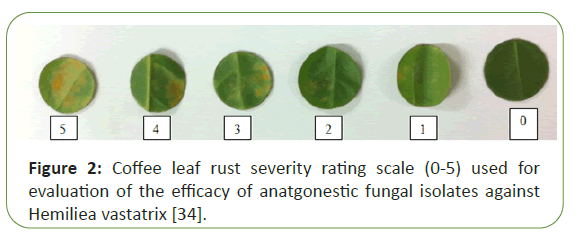
The coffee leaf rust severity of the disease was evaluated at 35 days after inoculation, using a rating scale from 0 to 5 (Figure 2). According to the percentage of leaf area with lesions: 0=0%; 1=0.1 – 5%; 2=5 – 25; 3=26% – 50%; 4=51%-75%, and 5>75% based on with little modification [34]. Disease index (DI) was calculated according to the equation:
DI (percent)=[Ʃ(r×a)/(R×A)] × 100
Where r is the rating value, a is the number of infected leaf discs with a rating of r, R is the maximum rating value and A is the total number of leaf disc tested.
Experimental design and statistical analysis
The experiments 1 and 2, aimed at determining the biocontrol potential of antagonist mycoparasites against CLR in terms of germination inhibition and reducing disease severity on leaf disc samples. The urediniospore germination inhibition potential experiments were arranged in completely randomized designs (CRD) with thirty treatments each (as set one and two) with four replications and we used sixty treatments in total. The leaf disc experiments were performed twice using a completely randomized design (CRD) with three replications, four discs with in the gearbox was used as one experimental unites (Figure 1). The F test was used for analyzing the data obtained from experiments 1 and 2 and to determine the degree of variance homogeneity between the replicates of each of the experiments. Data from all variables and parameters obtained from experiments were tested for Analysis of variance (ANOVA) assumptions and means from the treatments were compared using means separation test of least significant difference (LSD) with P ≤ 0.05 probability level, using SAS (version 9.2) software package [35].
Results
Germination inhibition potential of mycoparasitic isolates for Hemilea vastatirix
There was significant difference (p<0.05) among mycoparasitic antagonistic fugal isolates for inhibition of Hemilea vastatirixuredioniospore germination (Tables 1 and 2). From first experiment of set one the germination of the uredioniospore reached a maximum of 86.5%with zero inhibition in the control treatment (only sterile water treatment without antagonistic fungi). The maximum germination inhibition of Hemilea vastatirixuredioniospore was recorded with mycoparasitic antagonistic fugal isolates ofPseudocercospora sp. (ET603), Cladosporiumsp. (ET620), Cladosporiumsp. (ET566), Fusariumsp. (ET646), Alternariasp. (ET614) with inhibition percentage of88.28, 87.07, 83.45, 81.03 and 77.47 respectively (Table 1). Similarly, Acremoniumsp. (ET559), Simplicilliumsp. (ET553) and Fusariumsp. (ET642) also showed good antagonistic effect with the record of 67.75%, 66.39%and 65.33%germination inhibition of uredioniospore germination of Hemilea vastatirix, respectively.
| S. No | Cod | Isolate genera | Germination (%) | Inhibition (%) |
|---|---|---|---|---|
| 1 | ET551 | Simplicillium sp. | 54.02 | 37.55 |
| 2 | ET553 | Simplicillium sp. | 29.07 | 66.39 |
| 3 | ET554 | Simplicillium sp. | 71.79 | 17.01 |
| 4 | ET555 | Simplicillium sp. | 54.02 | 37.55 |
| 5 | ET558 | Acremonium sp. | 61.34 | 29.09 |
| 6 | ET559 | Acremo nium sp. |
27.9 | 67.75 |
| 7 | ET560 | Acremonium sp. | 55.07 | 36.34 |
| 8 | ET561 | Cladosporium sp. | 57.16 | 33.92 |
| 9 | ET564 | Cladosporium sp. | 54.23 | 37.31 |
| 10 | ET565 | Cladosporium sp. | 68.65 | 20.63 |
| 11 | ET566 | Cladosporium sp. | 14.32 | 83.45 |
| 12 | ET593 | Lecanicillium sp. | 69.56 | 19.58 |
| 13 | ET594 | Lecanicillium sp. | 64.75 | 25.14 |
| 14 | ET595 | Lecanicillium sp. | 57.25 | 33.82 |
| 15 | ET603 | Pseudocercospora sp. | 10.14 | 88.28 |
| 16 | ET614 | Alternaria sp. | 19.54 | 77.41 |
| 17 | ET620 | Cladosporium sp. | 11.18 | 87.07 |
| 18 | ET622 | Lecanicillium sp. | 50.89 | 41.17 |
| 19 | ET625 | Lecanicillium sp. | 71.25 | 17.63 |
| 20 | ET626 | Lecanicillium sp. | 69.25 | 19.94 |
| 21 | ET627 | Lecanicillium sp. | 71.75 | 17.05 |
| 22 | ET628 | Lecanicillium sp. | 78.75 | 8.96 |
| 23 | ET629 | Lecanicillium sp. | 65.25 | 24.57 |
| 24 | ET630 | Lecanicillium sp. | 66.25 | 23.41 |
| 25 | ET631 | Lecanicillium sp. | 63.75 | 26.3 |
| 26 | ET633 | Lecanicillium sp. | 37.3 | 56.87 |
| 27 | ET641 | Fusarium sp. | 38.35 | 55.67 |
| 28 | ET642 | Fusarium sp. | 29.99 | 65.33 |
| 29 | ET645 | Fusarium sp. | 49.84 | 42.38 |
| 30 | ET646 | Fusarium sp. | 16.41 | 81.03 |
| 31 | Cont | Control | 86.5 | 0 |
| Mean CV (%) LSD(0.05) |
50.82 | 41.25 | ||
| 20.06 | 21.72 | |||
| 6.45 | 6.45 | |||
Table 1: In-vitro germination inhibition result of antagonistic mycoparasits genera collected from Ethiopia (set one).
However, the least germination inhibition of Hemilea vastatirix uredioniospores were recorded with mycoparasitic antagonistic fugal species ofLecanicillium sp. (ET628), Simplicilliumsp. (ET554), Lecanicillium sp. (ET627), Lecanicillium sp. (ET625), Lecanicillium sp. (ET593), Lecanicillium sp. (ET626) with inhibition percentage of8.96, 17.01, 17.05, 17.63, 19.58 and 19.94 respectively (Table 1). The study revealed that more than 33.33% of the tested fungal antagonists at set one experiment showed promising potential with >50% inhibition of uredioniospore germination of Hemilea vastatirix. The remaining 46.67% of mycoparasitic antagonistic fugal species tested here showed mean percent uredioniospore germination reduction value ranged from 20.63 to 42.38%.
Overall, more than 70% of the tested mycoparsitic fungal isolates significantly (p<0.05) reduced germination of uredioniospores of Hemilea vastatirix, with >25% germination inhibition (Table 1).
Similarly, the first experiment of set two shows significant difference (p<0.05) among mycoparasitic antagonistic fugal isolates for inhibition of Hemilea vastatirix uredioniospore germination (Table 2). The maximum germination inhibition of Hemilea vastatirix uredioniospore was recorded with mycoparasitic antagonistic fugal isolates ofDigitopodiumsp. (ET568), Pleurodesmospora sp. (ET544), Lecanicilliumsp. (ET651), Lecanicilliumsp. (ET666), Lecanicillium sp. (ET669), Phomasp. (ET622), Digitopodiumsp. (ET567), Lecanicilliumsp. (ET600), Lecanicilliumsp. (ET665) with inhibition percentage of 88.86, 88.64, 88.64, 83.45, 87.47, 85.13, 83.69, 82.45, 81.62 and 78.11 respectively (Table 2). However, the least germination inhibition of Hemilea vastatirix uredioniospore from set two experiment was recorded with mycoparasitic antagonistic fugal species ofLecanicillium sp. (ET652), Lecanicillium sp. (ET601), Phomasp. (ET621), Phomasp. (ET615) with inhibition percentage of 17.65, 20.17, 23.08 and 25.21 respectively (Table 2). The maximum germination of the uredioniospore was recorded for control treatment which scored 89.25% with zero inhibition.
| No | Cod | Isolate genera | Germination (%) | Inhibition (%) |
|---|---|---|---|---|
| 1 | ET544 | Pleurodesmospora sp. | 10.14 | 88.64 |
| 2 | ET567 | Digitopodium sp. | 10.09 | 88.86 |
| 3 | ET568 | Digitopodium sp. | 22.68 | 74.59 |
| 4 | ET596 | Lecanicillium sp. | 37.3 | 58.2 |
| 5 | ET597 | Lecanicillium sp. | 38.35 | 57.03 |
| 6 | ET598 | Lecanicillium sp. | 29.99 | 66.4 |
| 7 | ET599 | Lecanicillium sp. | 49.84 | 44.15 |
| 8 | ET600 | Lecanicillium sp. | 16.41 | 81.62 |
| 9 | ET601 | Lecanicillium sp. | 71.25 | 20.17 |
| 10 | ET610 | Lecanicillium sp. | 38.56 | 56.8 |
| 11 | ET611 | Lecanicillium sp. | 57.54 | 35.53 |
| 12 | ET614 | Alternaria sp. | 10.14 | 88.64 |
| 13 | ET615 | Phoma sp. | 66.75 | 25.21 |
| 14 | ET616 | Phoma sp. | 55.75 | 37.54 |
| 15 | ET619 | Cercospora sp. | 58.2 | 34.79 |
| 16 | ET620 | Cladosporium sp. | 13.27 | 85.13 |
| 17 | ET621 | Phoma sp. | 68.65 | 23.08 |
| 18 | ET622 | Phoma sp. | 14.32 | 83.96 |
| 19 | ET651 | Lecanicillium sp. | 49.25 | 44.82 |
| 20 | ET651 | Lecanicillium sp. | 65 | 27.17 |
| 21 | ET652 | Lecanicillium sp. | 73.5 | 17.65 |
| 22 | ET653 | Lecanicillium sp. | 62.25 | 30.25 |
| 23 | ET655 | Lecanicillium sp. | 47.5 | 46.78 |
| 24 | ET656 | Lecanicillium sp. | 48.25 | 45.94 |
| 25 | ET657 | Lecanicillium sp. | 63.75 | 28.57 |
| 26 | ET665 | Lecanicillium sp. | 19.54 | 78.11 |
| 27 | ET666 | Lecanicillium sp. | 11.18 | 87.47 |
| 28 | ET667 | Lecanicillium sp. | 50.89 | 42.98 |
| 29 | ET673 | Lecanicillium sp. | 64.25 | 28.01 |
| 30 | ET675 | Fusarium sp. | 43.7 | 51.04 |
| 31 | Cont | Control | 89.25 | 0 |
| Mean CV (%) LSD(0.05) |
45.41 | 49.12 | ||
| 22.26 | 20.52 | |||
| 6.25 | 6.26 | |||
Table 2: In-vitro germination inhibition result of selected antagonistic mycoparasits genera collected from Ethiopia (Set two).
The result revealed that more than 43.33% of the tested fungal antagonists at set two experiment showed promising potential with >50% inhibition of uredioniospore germination of Hemilea vastatirix. The remaining 66.67% of mycoparasitic antagonistic fugal species tested at set two showed mean percent uredioniospore germination reduction value ranged from 25.21 to 74.29 percent. Overall, more than 90 percent of the tested mycoparsitic fungal isolates significantly (p<0.05) reduced germination of uredioniospores of Hemilea vastatirix, with >25% germination inhibition (Table 2). Here we can notice that there is antagonistic variation among mycoparasites and 23.3%of them show significant reduction of the germination of uredioniospores, with >75 percent germination inhibition but no antagonistic fugal isolates completely inhibited the germination of Hemilea vastatirix uredioniospores (Table 2).
Antagonistic potential of mycoparasitic isolates on severity of coffee leaf rust (Hemilea vastatirix) on leaf disc Test
In order to evaluate the biocontrol potential of mycoparasitic isolates against coffee leaf rust (Hemilea vastatirix), their spore suspensions were inoculated on coffee leaf disc by applying the suspension with different time of application (inoculation simultaneously the test pathogen and H. vastatirix, 24 and 72 hs after inoculation) and measuring severity of the disease on the leaf disc. The results showed that there was significant (p<0.05) interaction effect among the fungal antagonists and the time of applications in reducing severity of coffee leaf rust disease (Tables 3 and 4). The disease severity index reached maximum value of 100 percent for the treatment Acremoniumsp. (ET558) on the test coffee cultivar “cv. Catuaí-Vermelho IAC 144” leaf disc, which shows zero efficacy. From this experiment of set one, the a maximum efficacy or severity reduction were noticed from 0 hour inoculation experiment with mycoparasitic antagonistic fugal isolates ofFusariumsp. (ET642), Fusariumsp. (ET645); Cladosporiumsp. (ET620), Cladosporiumsp. (ET565), Pseudocercospora sp. (ET603), with efficacy percentage of88.30, 80.00, 60.00, 58.30 and 56.70 respectively (Table 3) and (Figure 2). However, the least efficacy or high disease severity was observed on antagonistic fugal isolates of Acremoniumsp. (ET558), Acremoniumsp. (ET559), Simplicilliumsp. (ET553), Acremoniumsp. (ET560), and Simplicilliumsp. (ET554) with disease severity index percentage of 100.0, 96.7, 95.0, 93.3 and 88.3, respectively (Table 3).
| No | Cod | Genera isolate | 0 h | 24 h | 72 h | |||
|---|---|---|---|---|---|---|---|---|
| Severity index | Efficay | Severity index | Efficay | Severity index | Efficay | |||
| 1 | ET551 | Simplicillium sp. | 63.3de | 26.7f | 65.0ed | 35.0ef | 53.3ef | 46.7ef |
| 2 | ET553 | Simplicillium sp. | 95.0a | 5.0h | 93.3a | 6.7jk | 91.6ab | 8.4gh |
| 3 | ET554 | Simplicillium sp. | 88.3ab | 11.7g | 73.3bc | 26.7h | 56.7ef | 43.3de |
| 4 | ET555 | Simplicillium sp. | 78.3bc | 21.7hi | 70.0c | 30.0ef | 60.0e | 40.0de |
| 5 | ET558 | Acremonium sp. | 100.0a | 0.0h | 90.0a | 10.0ij | 83.3bc | 16.7hg |
| 6 | ET559 | Acremonium sp. | 96.7a | 3.3h | 91.7a | 8.3jk | 73.3cd | 26.7gf |
| 7 | ET560 | Acremonium sp. | 93.3a | 6.7gh | 91.7ab | 8.3h | 88.3ab | 11.7ij |
| 8 | ET561 | Cladosporium sp. | 71.7cd | 28.3gh | 70.7d | 29.3fg | 68.3cd | 31.7ef |
| 9 | ET564 | Cladosporium sp. | 65.0ed | 35.0ef | 43.3fg | 56.7cd | 33.3h | 66.7cd |
| 10 | ET565 | Cladosporium sp. | 41.7fg | 58.3cd | 26.7h | 73.3ab | 13.3i | 86.7ab |
| 11 | ET566 | Cladosporium sp. | 60.0e | 40.0ef | 38.3g | 61.7c | 26.7h | 73.3ab |
| 12 | ET603 | Pseudocercospora sp. | 43.3g | 56.7cd | 38.3g | 61.7c | 33.3h | 66.7cd |
| 13 | ET614 | Alternaria sp. | 65.0cd | 35.0ef | 63.3ed | 36.7fg | 58.3ef | 41.7de |
| 14 | ET620 | Cladosporium sp. | 40.0g | 60.0bc | 31.7g | 68.3bc | 26.7h | 73.3c |
| 15 | ET627 | Lecanicillium sp. | 58.3ef | 41.7de | 45.0fg | 55.0de | 40.0g | 60.0bc |
| 16 | ET633 | Lecanicillium sp. | 56.7ef | 43.3de | 48.3fg | 52.7cd | 25.0h | 75.0bc |
| 17 | ET641 | Fusarium sp. | 78.0bc | 22.0hi | 23.3hi | 76.7ab | 18.3h | 81.7ab |
| 18 | ET642 | Fusarium sp. | 11.7hi | 88.3a | 15.0i | 85.0a | 6.7i | 92.3a |
| 19 | ET645 | Fusarium sp. | 20.0hi | 80.0a | 6.7i | 92.3a | 5.0i | 95.0a |
| 20 | ET646 | Fusarium sp. | 66.7de | 33.3ef | 53.3ef | 46.7de | 35.0gh | 65.0cd |
| 21 | cont | Control | 99.7a | 0.3h | 97.3a | 2.7k | 96.7a | 3.3h |
| Mean CV (percent) LSD (0.05) |
59.69 | 40.3 | 56.74 | 43.26 | 57.27 | 57.27 | ||
| 25.77 | 38.17 | 25.37 | 33.27 | 25.19 | 25.19 | |||
| 12.38 | 12.37 | 11.58 | 11.58 | 11.61 | 11.61 | |||
Note:- 0h- an aliquot of 25 μl of the antagonist suspension plus H; vastatrix uredinial suspension were deposited simultaneously on leaf disc ;24 h- an aliquot of 25 μl of the antagonist suspension placed on leaf disc 24 hs before depositing the urediniospore suspension of H. vastatrix; 72 h- an aliquot of 25 μl of the antagonist suspension placed on leaf disc 72 hs before depositing the urediniospore suspension of H. vastatrix: *Means with different letters are significantly different across the column.
Table 3: Interaction effect of Antagonistic fungal isolates and their time of application on CLR severity under leaf disc experiment (set one).
From twenty antagonistic mycoparsite isolates which tested as set one, more than 50.0% of the tested fungal antagonists of leaf disc experiment showed promising potential with >30% efficacy for coffee leaf rust (Hemilea vastatirix) control. In this trial 65.0% of mycoparasitic antagonistic fungal species showed percent severity index reduction value ranged from 26.7 to 88.3% (Table 3). From 24 hour after inoculation of set one, more than 80 percent of the tested mycoparsitic fungal isolates significantly (p<0.05) reduced severity index of Hemilea vastatirix, with >25%efficacy (Table 3). Moreover, there were antagonistic variation among mycoparasites and 35.0% of them significantly reduced the severity index of CLR, with >70% efficacy at 72 h after inoculation. Apart from the above mycoparasitic antagonistic fungal isolates which can significantly reduced the severity index percent of coffee leaf rust at 0 hs inoculation, five more isolates were show highest efficacy and significantly reduce the severity index of CLR with more than 65 percent at 72 hs after inoculation treatment. Those fungal isolates with high potential antagonistic capacity after 72 hs treatment were Fusariumsp. (ET641), Lecanicilliumsp. (ET633), Cladosporiumsp. (ET566), Cladosporiumsp. (ET564), Fusariumsp. (ET646) and Lecanicilliumsp. (ET627) with efficacy percent of 81.7, 75.0, 73.3, 66.7, 65.0 and 60.0, respectively. These isolates revealed good antagonistic activity when they were treated prior to the landing of the disease pathogen on the host.This result indicated that the time of bioagent application have significant difference on the efficacy of the treatment (Table 3).
Similarly, the set two or the second batch of leaf disc experiment revealed that there was significant (p<0.05) interaction effect among twenty one fungal antagonists used as a treatment and the time of applications in reducing severity of coffee leaf rust disease (Table 4). From this experiment of 0 hour inoculation, the a maximum disease severity reduction were noticed from treatment ofDigitopodiumsp. (ET568), Digitopodiumsp. (ET567), Fusariumsp. (ET675), Lecanicillium sp. (ET657), Lecanicilliumsp. (ET656), and Lecanicilliumsp. (ET651), with the efficacy percent of 80.0, 77.3, 74.0,68.3, 67.7 and 63.3 respectively.Moreover, with 72 hours inoculation more than 57% of treatments reduced the severity of coffee leaf rust with efficacy percent of >60%. Here we noticed that, the newly discovered two mycoparasit of Hemilea vastatirix called Digitopodiumsp. significantly and consistently reduce the severity of coffee leaf rust with efficacy percent of 80 and 81% at 72 hs inoculation on leaf disc experiment. However, the least efficacy or high disease severity was observed on antagonistic fugal isolates of Phomasp. (ET615), Phomasp. (ET614), Lecanicilliumsp. (ET623),Phomasp. (ET616) and Pleurodesmosporasp. (ET544) with disease severity index percentage of 100.0, 95.0, 88.3, 80.0 and 76.7, respectively (Table 4) (Figure 2).
| No | Cod | Genera isolate | 0 h | 24 h | 72 h | |||
|---|---|---|---|---|---|---|---|---|
| Severity index | Efficay | Severit index | Efficay | Severity index | Efficay | |||
| 1 | ET614 | Alternariasp. | 95.0a | 5.0j | 88.7b | 11.3jk | 73.3b | 26.7hi |
| 2 | ET619 | Cercospora sp. | 71.7c-e | 28.3gh | 70.0b | 30.0gh | 36.7i | 63.3bc |
| 3 | ET637 | Fusarium sp. | 70.0c-e | 30.0fg | 36.7h-j | 63.3cd | 36.7i | 63.3bc |
| 4 | ET567 | Digitopodium sp. | 23.3i | 77.3a | 21.0k | 79.0a | 18.0k | 82.0a |
| 5 | ET568 | Digitopodium sp. | 20.0ij | 80.0a | 20.0k | 80.0a | 19.0jk | 81.0a |
| 6 | ET675 | Fusarium sp. | 26.0jk | 74.0ab | 23.3j | 76.7ab | 21.7j | 78.3ª |
| 7 | ET610 | Lecanicillium sp. | 75.0cd | 25.0hi | 53.3gh | 46.7d | 45.0e-g | 55.0de |
| 8 | ET623 | Lecanicillium sp. | 88.3ab | 11.7ij | 75.0dc | 25.0hi | 71.7cd | 28.3gh |
| 9 | ET644 | Fusariumsp. | 53.3fg | 46.7ef | 35.0i | 65.0bc | 33.3h-j | 66.7cd |
| 10 | ET631 | Lecanicilliumsp. | 63.3e-g | 26.7hi | 51.7fg | 48.3de | 48.3d-f | 51.7ef |
| 11 | ET652 | Lecanicillium sp. | 51.7h | 42.3ef | 50.0g | 50.0cd | 48.3d-f | 51.7ef |
| 12 | ET653 | Lecanicillium sp. | 50.0g | 50.0cd | 43.3gh | 56.7de | 38.3i | 62.7cd |
| 13 | ET655 | Lecanicillium sp. | 55.0fg | 45.0de | 51.7de | 48.3f | 48.3gh | 51.7cd |
| 14 | ET657 | Lecanicillium sp. | 31.7ij | 68.3bc | 31.7ij | 68.3bc | 31.7ij | 68.3bc |
| 15 | ET651 | Lecanicillium sp. | 36.7hg | 63.3cd | 36.7i | 63.3bc | 23.3jk | 76.7ab |
| 16 | ET656 | Lecanicillium sp. | 33.3i | 67.7bc | 30.0ij | 70.0ab | 20.0k | 80.0a |
| 17 | ET615 | Phoma sp. | 100a | 0.0k | 93.3ª | 6.7ijk | 88.3ab | 11.7ij |
| 18 | ET616 | Phoma sp. | 80.0bc | 20.0i | 66.7bc | 33.3gh | 55.0fg | 45.0de |
| 19 | ET621 | Phoma sp. | 68.3de | 32.7fg | 65.0d-f | 35.0fg | 38.3f-h | 61.7de |
| 20 | ET622 | Phoma sp. | 70.0de | 30.0gh | 61.7ef | 38.3f | 31.7h-j | 68.3bc |
| 21 | ET544 | Pleurodesmospora sp. | 66.7ed | 23.3ij | 58.3cd | 41.7fg | 51.7fg | 48.3de |
| 22 | - | Control | 100.0a | 0.0k | 99.7a | 0.3k | 99.7ª | 0.3k |
| Mean | 58.04 | 41.96 | 54.32 | 45.68 | 55.7 | 44.28 | ||
| CV (percent) | 24.44 | 33.81 | 24.61 | 29.26 | 23.44 | 29.49 | ||
| LSD (0.05) | 11.41 | 11.41 | 10.75 | 10.75 | 10.5 | 10.5 | ||
Note:- 0h-an aliquot of 25 μl of the antagonist suspension plus H. vastatrix uredinial suspension were deposited simultaneously on leaf disc ;24h-an aliquot of 25 μl of the antagonist suspension placed on leaf disc 24 hs before depositing the urediniospore suspension of H. vastatrix; 72h-an aliquot of 25 μl of the antagonist suspension placed on leaf disc 72 hs before depositing the urediniospore suspension of H. vastatrix: *Means with different letters are significantly different across the column.
Table 4: Interaction effect of Antagonistic fungal isolates and their time of application on CLR severity under leaf disc experiment (set two).
From twenty one antagonistic mycoparsitic isolates which tested as set two, more than 85.0% of the tested fungal antagonists of leaf disc experiment showed promising potential with >45% efficacy for coffee leaf rust (Hemilea vastatirix) control. From 24 hours and 72 hours after inoculation treatment of set two, more than 85% tested mycoparsitic fungal isolates significantly (p<0.05) reduced severity index of Hemilea vastatirix, with >30%efficacy (Table 4). Apart from the above mycoparasitic antagonistic fungal isolates which can significantly reduced the severity index percent of coffee leaf rust at 0 hour inoculation, six more isolates were show highest efficacy and significantly reduce the severity index of CLR with more than 60% at 72 hours after inoculation treatment. Those fungal isolates with high potential antagonistic capacity during 72 hs treatment were Phomasp. (ET621), Lecanicilliumsp. (ET653), Cercosporasp. (ET619), Fusarium sp. (ET637), Fusariumsp. (ET644) and Lecanicilliumsp. (ET657) with efficacy percent of 61.7, 62.7, 63.3, 63.3, 66.7 and 68.3, respectively. These isolates revealed good antagonistic potential when they were treated prior to the landing of the disease pathogen (Table 4) (Figure 2).
Figure 2: Coffee leaf rust severity rating scale (0-5) used for evaluation of the efficacy of anatgonestic fungal isolates against Hemiliea vastatrix [34].
In the current study the result revealed that the application of antagonistic fungal species were found to reduce coffee leaf rust (H. vastatrix) germination and disease severity index percent compared to the untreated control (Figure 3). Indicating that biological control has considerable promise for reducing H. vastatrix.
Figure 3:Coffee leaf rust severity on leaf disc after 35 days of inoculation with different antagonistic fugal isolates such as Digitopodium sp., Fusarium sp., Lecanicillium sp. and control (inoculated with H. vastatrix uridionospores only).
Discussion
In this study the biocontrol potential of mycoparasiests associated with pustules of H. vastatrix obtained from samples collected from major coffee growing areas of Ethiopia was investigated. This was based on germination inhibition test and CLR severity reduction test on coffee leaf disc. These are the first results that report the effect of different mycoparasitic antagonistic generas as the main biological control agent for H. vastatrix in Ethiopia. In this study different genera of mycoparasic isolates namely Alternaria, Cercospora, Fusarium, Digitopodium, Lecanicillium, Phoma, Pleurodesmospora, Simplicillium, Acremonium and Cladosporium were collected, identified and subjected for biocontrol potential against coffee leaf rust. Most of previous studies and publications referring to the biological control of coffee leaf rust focus on the use of Lecanicillium lecanii as a biological control agent [36- 38]. The reason for this is partly to the fact that some related studies were made before the division of the genus Verticillium [35], and in other cases, there is no taxonomic or molecular identification of the isolated mycoparasites from pustules of CLR, and the assumption is made that it is Lecanicillium , due to the morphological characteristics they share.
According to James et al. [19], Lecanicillium can naturally parasite rust pustules in levels near 9 percent in coffee plantations under the shade in Costarica, Latin America. On the other hand [36] evaluated different strains of Verticillium isolated in the same region applied on greenhouse plants, and did not find differences in the incidence of rust pustules; these authors recorded a natural parasitism of 10.5%. Moreover, Zare and Gams [35], found that applying Verticillium can reduce the germination of uredospores by 60%. Other studies have shown that the presence of Verticillium psalliotae on coffee leaves reduces the severity of the damage caused by rust. Likewise, the germination of uredospores was affected significantly when they were put in contact with conidia of the mycoparasite [37].
Significant reductions in CLR severity was achieved with most of the isolates obtained during the survey. Although one hundred and one isolates of antagonistic mycoparasities were obtained during our surveys, the best performance in terms of rust control were with fifteen isolates. The ability of most antagonistic isolates of reducing the CLR disease index was higher when they were applied 72 and 24 hours before with H. vastatrixin the leaf disc study. In both germination inhibition and leaf disc experiment fifteen isolates significantly reduced the formation of pustules and sporulation of H. vastatrix and consequently, the severity of the disease. Some of them were Digitopodiumsp. (ET568), Digitopodiumsp. (ET567), Fusariumsp. (ET675), Lecanicillium sp. (ET657), Lecanicilliumsp. (ET656), and Lecanicilliumsp. (ET651). Nevertheless the best results were obtained when Digitopodiumsp. (ET568) and Digitopodiumsp. (ET567) was applied simultaneously with H. vastatrix.
This study has emerged from preliminary evaluations of potential biocontrol candidates as a species deserving further evaluation. Similarly, and also ironically, some of the antagonistic candidates belong to some genera containing several important plant pathogens. Moreover, it is possible, that someof Fusariu sp. and Alternaria sp. are known plant pathogens. For instance some of Aletrnaria sp. are a seed pathogen of pumpkin Paul et al. [38] and a pathogen of cumin (Holliday, 1980). However, this species has never been reported in the literature, even as an opportunistic pathogen of coffee [3] and it is likely to be inherently safe for use in coffee plantations.
The precise taxonomic identification of natural enemies is a fundamental requirement in any biological control project. The precise identification and characterization of each species of potential biocontrol agent involved is necessary to allow for the discrimination between the various species to play and the selection of the best biocontrol candidate. In the particular case of Hemileia vastatrix clarifying the identity of the antagonistic fungi is critica for discriminating and understanding the role of each fungus within the large diversity of uredinophilous species which is being found [25,37]. The finding of several isolates of anatagonestic fungal species on H.vastatrix pustules at various localities was unexpected. Only recently a number of anatagonestic fungal species was reported attacking a rust fungus rather than Lecanicillium lacani. Only recently a species of antagonistic mycoparasitic species attack rust species were known.
Intensified coffee farm management as use of chemical pesticides to improve yields affect the biodiversity conservation including antagonistic agents [37-40]. The majore aspect in this nexus is the question of sustaining indigenous antagonistic bioagents as natural pest control. Much would be gained if it would be possible to develop management options with less need of chemical inorganic pesticide use [41], while still enabling coffee producing farmers to improve their coffee yield and revenues. Environmentally safe and sustainable management approaches are mandatory to combat sudden outbreaks of diseases and insect pests in response to climate change [42]. However, use of indigenous bioagents for the controls of plant diseases are not widely implemented in Ethiopia, despite the promising potential in coffee disease and pest managment [43,44]. There is also evidence from other systems suggesting that mycoparasites can play a key role as potential biological control agents [45-47].
Conclusion
In conclusion, although there is no example of the practical use of antagonist fungi against CLR in the field at this time, some promising results have been published by other authors in the past, which serve as a stimulus for the continuation of the present work for instance, claimed to have demonstrated the effectiveness of Verticillium lecanii against H. vastatrix in field conditions and claimed that the application of their fungus led to a reduction in the rate of disease when applications were intercalated with copper oxychloride sprays. Unfortunately, the promising results obtained by González and Martinez have never been translated into novel broadly used biocontrol tools, much needed by coffee growers in Ethiopia Africa and elsewhere. Perhaps the present approach, based on on a broader evaluation of potential candidates, obtained from the vast diversity of urediniophilous fungi collected during our surveys, will pave the ground towards the implementation of biological control as a management tool to tackle CLR epidemics.
Acknowledgement
The authors would like to express their gratitude to Ethiopian Institute of Agricultural Research and Jimma University for logistics support to this research work.K.B.B. also thanks the Mrs Tsion Taye (representative of NKG) for providing funding for his split-PhD training period at UFV, Brazil.Moreover, we want to thank the team of plant pathology laboratory research at Federal University of Vicosa, for the support during the research activity.
Author’s Contribution
KBB, GA and WG designed the study. KB conducted the field, laboratory work and drafted the manuscript. All authors edit and wrote the manuscript and gave final approval for publication.
References
- Batista KD, Araújo WL, Antunes WC, Cavatte PC, Moraes GA, et al. (2012) Photosynthetic limitations in coffee plants are chiefly governed by diffusive factors. Trees 26: 459-468.
- Somarriba E, Harvey CA, Samper M, Anthony F, González J, et al. (2004) Biodiversity conservation in neotropical coffee (Coffea arabica) plantations. Agroforestry and biodiversity conservation in tropical landscapes. Island Press, Washington, DC 25: 198-226.
- Zhang LX, Li SS, Tan GJ, Shen JT, He T. (2012) First report of Nigrospora oryzae causing leaf spot of cotton in China. Plant disease 96: 1379
- Belachew K, Senbeta GA, Garedew W, Barreto RW, Del Ponte EM. (2020) Altitude is the main driver of coffee leaf rust epidemics: a large-scale survey in Ethiopia. Tropical Plant Pathology 45: 511-521.
- Menezes-Silva PE, Sanglard LM, Ávila RT, Morais LE, Martins SC, et al. (2017) Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. Journal of Experimental Botany 68: 4309-4322.
- Kushalappa AC, Eskes AB. (1989) Coffee rust: epidemiology, resistance, and management. CRC Press
- McCook S. (2006) Global rust belt: Hemileia vastatrix and the ecological integration of world coffee production since 1850. Journal of Global History 1: 177-195.
- Bhat SS, Naidu R, Daivasikamani S , Nirmala K (2000) Integrated disease management in coffee. In: IPM System in Agriculture – Cash Crops 6.
- Capucho AS, Zambolim L, Cabral PG, Maciel-Zambolim E, Caixeta ET. (2013) Climate favourability to leaf rust in Conilon coffee. Australasian Plant Pathology 42: 511-514.
- Junior JH, Zambolim L, Aucique-Pérez CE, Resende RS, Rodrigues FA. (2015) Photosynthetic and antioxidative alterations in coffee leaves caused by epoxiconazole and pyraclostrobin sprays and Hemileia vastatrix infection. Pesticide biochemistry and physiology 123: 31-39.
- Zambolim L. (2016) Current status and management of coffee leaf rust in Brazil. Tropical Plant Pathology 41: 1-8.
- Dangour A, Green R, Sutherland J, Watson L, Wheeler T. (2015) Health impact related to food and nutrition insecurity. Climate Change and Public Health. Oxford University Press, New York
- Várzea VM, Marques DV. (2005) Population variability of Hemileia vastatrix vs. coffee durable resistance. Durable resistance to coffee leaf rust 53-74.
- Cabral PG, Zambolim EM, Zambolim L, Lelis TP, Capucho AS, et al. (2009) Identification of a new race of Hemileia vastatrix in Brazil. Australasian Plant Disease Notes 4: 129-130.
- Capucho AS, Zambolim L, Cabral PG, Maciel-Zambolim E, Caixeta ET. (2013) Climate favourability to leaf rust in Conilon coffee. Australasian Plant Pathology 42: 511-514.
- Daivasikamani S. (2009) Biological control of coffee leaf rust pathogen, Hemileia vastatrix Berkeley and Broome using Bacillus subtilis and Pseudomonas fluorescens. Journal of Biopesticides 2: 94-8.
- Zambolim L. (2016) Current status and management of coffee leaf rust in Brazil. Tropical Plant Pathology 41: 1-8.
- Ibanez M, Blackman A. (2016) Is eco-certification a win–win for developing country agriculture? Organic coffee certification in Colombia. World Development 82: 14-27
- James TY, Marino JA, Perfecto I, Vandermeer J. (2016) Identification of putative coffee rust mycoparasites via single-molecule DNA sequencing of infected pustules. Applied and Environmental Microbiology 82: 631-639.
- Toniutti L, Breitler JC, Etienne H, Campa C, Doulbeau S, et al. (2017) Influence of environmental conditions and genetic background of arabica coffee (C. arabica L) on leaf rust (Hemileia vastatrix) pathogenesis. Frontiers in plant science 8: 2025.
- Daivasikamani S. (2009) Biological control of coffee leaf rust pathogen, Hemileia vastatrix Berkeley and Broome using Bacillus subtilis and Pseudomonas fluorescens. Journal of Biopesticides 2: 94-98.
- Bettiol W, Saito ML, Brandão MS. (1994) Control of coffee leaf rust with products based on Bacillus subtilis. Summa Phytopathologica 20: 119-122.
- Haddad F, Maffia LA, Mizubuti ES, Teixeira H. (2009) Biological control of coffee rust by antagonistic bacteria under field conditions in Brazil. Biological Control 49: 114-119.
- Carrinl G, Rico-Gray V. (2002) Mycoparasites on the coffee rust in Mexico. Fungal Diversity. 11: 49-60.
- James TY, Marino JA, Perfecto I, Vandermeer J. (2016) Identification of putative coffee rust mycoparasites via single-molecule DNA sequencing of infected pustules. Applied and Environmental Microbiology 82: 631-639.
- Dhingra OD, Sinclair JB.( 1985.) Basic plant pathology methods. CRC Press, Inc.
- Rayner RW. (1970) A mycological colour chart. Commonwealth Mycological Institute and British Mycological Society. Kew.
- Capucho AS, Zambolim EM, Freitas RL, Haddad F, Caixeta ET, et al.( 2011) Identification of race XXXIII of Hemileia vastatrix on Coffea arabica Catimor derivatives in Brazil. Australasian Plant Disease Notes 7: 189-191.
- Silva-Castro I, Barreto RW, Rodriguez MC, Matei PM, Martín-Gil J. (2018) Control of Coffee Leaf Rust by chitosan oligomers and proposes. In Agriculture for Life, Life for Agriculture” Conference Proceedings 4: 311-315.
- Kushalappa AC, Eskes AB. (1989) Advances in coffee rust research. Annual Review of Phytopathology 27: 503-531.
- Salcedo SS. (2018) Biological control of the coffee leaf rust fungus Hemileia vastatrix with an antagonist species of Calonectria in planta: disease control, biochemical and physiological effects. PhD thesis at University Federal of Viçosa.
- Kushalappa AC, Eskes AB. (1989) Coffee rust: epidemiology, resistance, and management. CRC Press
- SAS. (2008). SAS user’s guide (version 9.2), SAS Institute, Cary, NC.USA.
- Shiomi HF, Silva HS, Melo IS, Nunes FV, Bettiol W. (2006) Bioprospecting endophytic bacteria for biological control of coffee leaf rust. Scientia Agricola 63: 32-39.
- Zare R, Gams WJ. (2001) A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. Nova Hedwigia 73: 1-50.
- Canjura ES, Krauss U, Somarriba E. (2002) Massive reproduction of Verticillium sp. Coffee hyperparasite, Hemileia vastatirx. Integrated Pest and Agroecology Management 66: 274.
- Vandermeer J, Perfecto I, Liere H. (2009) Evidence for hyperparasitism of coffee rust (Hemileia vastatrix) by the entomogenous fungus, Lecanicillium lecanii, through a complex ecological web. Plant Pathology 58: 636-641.
- Chala J, Chemeda F, Girma A, Holger H. (2010) Coffee leaf rust epidemics (Hemileia vastatrix) in montane coffee (Coffea arabica L.) forests in southwestern Ethiopia. East African journal of sciences 4: 86-95.
- Paul NC, Deng JX, Lee HB, Yu SH. (2015) Characterization and pathogenicity of Alternaria burnsii from seeds of Cucurbita maxima (Cucurbitaceae) in Bangladesh. Mycobiology 43: 384-391.
- Geeraert L, Hulsmans E, Helsen K, Berecha G, Aerts R, et al. (2019) Rapid diversity and structure degradation over time through continued coffee cultivation in remnant Ethiopian Afromontane forests. Biological Conservation 236: 8-16
- Lewis WJ, Van Lenteren JC, Phatak SC, Tumlinson JH. (1997) A total system approach to sustainable pest management. Proceedings of the National Academy of Sciences 94: 12243-12248.
- Batista KD, Araújo WL, Antunes WC, Cavatte PC, Moraes GA, et al. (2012) Photosynthetic limitations in coffee plants are chiefly governed by diffusive factors. Trees 26: 459-468.
- Avelino J, Cristancho M, Georgiou S, Imbach P, Aguilar L, et al. ( 2015) The coffee rust crises in Colombia and Central America impacts, plausible causes and proposed solutions. Food Security 7: 303-321.
- Bailey BA, Bae H, Strem MD, Crozier J, Thomas SE, et al. (2008) Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biological control 46: 24-35.
- Gleason FH, Lilje O, Marano AV, Sime-Ngando T, Sullivan BK, et al. ( 2014) Ecological functions of zoosporic hyperparasites. Frontiers in microbiology 5: 244
- Parratt SR, Laine AL. (2016) The role of hyperparasitism in microbial pathogen ecology and evolution. The ISME journal 10: 1815-1822.
- González E, Martinez B (1998) Control biologico de la roya del cafeto (Hemileia vastatrix Berk y Br.) con Verticillium lecanii (Zimm) Viegas. Revista Protección Vegetal 13: 81-104.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences