ISSN : 0976-8505
Der Chemica Sinica
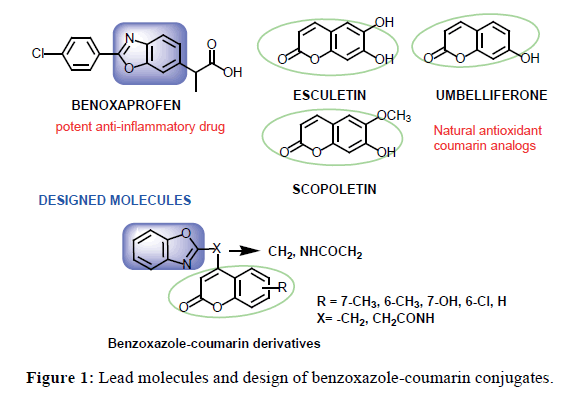
Benzoxazole-Coumarin Derivatives: Potential Candidates for Development of Safer Anti-inflammatory Drugs
Richa Minhas, Sonali Sandhu, Yogita Bansal* and Gulshan Bansal
Department of Pharmaceutical Sciences and Drug Research, Punjabi University Patiala, India.
Abstract
Many naturally existing antioxidants contain coumarin nucleus as a common pharmacophore whereas benzoxazole is identified as pharmacophore for anti-inflammatory activity from benoxaprofen, a potent anti-inflammatory drug. These two therapeutically important nuclie were coupled to generate two series of benzoxazole-coumarin derivatives (4a-4e and 5a-5e) for the development of novel compounds having potent anti-inflammatory and antioxidant activities with insignificant or no ulcerogenic potential. All compounds were evaluated for antioxidant, anti-inflammatory, ulcerogenic potential and oxidative stress induction. Compound 5e has been found to be the most potent antioxidant among these and in comparison to standard drug, BHT. Anti-inflammatory activity evaluation though in vitro hRBC method and in vivo rat paw edema model revealed that 5c exhibits maximum inhibition of hRBC lysis (92.5%) and inhibition of paw edema (52.7%). Results of in vivo biochemical estimations and evaluation of ulcerogenic potential shows that 5c is maximally safe to gastric mucosa. It is also proposed to cause no phototoxicity owing to radical scavenging ability of coumarin nucleus in it. Findings of the present study revealed that compound 5c may be taken as lead benzoxazole-coumarin derivative for development of safe anti-inflammatory agents.
Keywords
Coumarin, Benzoxazole, Anti-inflammatory, Antioxidant, Ulcerogenicity
Abbreviations
DCC: Dicyclohexylcarbodiimide; DCM: Dichloromethane; DMAP: 4-dimethylaminopyridine; DCU: Dicyclohexylurea; SCMC: Sodium Carboxy Methyl Cellulose; DPPH: 2,2-diphenyl-1-picrylhydrazyl; BHT: Butylhydroxytoluene; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; TMS: Tetramethylsilane; DMSO: Dimethyl Sulphoxide; HRBC: Human Red Blood Cells; GSH: Glutathione; CAT: Catalase; TBARS: Thiobarbituric Acid Reactive Substances; OPA: o-Phosphoric Acid; PPA: Polyphosphoric Acid.
Introduction
Coumarin is a unique scaffold in medicinal chemistry owing to its natural existence and synthetic feasibility. It attracts continuous attention of medicinal chemists because of its wide array of biological activities. Numerous coumarin derivatives are reported to possess anticancer [1], antimicrobial [2,3], antioxidant, anti-inflammatory [4] anti-Alzheimer’s [5] and anti-hyperlipidemic activities [6]. Naturally existing coumarin derivatives possess good antioxidant activity and provide an opportunity for development of new antioxidants. Scopoletin, umbelliferone and esculetin (Figure 1) are some of the natural coumarin analogs having antioxidant and anti-inflammatory activities [7]. Benzoxazole is another heterocycle, whose derivatives exhibit a wide range of biological activities such as antiallergic [8] antiproliferative [9], antitumour [10], anti-HIV [11], anti-tuberculosis [12] and antibacterial [13]. This nucleus possesses anti-inflammatory, analgesic and kinase inhibitory activities in itself [14-16]. Benoxaprofen (Figure 1), a potent non-steroidal anti-inflammatory drug (NSAID) having benzoxazole as core structural component, was introduced in 1982. This drug was withdrawn from clinics because of phototoxicity caused by its free radical decarboxylated derivative generated in vivo [17]. Other prominent general side effects associated with use of NSAIDs for alleviating inflammation include gastrointestinal irritation and kidney damage [18]. Therefore, search for development of novel anti-inflammatory agents devoid of such side effects has been attracting attention of medicinal chemists. Coupling of a NSAID with antioxidant molecule has proven to be a fruitful strategy in eliminating or decreasing ulcerogenic potential of the NSAID [19].
In continuity to the previous efforts to develop safer and potent anti-inflammatory agents, we have developed benzimidazole-coumarin derivatives that exhibit potent anti-inflammatory and antioxidant activities with significantly low ulcerogenic potential [20]. The present study reveals coupling of benzoxazole, an anti-inflammatory pharmacophore, with coumarin, an antioxidant and anti-inflammatory pharmacophore, to produce benzoxazole-coumarin derivatives (Figure 1) as potent and safe anti-inflammatory compounds.
Materials and Methods
Chemical reactions were monitored by TLC using silica-gel precoated aluminium plates (Merck, Germany) visualized in a UV chamber at short and long wavelength. Melting points were recorded using open capillary tubes and were uncorrected. Infrared spectra were recorded on a Perkin Elmer Fx IR Spectrometer using KBr disc with vibrational frequencies reported in cm-1. 1H-NMR and 13C-NMR spectra were recorded in DMSO-d6 on Bruker Avance II 400 MHz NMR spectrophotometer. Chemical shifts were reported as δ (ppm) using tetramethylsilane (TMS) as internal standard with multiplicities and number of protons. Coupling constant (J) values are reported when required. Mass spectral analyses were performed on a Thermoscientific (Model LTQ, XL) mass spectrometer [21].
Wistar rats (150-200 g) were used for evaluation of pharmacological activities. The animals were allowed food and water ad libitum. They were housed in cages at room temperature (about 25°C) with a 12 h light/dark cycle. The animals were randomly allocated into groups at the beginning of the experiments. All test compounds and the reference drugs were administered i.p. suspended in 0.5% sodium carboxymethylcellulose solution (SCMC). The experimental protocol was duly approved by Institutional Animal Ethical Committee.
Chemistry
Coumarin-4-acetic acid derivatives (2a-2e)
Coumarin-4-acetic acid is a known compound, and synthetic methods for its synthesis are reported in literature. In the present study, we have taken two methods [22,23] as lead to synthesize variedly substituted coumarin-4-acetic acids. The methods were modified and optimized to get the best yields.
Method A: A mixture of 2.1 g (10 mmol) of citric acid monohydrate and 2.8 mL of conc. H2SO4 in conical flask was stirred for 1 h, and was heated to remove carbon monoxide. Solution was cooled to 0°C and 1.1 mL of conc. H2SO4 was added dropwise, maintaining the temperature below 10°C. Substituted phenol (8 mmol) was added and reaction mixture was stored at 0°C for 16 h. The reaction mixture was poured onto ice and precipitates formed were separated. These were transferred into a saturated sodium bicarbonate solution, stirred at 80°C for 15 min, and filtered. The filtrate was acidified dropwise by addition of conc. H2SO4. The precipitates obtained were separated and recrystallized from ethanol.
Method B: A mixture of 1.92 g (10 mmol) of anhydrous citric acid was heated with 3.2 mL conc. H2SO4 at 60-65°C with constant stirring. The contents were cooled to 0°C, and substituted phenol (10 mol) was added to the solution under vigorous stirring at 0-5° C over a period of 1-1.5 h. Stirring was continued at 5°C for 2 h. Temperature of the mixture was raised slowly to 30°C, and stirred continually for 24 h. The solution was poured into ice cold water, the precipitates produced were filtered, and recrystallized from aqueous ethanol.
2-(6-Methyl-2-oxo-2H-chromen-4-yl)acetic acid (2a):
Method A, 78% yield, m.p. 190-191°C, IR (KBr, cm-1): 3106 (Ar C-H), 2832 (Al C-H), 1705 (C=O), 1688 (C=O lactone), 1610 (C=C), 1141 (C-O), 1416 (C-O-H). 1H-NMR (400 MHz, DMSO-d6): δ 12.08 (1H, s, COOH), 2.50- 2.52 (3H, m, CH3), 3.86 (2H, s, CH2), 6.39 (1H, s, H-3), 7.17-7.21 (2H, m, H-7 and H-5), 7.57 (1H, s, H-8). 13C-NMR (DMSO-d6, δ): 160.8 (C-2), 112.5 (C-3), 155.4 (C-4), 120.9 (4a), 127.6 (C-5), 125.1 (C-6), 127.8 (C-7), 116.9 (C-8), 150.5 (8a), 37.0 (-CH2), 171.2 (C=O), 21.7 (CH3). MS (+ESI) [M+H]+: 219.06.
2-(7-Methyl-2-oxo-2H-chromen-4-yl)acetic acid (2b)
Method A, 84% yield, m.p. 191-192°C, IR (KBr, cm-1): 3134 (Ar C-H), 2921 (Al C-H), 1710 (C=O), 1693 (C=O lactone), 1615 (C=C), 1054 (C-O), 1248 (C-O-H). 1H-NMR (400 MHz, DMSO-d6): δ 12.6 (1H, s, COOH), 2.19-2.23 (3H, m, CH3), 3.89 (2H, s, CH2), 6.30 (1H, s, H-3), 7.21-7.25 (2H, m, H-8 and H-6), 7.52 (1H, s, H-5). 13C-NMR (DMSO-d6, δ): 161.8 (C-2), 113.5 (C-3), 154.0 (C-4), 121.0 (4a), 125.9 (C-5), 135.3 (C-6), 122.0 (C-7), 117.0 (C-8), 153.5 (8a), 37.0 (-CH2), 171.0 (C=O), 21.7 (CH3). MS (+ESI) [M+H]+: 219.06.
2-(7-Hydroxy-2-oxo-2H-chromen-4-yl)acetic acid (2c):
Method B, 75% yield, m.p. 201-202°C, IR (KBr, cm-1): 3479-3376 (OH), 3112 (Ar C-H), 2938 (Al C-H), 1715 (C=O), 1701 (C=O lactone), 1580 (C=C), 1056 (C-O), 1390 (C-O-H). 1H-NMR (400 MHz, DMSO-d6): δ 10.27 (1H, s, COOH), 5.26 (1H, s, OH), 3.74 (2H, s, CH2), 6.16 (1H, s, H-3), 6.72-6.80 (2H, m, H-6 and H-8), 7.47-7.49 (1H, d, H-5, J=6.0 Hz). 13C-NMR (DMSO-d6, δ): 160.2 (C-2), 112.5 (C-3), 152.4 (C-4), 120.1 (4a), 124.8 (C-5), 135.2 (C-6), 122.9 (C-7), 104.1 (C-8), 150.5 (8a), 37.0 (-CH2), 171.5 (C=O). MS (+ESI) [M+H]+: 221.04.
2-(6-Chloro-2-oxo-2H-chromen-4-yl)acetic acid (2d):
Method B, 69% yield, m.p. 210-211°C, IR (KBr, cm-1): 3075 (Ar C-H), 2863 (Al C-H), 1709 (C=O), 1692 (C=O lactone), 1591 (C=C), 1087 (C-O), 1368 (C-O-H). 1H-NMR (400 MHz, DMSO-d6): δ 11.06 (1H, s, COOH), 3.86 (2H, s, CH2), 6.52 (1H, s, H-3), 7.89-7.91 (1H, m, H-7), 7.56-7.59 (1H, m, H-8), 7.79 (1H, s, H-5). 13C-NMR (DMSO-d6, δ): 161.1 (C-2), 110.8 (C-3), 154.4 (C-4), 122.1 (4a), 127.2 (C-5), 140.7 (C-6), 128.0 (C-7), 126.1 (C-8), 150.5 (8a), 37.0 (-CH2), 170.9 (C=O). MS (+ESI) [M+H]+: 240.10.
2-(2-Oxo-2H-chromen-4-yl)acetic acid (2e):
Method B, 57% yield, m.p. 169-170°C, IR (KBr, cm-1): 3125 (Ar C-H), 2925 (Al C-H), 1718 (C=O), 1698 (C=O lactone), 1595 (C=C), 1072 (C-O), 1418 (C-O-H). 1H-NMR (400 MHz, DMSO- d6): δ 12.81 (1H, s, COOH), 3.86 (2H, s, CH2), 6.52 (1H, s, H-3), 7.47-7.51 (2H, d, H-5 and H-8, J= 8.0 Hz), 7.82-7.85 (2H, m, H-6 and H-7). 13C-NMR (DMSO-d6, δ): 161.0 (C-2), 111.8 (C-3), 155.4 (C-4), 128.1 (4a), 128.2 (C-5), 137.7 (C-6), 128.5 (C-7), 118.1 (C-8), 150.0 (8a), 37.0 (-CH2), 170.9 (C=O). MS (+ESI) [M+H]+: 205.05.
2-Amino benzoxazole (3)
It was synthesized by a method developed by slight modifications in a method reported for synthesis of 2-aminobenzimidzoles [24]. Briefly, solutions of cynogen bromide (2.18 g) and o-phenylenediamine (2.1 g) prepared separately in 25 mL of 50% aqueous methanol were mixed in 250 mL conical flask and stirred at room temperature for 48 h. Methanol was recovered under vacuum on water bath. The leftover was cooled to room temperature and made alkaline with aqueou ammonia. Compound 3 was separated as precipitates which were recrystallized from aqueous ethanol. 86% yield, m.p. 129-130°C, IR (KBr, cm-1): 3454 (N-H), 3108 (Ar C-H), 1653 (C=N), 1558 (N-H), 1503 (C=C), 1279 (C-N), 1104 (C-O). 1H-NMR (400 MHz, DMSO-d6): δ 7.10-7.18 (2H, dd, H-7 and H-4, J=6.0 Hz, J=7.0 Hz), 7.23-7.26 (2H, m, H-6 and H-5), 6.96 (2H, s, NH2). 13C-NMR (DMSO-d6, δ): 164.3 (C-2), 143.6 (3a'), 115.5 (C- 4), 124.8 (C-5), 123.6 (C-6), 110.6 (C-7), 148.4 (7a'). MS (+ESI) [M+H]+: 135.12.
Test compounds 4a-4e
These were synthesized by using a procedure developed by modifications in a method reported for synthesis of benzazole derivatives as antimicrobials [25]. In general, 1.0 mmol of each of the coumarin-4-acetic acid derivative (3a-3e) and 1.5 mmol of 2-aminophenol were mixed with PPA/OPA (4g/mmol) in a round bottom flask, and refluxed for 15 min. The reaction mixture was cooled, neutralized with 0.1 N solution of sodium hydroxide, and extracted with 15 mL of dichloromethane. The organic layer was separated and evaporated in china dish. The residue was recrystallized from aqueous ethanol.
4-(Benzo[d]oxazol-2-ylmethyl)-6-methyl-2H-chomen-2-one (4a):
76% yield, m.p. 180-181°C, IR (KBr, cm-1): 3081 (Ar C-H), 2912 (Al C-H), 1698 (C=O lactone), 1610 (C=N), 1564 (Ar C=C), 1143 (C-O), 738 (Ar C-H). 1H-NMR (400 MHz, DMSO-d6): δ 4.40 (2H, s, CH2), 2.40-2.41 (3H, m, CH3), 6.4 (1H, s, H-3), 7.25-7.27 (1H, m, H-7), 7.71-7.73 (1H, m, H-8), 7.34-7.36 (3H, m, H-5, H-4' and H-7'), 7.49-7.51 (2H, m, H-5' and H-6'). 13C-NMR (DMSO-d6, δ): 160.8 (C-2), 112.5 (C-3), 155.0 (C-4), 121.4 (4a), 126.8 (C-5), 135.3 (C-6), 131.7 (C-7), 116.0 (C-8), 150.5 (8a), 21.3 (CH3), 40.2 (CH2), 152.4 (C-2'), 140.9 (3a'), 119.0 (C-4'), 124.2 (C- 5'), 123.6 (C-6'), 110.3 (C-7'), 150.1 (7a'). MS (+ESI) [M+H]+: 292.09.
4-(Benzo[d]oxazol-2-ylmethyl)-7-methyl-2H-chomen-2-one (4b):
79% yield, m.p. 186-187°C, IR (KBr, cm-1): 3050 (Ar C-H), 2908 (Al C-H), 1694 (C=O lactone), 1611 (C=N), 1561 (Ar C=C), 1144 (C-O), 747 (Ar C-H). 1H-NMR (400 MHz, DMSO-d6): δ 4.39 (2H, s, CH2), 2.42-2.43 (3H, m, CH3), 6.4 (1H, s, H-3), 7.17 (1H, s, H-8), 7.09-7.11 (1H, d, H-6, J=4.0 Hz), 7.48-7.51 (1H, m, H-5), 7.33-7.35 (2H, m, H-4' and H-7'), 7.69-7.71 (2H, m, H-5' and H-6'). 13C-NMR (DMSO-d6, δ): 161.3 (C-2), 111.4 (C-3), 155.6 (C-4), 119.9 (4a), 125.8 (C-5), 125.3 (C-6), 141.7 (C-7), 117.0 (C-8), 152.9 (8a), 21.0 (CH3), 39.4 (CH2), 152.4 (C-2'), 141.9 (3a') 119.1 (C-4'), 124.6 (C-5'), 123.6 (C-6'), 111.0 (C-7'), 150.0 (7a'). MS (+ESI) [M+H]+: 292.04.
4-(Benzo[d]oxazol-2-ylmethyl)-7-hydroxy-2H-chomen-2-one (4c):
64% yield, m.p. 195-196°C, IR (KBr, cm-1): 3157 (Ar C-H), 2967 (Al C-H), 1691 (C=O lactone), 1659 (C=N), 1599 (Ar C=C), 1128 (C-O), 973 (Ar C-H). 1H-NMR (400 MHz, DMSO-d6): δ 3.40 (2H, s, CH2), 6.56 (1H, s, H-3), 5.35 (1H, m, OH), 6.77 (1H, s, H-8), 7.76-7.79 (1H, m, H-5), 7.51-7.53 (1H, m, H-6), 7.39-7.40 (2H, m, H-4' and H-7'), 7.79-7.81 (2H, m, H-5' and H-6'). 13C-NMR (DMSO-d6, δ): 159.9 (C-2), 113.4 (C-3), 155.6 (C-4), 116.1 (4a), 125.8 (C-5), 115.3 (C-6), 159.1 (C-7), 104.0 (C-8), 154.2 (8a), 40.4 (CH2), 151.9 (C-2'), 141.5 (3a'), 119.1 (C-4'), 124.8 (C- 5'), 123.6 (C-6'), 110.9 (C-7'), 151.7 (7a'). MS (+ESI) [M+H]+: 294.07.
4-(Benzo[d]oxazol-2-ylmethyl)-6-chloro-2H-chomen-2-one (4d):
59% yield, m.p. 210-211°C, IR (KBr, cm-1): 3159 (Ar C-H), 2916 (Al C-H), 1688 (C=O lactone), 1680 (C=N), 1600 (Ar C=C), 1120 (C-O), 792 (Ar C-H), 1042 (1042). 1H-NMR (400 MHz, DMSO-d6): δ 3.25 (2H, s, CH2), 6.19 (1H, s, H-3), 7.09 (1H, s, H-8), 7.31-7.33 (1H, d, H-7, J=6.0 Hz), 7.68-7.70 (1H, m, H-5), 7.31-7.33 (2H, m, H-4' and H-7'), 7.72-7.74 (2H, m, H-5' and H-6'). 13C-NMR (DMSO-d6, δ): 160.0 (C-2), 112.1 (C-3), 155.4 (C-4), 122.1 (4a), 126.1 (C-5), 130.3 (C-6), 129.1 (C-7), 124.0 (C-8), 152.2 (8a), 39.4 (CH2), 152.4 (C-2'), 141.9 (3a'), 119.1 (C-4'), 124.6 (C- 5'), 123.6 (C-6'), 111.0 (C-7'), 150.0 (7a'). MS (+ESI) [M+H]+: 313.12.
4-(Benzo[d]oxazol-2-ylmethyl)-2H-chomen-2-one (4e):
61% yield, m.p. 174-175°C, IR (KBr, cm-1): 3080 (Ar C-H), 2901 (Al C-H), 1690 (C=O lactone), 1654 (C=N), 1564 (Ar C=C), 1073 (C-O), 801 (Ar C-H). 1H-NMR (400 MHz, DMSO-d6): δ 3.26 (2H, s, CH2), 6.25 (1H, s, H-3), 7.45- 7.48 (2H, m, H-8 and H-5), 7.68-7.70 (2H, m, H-6 and H-7), 7.41-7.43 (2H, m, H-4' and H-7'), 7.71-7.73 (2H, m, H-5' and H-6'). 13C-NMR (DMSO-d6, δ): 160.8 (C-2), 112.1 (C-3), 155.4 (C-4), 121.1 (4a), 128.1 (C-5), 125.3 (C-6), 128.1 (C-7), 115.0 (C-8), 153.2 (8a), 39.8 (CH2), 152.1 (C-2'), 140.9 (3a'), 116.1 (C-4'), 126.4 (C-5'), 126.3 (C-6'), 109.5 (C-7'), 151.2 (7a'). MS (+ESI) [M+H]+: 278.08.
Test compounds 5a-5e
These were synthesized by modifications in a method reported earlier by our laboratory for generating an amide bond between –COOH and -NH2 groups [26]. A suspension of 3 (10 mmol) and DCC (10 mmol) in 100 mL of dried dichloromethane was vigorously stirred for 30 min under nitrogen. A solution of 2-aminobenzoxazole (10 mmol) and dimethylaminopyridine (0.05 g) in 30 mL of dried dichloromethane and freshly distilled pyridine (50 mL) was added to the stirred suspension at 0° C in 15 min. The reaction mixture was stirred at 0° C for 2 h and then at room temperature overnight. The mixture was filtered to remove dicyclohexylurea (DCU), and the filtrate was evaporated under vacuum to yield dry solid which was dissolved in dried ethyl acetate with heating. The residue was filtered, and the filtrate was washed with distilled water. The ethyl acetate layer was dried over magnesium sulphate and solvent recovered under reduced pressure. The resulting crude solid was recrystallized from methanol to yield corresponding amide.
N-(Benzo[d]oxazol-2-yl)-2-(6-methyl-2-oxo-2H-chomen-4-yl)acetamide (5a):
83% yield, m.p. 221-222°C, IR (KBr, cm-1): 3300-3500 (N-H), 3173 (Ar C-H), 2923 (Al C-H), 1663 (C=O), 1688 (C=O lactone), 1645 (C=N), 1609 (Ar C=C), 1065 (C-O), 1065 (C-N), 3397 (N-H). 1H-NMR (400 MHz, DMSO-d6): δ 2.85 (2H, s, CH2), 1.30-1.34 (3H, m, CH3), 6.37 (1H, s, H-3), 7.25-7.27 (1H, m, H-7), 7.30-7.31 (1H, m, H-8), 7.74- 7.76 (3H, m, H-5, H-5' and H-6'), 7.39-7.41 (2H, d, H-4' and H-7'), 9.15 (1H, s, NH). 13C-NMR (DMSO-d6, δ): 161.0 (C-2), 112.1 (C-3), 154.9 (C-4), 120.1 (4a), 127.1 (C-5), 135.3 (C-6), 131.8 (C-7), 116.0 (C-8), 153.8 (8a), 165.1 (C=O), 42.8 (CH2), 21.9 (CH3), 152.1 (C-2'), 148.9 (3a'), 110.1 (C-4'), 123.4 (C-5'), 124.3 (C-6'), 115.5 (C-7'), 143.2 (7a'). MS (+ESI) [M+H]+: 335.10.
N-(Benzo[d]oxazol-2-yl)-2-(7-methyl-2-oxo-2H-chomen-4-yl)acetamide (5b):
81% yield, m.p. 225-226°C, IR (KBr, cm-1): 3300-3500 (N-H), 3096 (Ar C-H), 2892 (Al C-H), 1673 (C=O), 1693 (C=O lactone), 1657 (C=N), 1580 (Ar C=C), 1098 (C-O), 1069 (C-N), 3418 (N-H). 1H-NMR (400 MHz, DMSO-d6): δ 2.80 (2H, s, CH2), 1.34-1.36 (3H, m, CH3), 6.41 (1H, s, H-3), 7.29-7.32 (2H, m, H-6 and H-8), 7.57 (1H, m, H-5), 7.80-7.82 (2H, m, H-5' and H-6'), 7.36-7.39 (2H, d, H-4' and H-7', J=6.0 Hz), 9.04 (1H, s, NH). 13C-NMR (DMSO-d6, δ): 160.6 (C-2), 112.7 (C-3), 154.9 (C-4), 118.1 (4a), 125.1 (C-5), 125.3 (C-6), 143.8 (C-7), 117.2 (C-8), 153.8 (8a), 165.4 (C=O), 42.9 (CH2), 21.3 (CH3), 152.0 (C-2'), 149.0 (3a'), 110.5 (C-4'), 122.4 (C-5'), 124.4 (C-6'), 115.6 (C-7'), 143.8 (7a'). MS (+ESI) [M+H]+: 335.10.
N-(Benzo[d]oxazol-2-yl)-2-(7-hydroxy-2-oxo-2H-chomen-4-yl)acetamide (5c):
76% yield, m.p. 234-235°C, IR (KBr, cm-1): 3300-3500 (N-H), 3116 (Ar C-H), 2850 (Al C-H), 1661 (C=O), 1694 (C=O lactone), 1661 (C=N), 1453 (Ar C=C), 1060 (C-O), 1127 (C-N), 3263 (N-H). 1H-NMR (400 MHz, DMSO-d6): δ 3.51 (2H, s, CH2), 6.41 (1H, s, H-3), 7.41-7.45 (2H, m, H-6 and H-8), 7.61 (1H, m, H-5), 7.63-7.66 (2H, m, H-5' and H-6'), 7.31-7.33 (2H, d, H-4' and H-7', J=6.0 Hz), 9.21 (1H, s, NH), 5.31 (1H, m, OH). 13C-NMR (DMSO-d6, δ): 160.9 (C-2), 111.9 (C-3), 155.2 (C-4), 113.2 (4a), 126.3 (C-5), 112.3 (C-6), 158.8 (C-7), 102.2 (C-8), 154.8 (8a), 164.4 (C=O), 42.3 (CH2), 22.1 (CH3), 152.3 (C-2'), 148.6 (3a'), 111.5 (C-4'), 123.4 (C-5'), 123.8 (C-6'), 114.9 (C-7'), 144.0 (7a'). MS (+ESI) [M+H]+: 337.08.
N-(Benzo[d]oxazol-2-yl)-2-(6-chloro-2-oxo-2H-chomen-4-yl)acetamide (5d):
58% yield, m.p. 215-216°C, IR (KBr, cm-1): 3300-3500 (N-H), 3078 (Ar C-H), 2956 (Al C-H), 1675 (C=O), 1699 (C=O lactone), 1649 (C=N), 1483 (Ar C=C), 1067 (C-O), 1234 (C-N), 3478 (N-H), 1068 (C-Cl). 1H-NMR (400 MHz, DMSO-d6): δ 2.88 (2H, s, CH2), 6.33 (1H, s, H-3), 7.28-7.30 (2H, m, H-7 and H-8), 7.53 (1H, m, H-5), 7.73-7.76 (2H, m, H-5' and H-6'), 7.36-7.40 (2H, d, H-4' and H-7'), 9.19 (1H, s, NH). 13C-NMR (DMSO-d6, δ): 160.9 (C-2), 111.9 (C- 3), 155.2 (C-4), 122.2 (4a), 126.8 (C-5), 131.3 (C-6), 129.8 (C-7), 122.2 (C-8), 153.8 (8a), 164.3 (C=O), 42.1 (CH2), 22.7 (CH3), 152.7 (C-2'), 144.3 (3a'), 116.5 (C-4'), 124.4 (C-5'), 123.3 (C-6'), 114.1 (C-7'), 144.1 (7a'). MS (+ESI) [M+H]+: 356.04.
N-(Benzo[d]oxazol-2-yl)-2-(2-oxo-2H-chomen-4-yl)acetamide (5e):
53% yield, m.p. 229-230°C, IR (KBr, cm-1): 3300-3500 (N-H), 3056 (Ar C-H), 2999 (Al C-H), 1679 (C=O), 1687 (C=O lactone), 1650 (C=N), 1515 (Ar C=C), 1101 (C-O), 1270 (C-N), 3401 (N-H). 1H-NMR (400 MHz, DMSO-d6): δ 2.78 (2H, s, CH2), 6.47 (1H, s, H-3), 7.65-7.67 (2H, m, H-5 and H-8), 7.16-7.19 (2H, m, H-6 and H-7), 7.79-7.81 (2H, m, H-5' and H-6'), 7.45-7.47 (2H, d, H-4' and H-7'), 9.12 (1H, s, NH). 13C-NMR (DMSO-d6, δ): 160.8 (C-2), 112.9 (C-3), 155.2 (C-4), 122.4 (4a), 128.8 (C-5), 125.3 (C-6), 128.5 (C-7), 116.2 (C-8), 151.8 (8a), 164.0 (C=O), 42.1 (CH2), 22.9 (CH3), 152.1 (C-2'), 143.9 (3a'), 116.9 (C-4'), 124.5 (C-5'), 123.7 (C-6'), 115.1 (C-7'), 145.2 (7a'). MS (+ESI) [M+H]+: 321.08.
Molecular Property CalculationsMolecular properties such as Lipophilicity (Log P), Total polar surface area (TPSA), molecular weight (MW), Hydrogen bond acceptors (nON), Hydrogen bond donors (nOHNH), number of violations (nviol), and number of rotatable bonds (nrotb) of the synthesized compounds were calculated using molinspiration calculations software [27]. These properties help in prediction of intestinal absorption, blood brain barrier permeability and oral bioavailability of the compounds according to Lipinski’s rule of 5.
Pharmacological StudyIn-vitro Antioxidant Activity
It was evaluated as hydrogen donating or radical scavenging ability using DPPH method [28] taking BHT and ascorbic acid as standard drugs. Briefly, a 700 μl solution of BHT, ascorbic acid and compounds in methanol was mixed with the same volume of DPPH solution (100 μM in methanol). The mixture was shaken vigorously and allowed to stand in dark at room temperature for 30 min, and absorbance was read at 517 nm. The standard solution was replaced with methanol in control. The antiradical activity was expressed as percent inhibition (I%) and calculated using the following equation:
Inhibition percentage (I%) = [(AbsControl – AbsTest)/AbsControl] × 100
Different concentrations of each test compound and standard drugs were used in order to obtain calibration curves. The antioxidant activity of each compound and drug was reported as EC50 (concentration required to obtain a 50% antioxidant capacity) values in μmol/mL using the method as developed by Locatelli et al [29].
In-vitro Anti-inflammatory Activity
All synthesized test compounds were screened for in-vitro anti-inflammatory activity using hRBC membrane stabilization method [30]. Blood collected from healthy volunteers was mixed with equal volume of sterilized Alsever’s solution (2% dextrose, 0.8% sodium citrate, 0.05% citric acid, and 0.42% sodium chloride) and centrifuged at 1500 rpm. The packed cells were washed with isotonic NaCl (0.85% w/v, pH 7.2) and a 10% v/v suspension of the packed cells was made with isotonic NaCl. Ibuprofen and mesalamine were used as the reference drugs. The assay mixtures contained the drug with concentration ranging from 20, 40, 60, 80 and 100 μg, 1.0 ml phosphate buffer (0.15 M, pH 7.4), 2 ml of hypotonic NaCl (0.36% w/v), and 0.5 ml hRBC suspension. Distilled water (2.0 ml) was used in the control. The mixtures were incubated at 37°C for 30 min and centrifuged. Absorbance (OD) of the supernatant solution was estimated at 500 nm. The percentage of hRBC membrane stabilization was calculated using the formula:
% prevention of lysis=100-[(OD of drug treated sample/OD of control) × 100]
In-vivo Anti-inflammatory Activity
It was evaluated using formalin-induced rat paw edema model [31]. Edema was induced on the right hind paw of rat by subplantar injection of 0.05 ml of solution of 2.5% formalin in 0.9% w/v NaCl. The test compounds were administered orally as suspension in 0.5% sodium carboxy methyl cellulose (SCMC) at a dose equimolar to the standard 1 h before the injection of formalin. Indomethacin was used as standard at the dose of 20 mg/kg. Volume of the paw was measured at 1h, 2h, 3h and 4h, and the activity was calculated as percent inhibition using the following formula:
Percent inhibition=100-[(oedema volume in treated/oedema volume in control) × 100]
Ulcerogenic Activity
Albino rats were divided into 4 groups of 6 animals each, and fasted for 20 h. The control group was administered vehicle (0.5% SCMC). The test groups and the standard group received the test compounds and indomethacin, respectively p.o. at a dose of 150 μmol/kg. The rats were again fasted for 2 h, and then fed for next 2 h. The same process was repeated for next 3 days. The rats were then sacrificed on fourth day. The stomach was removed, opened along the greater curvature, and washed slowly with 0.9% saline. It was examined for the severity of ulceration according to the following scale: 0 = normal grey coloured stomach, 0.5=pink to red colouration of stomach, 1=spot ulcer, 1.5=haemorrhagic ulcer, 2=ulcer<5, 3=ulcer>5, 4=ulcers with bleeding [32]. Mean ulcer score for each animal was calculated and reported as ulcer index.
In-vivo Oxidative Stress
Glandular parts of the extracted stomachs were homogenized in cold phosphate buffer (pH 7.4) for 2 min. The homogenized organ was centrifuged at 800 g for 10 min and then at 12000 g for 15 min. The resulting supernatant was used for biochemical estimations of GSH levels [33] CAT activity [34] and lipid peroxidation in terms of TBARS levels [35].
Results and Discussion
Chemistry
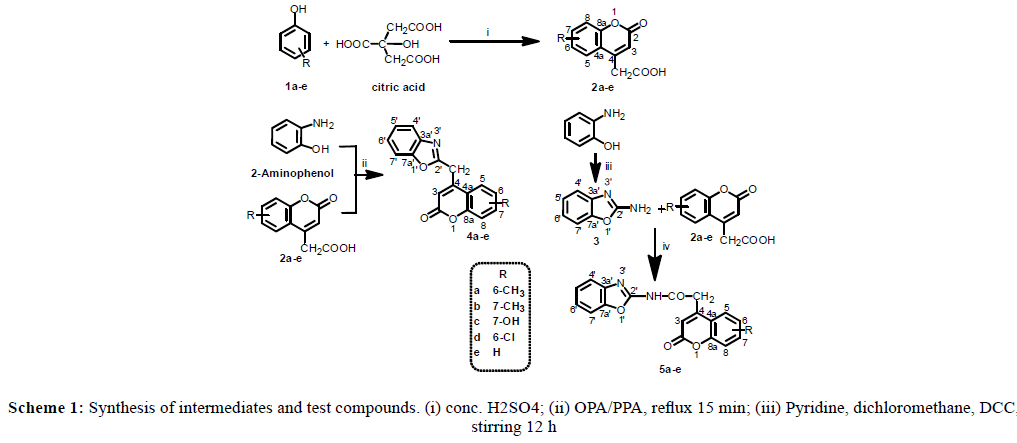
The compounds were synthesized as mentioned in Scheme 1. In first step, variedly substituted phenols (1a-1e) were condensed with citric acid to produce coumarin-4-acetic acid derivatives (2a-2e) as intermediates. 2-Aminobenzoxazole (3) was synthesized by stirring o-aminophenol and cyanogen bromide (CNBr) for 48 h in methanol at room temperature. The first series of test compounds (4a-4e) were synthesized by refluxing intermediates 2a-2e with o-aminophenol in the presence of catalyst for 15 min. Polyphosphoric acid (PPA) was used as catalyst for synthesis of compounds 4a and 4b whereas orthophosphoric acid (OPA) was used for synthesis of compounds 4c-4e, because PPA being stronger acid leads to oxidation of the products. The second series of test compounds 5a-5e was synthesized by coupling the two intermediates, 2a-2e and 3, under anhydrous conditions using dicyclohexylcarbodiimide (DCC) as coupling agent. Each intermediate and compound was purified by recrystallization from ethanol. Structure of each synthesized intermediate and compound was ascertained by various spectral techniques such as IR, 1H-NMR, 13C-NMR and mass In IR spectra of compounds 2a-2e, presence of a sharp band at 1700-1680 cm-1 and broad band at 1400-1100 cm-1, attributed to stretching frequency of a C=O bond and of C-(C=O)-O system in a six-membered lactone ring [21], indicated a coumarin nucleus in the compounds. Carboxylic acid group was ascertained by a sharp C=O stretch at 1720-1700 cm-1 and a broad O-H stretching band at 3640-3200 cm-1. In 1H-NMR spectra, the –COOH proton appeared as a broad peak at δ range of 12.08-10.27 whereas a 2 proton singlet at δ 2.96-2.01 was assigned to CH2 flanked by –COOH group and coumarin nucleus. The proton at C-3 of coumarin ring appeared as a 1 proton singlet at δ 6.52-6.16 whereas chemical shifts of the other protons (at C-5, C-6, C-7, and/or C-8) were found dependent on the substituent at C-6 or C-7 position. In general, the proton at C-8 was detected maximally downfield at δ ≈ 7.90. Structure of compound 3 was ascertained by a 2 protons double doublet at δ 7.14 due to protons at C-4’ and C-7’, and another 2 protons multiplet at δ 7.24 due to protons at C-5’ and C-6’ of benzoxazole nucleus. The two protons of -NH2 group were detected as a singlet at δ 6.96 that disappeared upon D2O exchange.
Formation of compounds 4 was ascertained by disappearance of C=O stretching band at 1720-1700 cm-1, which was noted due to-COOH in compounds 2, and concomitant appearance of C=N stretch at 1610-1680 cm-1 in their IR spectra. The amide linkage in compounds 5 was ascertained by two bands in the range of 1670-1661 and 3300-3500 cm-1 due to Amide I and N-H stretching, respectively. The lactone ring in both the series of compounds was detected by C=O and C-O stretching bands in the range of 1699-1687 and 1143-1060 cm-1, respectively. In 1H-NMR spectra, the protons on coumarin and benzoxazole rings present in compounds 4a-4e and 5a-5e were detected in the range of δ 7.81-6.25 similarly as in the corresponding reactants (3a-3e). The δ values of these protons were in consonant with the upfield or downfield shifts caused by electron donating group or electron withdrawing group in the compounds. The -NH- proton in compounds 5 appeared as a broad singlet at δ 9.04-9.21 confirming the presence of an amide linkage. The methyl groups in compounds 4a, 4b, 5a and 5b appeared at δ 2.30-2.41. In compounds 4c and 5c, –OH proton was observed at δ 5.35-5.20 confirming the presence of hydroxyl group. The signals due to NH and OH protons were confirmed by deuterium exchange experiments. Signals in 13C-NMR spectra of these compounds were in agreement with the signals in their 1H-NMR spectra. In general, the lactonic carbonyl carbon in compounds 4a-4e the carbonyl carbon of amide linkage in compounds 5a-5e was observed maximally downfield at δ range of 161.5-159.0 and 163.2- 165.4, respectively. C-8a of coumarin nucleus being adjacent to oxygen atom was noted at δ 115.0-124.1. The other carbons in coumarin nucleus were observed at different δ values depending on electron withdrawing or donating characters of the substituents attached. Carbons of benzoxazole nucleus appeared in δ range of 154.3-110.6. The molecular masses of all compounds were confirmed by mass spectral analysis in +ESI mode, where the parent ion peak for each compound was observed as M+1 peak corresponding to its molecular mass.
Molecular property calculations
Lipinski’s parameters of all the synthesized compounds are shown in Table 1. Total polar surface area (TPSA) is the parameter for optimizing ability of a drug to permeate cells. Molecules with polar surface area not greater than 140 Å sq can easily permeate cells. Since all test compounds have TPSA less than 140, these can permeate cell membrane.
| Compound | Log Pa | TPSAb | MWc | nONd | nOHNHe | nviolf | nrotb |
|---|---|---|---|---|---|---|---|
| 4a | 4.358 | 56.243 | 291.306 | 4 | 0 | 0 | 2 |
| 4b | 4.358 | 56.243 | 291.306 | 4 | 0 | 0 | 2 |
| 4c | 3.43 | 76.471 | 293.278 | 5 | 1 | 0 | 2 |
| 4d | 4.587 | 56.243 | 311.724 | 4 | 0 | 0 | 2 |
| 4e | 3.933 | 56.243 | 277.27 | 4 | 0 | 0 | 2 |
| 5a | 3.264 | 85.341 | 334.331 | 6 | 1 | 0 | 3 |
| 5b | 3.264 | 85.341 | 334.331 | 6 | 1 | 0 | 3 |
| 5c | 2.337 | 105.569 | 336.303 | 7 | 2 | 0 | 3 |
| 5d | 3.494 | 85.341 | 354.749 | 6 | 1 | 0 | 3 |
| 5e | 2.84 | 85.341 | 320.304 | 6 | 1 | 0 | 3 |
Table 1: Lipinski’s parameters of synthesized compounds.
Log P value according to Lipinski’s rule of five should not be greater than 5 for the molecules to be orally bioavailable. All test compounds have Log P less than 5, and hence are predicted to be orally bioavailable.
Pharmacology
In vitro antioxidant activity
It was evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) method wherein an antioxidant compound reacts with DPPH radical and decreases the colour intensity of the radical that is measured spectrophotometrically. Kinetic studies on the reaction between test compound and DPPH revealed that absorbance plateau was attained at 30 min. Hence, 30 min was taken as the incubation time for compounds for evaluating the activity. All test compounds decreased the colour intensity of DPPH solution, suggesting that the compounds possess radical scavenging ability. Compounds 5a-5e exhibited slightly improved antioxidant activity (0.17-2.2 μmol/mL) than compounds 4a-4e (0.9-2.40 μmol/ mL), which may be attributed to the acidic amide functionality in these compounds (Table 2). Compound 5e (EC50 0.17 μmol/mL) was the most potent and had significantly improved activity in comparison to Butyl hydroxyl toluene (BHT). The results revealed that an unsubstituted coumarin ring exhibit maximum antioxidant activity whereas a substituent on the ring leads to decrease in the antioxidant potential.
| Comp. | Antioxidant activity (EC50, µmol/mL) | hRBC membrane stabilization activity | EC50 (µg/ml) |
||||
|---|---|---|---|---|---|---|---|
| % prevention of membrane lysis | |||||||
| 20 µg/ml | 40 µg/ml | 60 µg/ml | 80 µg/ml | 100 µg/ml | |||
| 4a | 2.40 ± 0.67 | 30.1 ± 0.55a,b | 48.6 ± 0.50a,b | 62.0 ± 0.95a,b | 74.6 ± 0.61a,b | 86.5 ± 0.58b | 45.2 ± 0.54a,b |
| 4b | 2.25 ± 0.19 | 38.6 ± 0.89a,b | 50.9 ± 0.21b | 68.4 ± 0.31a,b | 78.5 ± 0.53a,b | 88.2 ± 0.30b | 39.4 ± 0.60b |
| 4c | 2.72 ± 0.32 | 40.9 ± 0.67a,b | 57.8 ± 0.19a,b | 76.4 ± 0.09a,b | 83.1 ± 0.14a,b | 90.5 ± 0.56a,b | 30.2 ± 0.90a,b |
| 4d | 1.15 ± 0.21 | 40.5 ± 0.41a,b | 56.5 ± 0.72a,b | 74.5 ± 0.46a,b | 81.4 ± 0.61a,b | 89.1 ± 0.22b | 32.1 ± 0.48a,b |
| 4e | 0.92 ± 0.54 | 27.6 ± 0.76a,b | 47.4 ± 0.50a,b | 63.9 ± 0.46a,b | 77.0 ± 0.21a,b | 84.6 ± 0.91a,b | 48.3 ± 0.25a,b |
| 5a | 2.26 ± 0.45 | 30.6 ± 0.48a,b | 45.2 ± 0.54a,b | 61.9 ± 0.55a,b | 72.6 ± 0.17b | 80.2 ± 0.28a,b | 50.7 ± 0.31a,b |
| 5b | 1.88 ± 0.76 | 32.4 ± 0.88a,b | 47.1 ± 0.09a,b | 64.3 ± 0.79b | 79.4 ± 0.77a,b | 83.6 ± 0.42a,b | 47.1 ± 0.92a,b |
| 5c | 2.37 ± 0.51 | 34.9 ± 0.54a,b | 52.2 ± 0.90b | 69.5 ± 0.81a,b | 84.5 ± 0.53a,b | 92.5 ± 0.67a,b | 37.6 ± 0.54b |
| 5d | 0.77 ± 0.12 | 31.6 ± 0.92a,b | 53.0 ± 0.45b | 70.8 ± 0.43a,b | 79.1 ± 0.22a,b | 87.9 ± 0.33b | 35.4 ± 0.69a,b |
| 5e | 0.17 ± 0.44 | 25.8 ± 0.63a,b | 44.1 ± 0.41a,b | 60.1 ± 0.63a,b | 69.2 ± 0.81a,b | 79.4 ± 0.71a,b | 52.5 ± 0.71a,b |
| Std 1 | 0.03 ± 0.001 | - | - | - | - | - | - |
| Std 2 | 23.4 ± 1.23 | - | - | - | - | - | - |
| Std 3 | - | 46.4 ± 0.11 | 52.3 ± 0.33 | 66.1 ± 0.89 | 72.5 ± 0.17 | 87.8 ± 0.40 | 37.8 ± 0.38 |
| Std 4 | - | 21.9 ± 0.38 | 30.6 ± 0.40 | 36.5 ± 0.39 | 40.7 ± 0.39 | 44.6 ± 0.36 | 150 ± 0.32 |
Table 2: Antioxidant and hRBC membrane stabilization activities of target compounds.
Anti-inflammatory activity
In vitro evaluation:
Inflammation is characterized with the release of lysosomal enzymes. Lysis of human red blood cell (hRBC) membrane causes the release of lysosomal enzymes, and hence it is taken as a measure of anti-inflammatory activity of a drug. Stabilization of hRBC membrane tends to reduce the lysosomal enzymes. Hence, the molecules that can cause hRBC membrane stabilization are expected to be anti-inflammatory. In the present study, this activity of each compound was evaluated, in the concentration range of 20-100 μg/mL, as % prevention of lysis of hRBC. Mesalamine and ibuprofen were taken as standard drugs. Mesalamine is selected as it is an active moiety in sulfasalazine, which is reported to be a potent membrane stabilizing agent, and ibuprofen is selected as a representative of NSAIDs.
EC50 values of all compounds were in the range of 30-50 μg/mL while those of mesalamine and ibuprofen were 37.8 and 150 μg/mL, respectively. Membrane stabilizing activity of all compounds was found concentration-dependent. At higher concentration, all compounds showed remarkable stabilizing effect. At 100 μg/mL, compounds 4c and 5c were found to exhibit maximum membrane stabilization with 90.5% and 92.5% protection against lysis in comparison to mesalamine and ibuprofen, which showed 87.8 and 44.6% protection, respectively (Table 2). The activities of compounds 4a, 4b, 4d and 5d are found almost equivalent to that of mesalamine. Compound 4c emerged as the most potent compound from the series with an EC50 value of 30.2 ± 0.90 μg/mL.
In vivo evaluation:
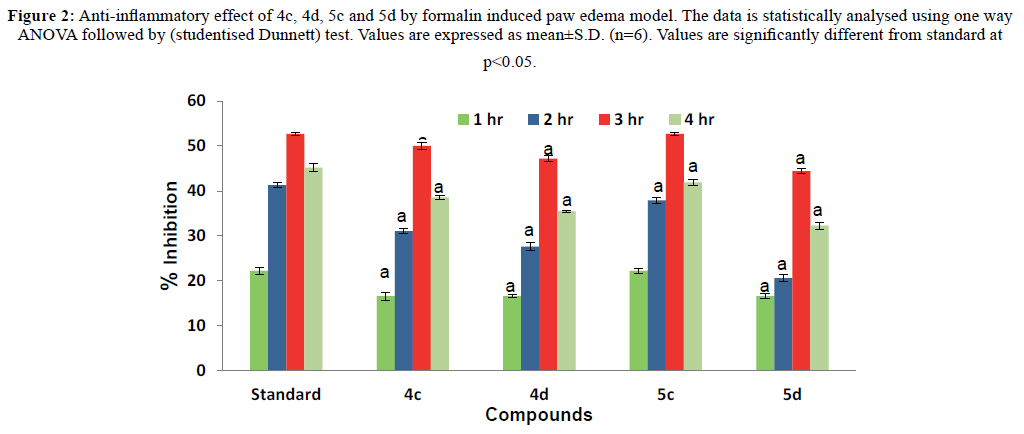
Compounds 4c, 4d, 5c and 5d showed maximum protection to hRBC, and hence these were selected for in vivo anti-inflammatory activity evaluation. The activity was evaluated through formalin induced rat paw edema model. The compounds were found to decrease the paw volume with respect to control group (Table 3), and the peak antiinflammatory inhibition was observed after 3 h. All four compounds showed inhibition of paw edema in the range of 44.4% to 52.7% (Figure 2) which is comparable to the reference drug indomethacin (52.7%). Compounds 5c and 5d exhibited better anti-inflammatory profile in comparison to compounds 4c and 4d. Among these, 5c possess maximum potency that was statistically insignificant from the standard drug. These results suggested that a hydroxy group and an amide linkage increase the potency.
| Compound | Paw volume (ml) | |||
|---|---|---|---|---|
| 1.0 h | 2.0 h | 3.0 h | 4.0 h | |
| 4c | 0.15 ± 0.012a | 0.20 ± 0.006a,b | 0.18 ± 0.010a | 0.19 ± 0.005a |
| 4d | 0.15 ± 0.010a | 0.21 ± 0.005a,b | 0.19 ± 0.009a | 0.20 ± 0.017a,b |
| 5c | 0.14 ± 0.009a | 0.18 ± 0.002a,b | 0.17 ± 0.012a | 0.18 ± 0.013a |
| 5d | 0.15 ± 0.008a | 0.23 ± 0.004a,b | 0.20 ± 0.007a | 0.21 ± 0.011a,b |
| Control | 0.18 ± 0.006 | 0.29 ± 0.005 | 0.36 ± 0.006 | 0.37 ± 0.010 |
| Indomethacin | 0.14 ± 0.005a | 0.17 ± 0.006a | 0.17 ± 0.008a | 0.17 ± 0.014a |
Table 3: In vivo anti-inflammatory activity of selected target compounds.
Ulcerogenic activity
Based on in vivo anti-inflammatory and in vitro antioxidant activities, compounds 4c and 5c were selected for evaluation of their ulcerogenic potential in terms of ulcer index and in vivo oxidative stress. Low ulcer index (1.08) of compound 5c suggested it to be safe on gastric mucosa in comparison to indomethacin (ulcer index 3.48) (Table 4). Decrease in catalase (CAT) and glutathione (GSH) levels, and increase in thiobarbituric acid reactive substances (TBARS) levels in animals treated with indomethacin vis-à-vis the control animals indicated that indomethacin induced the oxidative stress (Table 4). CAT, GSH and TBARS levels in animals treated with compounds 5c were almost equal to those in control group, which indicated that 5c did not exert oxidative stress on the tissues. These results suggested compound 5c to be maximally safe on gastric mucosa. The gastric safety parameters for 4c revealed that it was less safe than 5c but safer in comparison to indomethacin.
| Compound | Catalase (µM/mg/min) | TBARS (nM/mg) | Glutathione (µM/100mg) | Ulcer index |
|---|---|---|---|---|
| Control | 36.2 ± 0.9 | 1.63 ± 0.4 | 143.2 ± 5.6 | 0.66 ± 0.6 |
| Indomethacin | 16.8 ± 0.3a | 8.86 ± 0.9a | 69.0 ± 2.1a | 3.48 ± 0.8a |
| 4c | 22.5 ± 0.8a | 5.24 ± 0.3a,b | 86.6 ± 3.1a,b | 1.33 ± 0.3b |
| 5c | 35.6 ± 0.6b | 3.29 ± 1.04a,b | 112.1 ± 2.9a,b | 1.08 ± 0.9b |
Table 4: In vivo oxidative stress and ulcer index of 4c and 5c.
Structure-activity relationship
Based on the results of these studies, SAR has been proposed that may help in rational designing of novel safe antiinflammatory agents.
• Appendage of coumarin ring to benzoxazole incurs antioxidant activity, which is greatest when the two rings are attached through an amide linkage.
• An electron donating or electron releasing group on coumarin ring decreases the antioxidant potential.
• Anti-inflammatory activity depends on the nature of substituent on coumarin and follows the order OH>Cl>CH3. Higher anti-inflammatory activity due to -OH group may be attributed to its H-bond donating ability.
• An amide linker along with -OH group on coumarin ring exert an additive effect on anti-inflammatory and antioxidant activities.
Conclusion
Taking leads from anti-inflammatory potential of benoxaprofen and antioxidant effect of natural coumarin analogs, series of benzoxazole-coumarin derivatives (4 and 5) were designed, synthesized and evaluated for antioxidant, antiinflammatory and ulcerogenic activities. Compounds of series 5 emerged as maximally potent antioxidants. In vitro hRBC model revealed compound 4c and 5c to exert membrane stabilizing effects better than ibuprofen and mesalamine. Both 4c and 5c also displayed significant antioxidant activity. In vivo anti-inflammatory studies showed compound 5c to be maximally active and equipotent to indomethacin. Further, 5c proved safe on gastric mucosa, and induced negligible oxidative stress. Therefore, 5c can be used as lead derivative from the present study for development of safe anti-inflammatory agents..
Conflicts of Interest
There is no conflict of interest among authors.
Acknowledgements
The authors are thankful to Crystal Pharmaceutical (Ambala, India) for providing ibuprofen and indomethacin, and to Sun Pharma (Formerly Ranbaxy Research Laboratories) (Gurgaon, India) for providing mesalamine as generous gift samples to carry out the study. The authors are also thankful to Punjabi University (Patiala, India) for providing necessary infrastructure and funds to carry out the study. The authors (RM and SS) are thankful to All India Council for Technical Education (New Delhi, India) for providing fellowship.
References
- Riveiro ME, Moglioni A, Vazquez R, Gomez N, Facorro G, et al. (2008) Structural insights into hydroxycoumarin-induced apoptosis in U-937 cells. Bioorg Med Chem 16: 2665-2675.
- Gormley NA, Orphanides G, Meyer A, Cullis PM, Maxwell A (1996) The interaction of coumarin antibiotics with fragments of the DNA gyrase B protein. Biochem 35: 5083-5092.
- Manvar A, Bavishi A, Radadiya A, Patel J, Vora V, et al. (2011) Diversity oriented design of various hydrazides and their in vitro evaluation against Mycobacterium tuberculosis H37 Rv strains. Bioorg Med Chem lett 21: 4728-4731.
- Bansal Y, Sethi P, Bansal G (2013) Coumarin: a potential nucleus for anti-inflammatory molecules. Med Chem Res 22: 3049-3060.
- Anand P, Singh B, Singh N (2012) A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg Med Chem 20: 1175-1180.
- Madhavan GR, Balraju V, Mallesham B, Chakrabarti R, Lohray VB (2003) Novel coumarin derivatives of heterocyclic compounds as lipid-lowering agents. Bioorg Med Chem lett13: 2547-2551.
- Kostova I, Bhatia S, Grigorov P, Balkansky S, S Parmar V, et al. (2011) Coumarins as antioxidants. Cur Med Chem 18: 3929-3951.
- Nakano H, Inoue T, Kawasaki N, Miyataka H, Matsumoto H, et al. (2000) Synthesis and biological activities of novel antiallergic agents with 5-lipoxygenase inhibiting action. Bioorg Med Chem 8: 373-380.
- Garuti L, Roberti M, Pizzirani D, Pession A, Leoncini E, et al. (2004) Differential antiproliferative activity of new benzimidazole-4, 7-diones. Il Farmaco 59: 663-668.
- White AW, Curtin NJ, Eastman BW, Golding BT, Hostomsky Z, et al. (2004) Potentiation of cytotoxic drug activity in human tumour cell lines, by amine-substituted 2-arylbenzimidazole-4-carboxamide PARP-1 inhibitors. Bioorg Med Chem Lett 14: 2433-2437.
- Novelli F, Tasso B, Sparatore F, Sparatore A (1996) Synthesis and biological investigations of 2-(tetrahydropyran-2'-yl) and 2-(tetrahydrofuran-2'-yl) benzimidazoles. Farmaco(Societa chimica italiana: 1989) 52: 499-507.
- Klimensova V, Koci J, Waisser K, Kaustova J, Dahse M (2002) Some of 2-Benzylsulfanylbenzoxazole as Anti-inflammatory Agents. Bioorg Med Chem Lett 12: 3275-3278.
- Kazimierczuk Z, Andrzejewska M, Kaustova J, Klimešova V (2005) Synthesis and antimycobacterial activity of 2-substituted halogenobenzimidazoles. Eur J Med Chem 40: 203-208.
- Sondhi SM, Singh N, Johar M, Kumar A (2005) Synthesis, anti-inflammatory and analgesic activities evaluation of some mono, bi and tricyclic pyrimidine derivatives. Bioorg Med Chem 13: 6158-6166.
- Sondhi SM, Goyal RN, Lahoti AM, Singh N, Shukla R, et al. (2005) Synthesis and biological evaluation of 2-thiopyrimidine derivatives. Bioorg Med Chem 13: 3185-3195.
- Sondhi SM, Singh N, Lahoti AM, Bajaj K, Kumar A, et al. (2005) Synthesis of acridinyl-thiazolino derivatives and their evaluation for anti-inflammatory, analgesic and kinase inhibition activities. Bioorg Med Chem 13: 4291-4299.
- Lewis D, Ioannides C, Parke D (1990) A retrospective study of the molecular toxicology of benoxaprofen. Tox 65: 33-47.
- Kourounakis PN, Tsiakitzis K, Kourounakis AP, Galanakis D (2000) Reduction of gastrointestinal toxicity of NSAIDs via molecular modifications leading to antioxidant anti-inflammatory drugs. Tox 144: 205-210.
- Madhukar M, Sawraj S, Sharma PD (2010) Design, synthesis and evaluation of mutual prodrug of 4-biphenylacetic acid and quercetin tetramethyl ether (BPA–QTME) as gastrosparing NSAID. Eur J Med Chem 45: 2591-2596.
- Arora RK, Kaur N, Bansal Y, Bansal G (2014) Novel coumarin–benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharma Sin B 4: 368-375.
- Silverstein RM, Webster FX, Kiemle DJ, Bryce DL (2014) Spectrometric identification of organic compounds. John wiley and sons<22.
- Clinton R, Laskowski S (1949) Coumarins. I. Derivatives of Coumarin-3-and 4-Carboxylic Acids. J Amer Chem Soc 71: 3602-3606.
- Mashelkar U, Audi A (2006) Synthesis of some novel 4-substituted coumarins having potential biological activity (part II).
- Bansal Y, Kaur M, Silakari O (2015) Benzimidazole–ibuprofen/mesalamine conjugates: potential candidates for multifactorial diseases. Eur J Med Chem 89: 671-682.
- Yildiz-Oren I, Yalcin I, Aki-Sener E, Ucarturk N (2004) Synthesis and structure-activity relationships of new antimicrobial active multisubstituted benzazole derivatives. Eur J Med Chem 39: 291-298.
- Goyal A, Singh J, Pathak DP (2014) Synthesis and Pharmacological Evaluation of Some Novel Imidazole Derivatives for Their Potential Anti-Hypertensive Activity.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Del Rev 23: 3-25.
- Anagnostopoulou MA, Kefalas P, Papageorgiou VP, Assimopoulou AN, Boskou D (2006) Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). F Chem 94: 19-25.
- Locatelli M, Gindro R, Travaglia F, Coïsson JD, Rinaldi M, et al. (2009) Study of the DPPH-scavenging activity: Development of a free software for the correct interpretation of data. F Chem 114: 889-897.
- Rajakumar P, Rasheed AMA, Rabia AI, Chamundeeswari D (2006) Synthesis and study of anti-inflammatory activity of some novel cyclophane amides. Bioorg Med Chem Lett 16: 6019-6023.
- Fereidoni M, Ahmadiani A, Semnanian S, Javan M (2000) An accurate and simple method for measurement of paw edema. J Pharma Toxicol Meth 43: 11-14.
- Cioli V, Putzolu S, Rossi V, Barcellona PS, Corradino C (1979) The role of direct tissue contact in the production of gastrointestinal ulcers by anti-inflammatory drugs in rats. Toxicol Appl Pharmacol 50: 283-289.
- Bergmeyer H, Gawehn K, Grassl M (1974) Aldolase from rabbit muscle. Methods of Enzymatic Analysis, Academic Press, New York: 430.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882-888.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences