Bacillus subtilis (Bbv57) Strain Controls Post Flowering Stalk Rot of Maize caused by Fusarium verticillioides
Radhajeyalakshmi Raju1*, Lakshmi Narayanan Subramanian1, Senthilvel Vaithiyanathan2, and Karthikeyan Gandhi2
1 Department of Plant Pathology, Tamil Nadu Agricultural University, Vagarai, Tamil Nadu, India
2 Department of Plant Pathology, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India
- *Corresponding Author:
- Radhajeyalakshmi Raju
Department of Plant Pathology,
Tamil Nadu Agricultural University,
Vagarai,
Tamil Nadu,
India,
E-mail: radhajeyalakshmi@hotmail.com
Received Date: October 14, 2021; Accepted Date: October 28, 2021; Published Date: November 4, 2021
Citation: Raju R, Subramanian LN, Vaithiyanathan S, Gandhi K (2021) Bacillus subtilis (Bbv57) Strain Controls Post Flowering Stalk Rot of Maize caused by Fusarium verticillioides. J Res Plant Pathol Vol.4 No.5: 01.
Abstract
Among the treatments implied under artificial epiphytotic conditions, Seed treatment (10 g/kg) with Bacillus subtilis (Bbv57) and Soil Application (2.5 kg/ha) recorded lower Fusarium Post Flowering Stalk Rot incidence (3.8 %) compared with Untreated control (8.9%). Among the treatments implied at infected fields of Vagarai and Keeranur, Seed treatment (10 g/kg) with Bacillus subtilis (Bbv57) and Soil Application (2.5 kg/ha) recorded lower Fusarium Post Flowering Stalk Rot incidence (2.0% and 1.04%) compared with Untreated control (7.5% and 3.56%) in both hot spot areas with yield of 5 to 6 t/ha. This study indicated that, Bacillus subtilis (Bbv57) was found to be a potential bio control agent for controlling stalk rot of maize.
Keywords
Post flowering stalk rot; Bacillus subtilis; Fusarium verticillioides
Introduction
The most important diseases associated with maize are caused by fungi, which are primarily represented by the genus Fusarium. In India, the disease is prevalent in most of the maize growing areas, particularly in rain fed areas viz., Jammu and Kashmir, Punjab, Haryana, Delhi, Rajasthan, Madhya Pradesh, Uttar Pradesh, Bihar, West Bengal, Andhra Pradesh, Tamil Nadu and Karnataka, where water stress occurs after flowering stage of the crop [1]. In September 2012, symptoms of Fusarium stalk rot were observed on maize cultivar PAC 740 (Advanta Ltd., Hyderabad, India) grown in a field in Tinsukia, Assam, northeast India (27.5° N; 95.37° E; elev. 116 m) [2]. Reported that incidence of Post Flowering Stalk Rot complex (Charcoal rot, Fusarium stalk rot, late wilt) varying from 5 to 40 per cent at different parts of the country. The annual loss due to maize diseases in India was estimated to the tune of 13.2 to 39.5% [3]. The disease incidence ranged from 10 to 42% in Karnataka [4]. The estimated loss due to Fusarium stalk rot has been reported as 38% in total yield [5].
The infection of maize by Fusarium verticillioides can result in highly variable disease symptoms ranging from asymptomatic plants to severe rotting and wilting. Systemic infection can start from fungal conidia or mycelia that are either carried inside the seeds or on the seed surface [6,7]. The fungus develops inside the young plant, moving from the roots to the stalk and finally to the cob and kernals described the transmission of F.verticillioides from seed to kernals in four steps that include (i) seed to seedling transmission, (ii) colonization of the stalk, (iii) movement into the ear, and (iv) spread within the ear. Irrespective public and private bred hybrids all are succumbing to severe stalk rot. Fusarium stalk rot was observed in the plant age group of 55 to 65 days which coincides with tasseling and silking and immediately followed grain formation stage. The soil borne pathogen led to breakage of stalk rotting, lodging and premature death of the infested plants. Hence, the present study was undertaken with an objective to work out management strategies using Bacillus as one of the components for this threatening disease.
Materials and Methods
Mass multiplication of inoculum for fusarium stalk rots (Fusarium verticillioides)
The present investigation was carried out during 2019-2020 at Maize Research Station, Vagarai, Tamil Nadu Agricultural University, which is located at 10.58⁰ N latitude, 77.57⁰ E longitudes with an altitude of 254 MSL in the Dindigul region of Tamil Nadu state.
Isolations were done by plating surface sterilized (4 per cent sodium hydrochloride) small pieces of infected tissues on Potato Dextrose Agar (PDA) medium. Purification of cultures was made by hyphal tip method. The fungal hyphae are aseptically transferred to culture plates containing the sterile PDA medium to get stock culture. The culture colour of F. verticillioides is pinkish white and it can be identified by confirming the shape of microconidia. The inoculum was increased on toothpicks, which are boiled several times thoroughly in water to remove resin, gum or any toxic substances that might inhibit the growth of the fungus. After washing they were dried into sun. Keeping the tapering end upwards, the dried toothpicks were staked loosely in screw capped jars. Prior to autoclaving, potato dextrose broth is added. The level of broth was adjusted to one-third length of toothpicks after autoclaving. Subsequently, the sterilized jar was seeded with fungus and incubated 280˚C for one week. Abundant mycelial growth was spread on the toothpicks.
Inoculation of Fusarium verticillioides on maize
Inoculations should be made with of 45-50 days old plants just after flowering stage, in the lower internodes (second) above the soil level. The toothpicks is inserted diagonally after pricking and making 2 cm hole with the help of jabber in the desired internodes. Disease symptoms appeared in the inoculated plants about 20-25 days after inoculation. The disease intensity and severity is recorded following 1-9 rating scale [8]. (1) Healthy or slight discolouration at the site of inoculation; (2) Up to 50% of the inoculated internode is discoloured; (3) 51-75% of the inoculated internode is discoloured; (4) 76-100% of the inoculated internode is discoloured; (5) Less than 50% discolouration of the adjacent internode; (6) More than 50% discolouration of the adjacent internode; (7) discolouration of three internodes; (8) discolouration of four internodes; (9) discolouration of five or more internodes and premature death of plant.
Field experiments on the bio efficacy of Bbv57 on Fusarium stalk rot of maize
To assess the field performance of bio agents, the trials were conducted at Maize Research Station, Vagari, Dindigul region of Tamil Nadu namely in randomized block design with three replications maintaining spacing of 60 cm between rows and 20 cm between plants. The bioagents T.asperellam (1 x 104 cfu/g) and B.subtilis (1 x 105 cfu/g) were applied in the soil before sowing @ 200 g/m2. Unamended plots served as check. Seeds were treated with the slurry of carbendazim (0.2%) and bio agents of talc based formulation viz., T.asperellam (4 g/kg), B.subtilis (10 g/ kg). Soil application of T.asperellamand B.subtilis was done @2.5 kg/ha just before sowing. After treating with fungicides, maize seeds were allowed to air dried before sowing. Bio agent treated seeds were kept overnight in moist chamber so as to enable the antagonists to establish on seed surface. The fungi toxicants were applied in the form of spray on the above ground parts of the plants. Two foliar sprays, first two days after inoculation followed by the second 15 days later were made. Observations on disease severity were recorded 45 days after tooth pick inoculation of the pathogen following 1-9 scale devised by [8]. Disease scoring was done until cob development stage.
Field experiments on the bio efficacy of Bbv57 on Fusarium stalk rot of maize in hotspot areas
Two hot spot areas were selected during Kharif 2020 and summer 2021 in the Fusarium infected field at MRS, Vagarai, and in where 10-15% disease incidence was observed and summer 2021 at Keeranur, Palani (Tk.), where 3%-10% disease incidence was noticed. The crop was sown in randomized block design with three replications maintaining spacing of 60 cm between rows and 20 cm between plants. The bio agents T.asperellam (1 x 104 cfu/g) and B.subtilis (1 x 105 cfu/g) were applied in the soil before sowing @ 200 g/m2. Unamended plots served as check. Seeds were treated with the slurry of carbendazim (0.2%) and bio agents of talc based formulation viz., T.asperellam (4 g/kg), B.subtilis (10 g/kg). Soil application of T.asperellam and B.subtilis was done @ 2.5 kg/ha just before sowing. After treating with fungicides, maize seeds were allowed to air dried before sowing. Bio agent treated seeds were kept overnight in moist chamber so as to enable the antagonists to establish on seed surface. The fungi toxicants were applied in the form of seed treatment and soil application on the above ground parts of the plants at 45 days of sowing. Observations on disease severity were recorded 45 days after sowing until cob development stage.
Results and Discussion
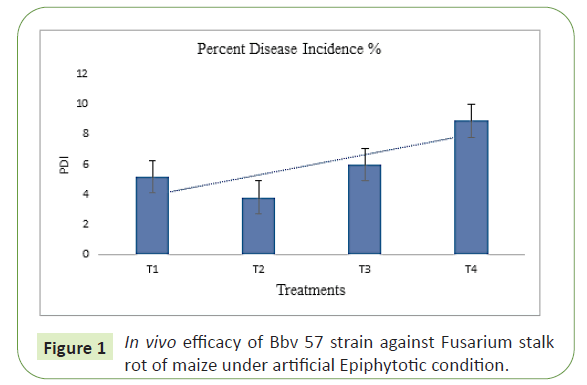
In vivo studies on the efficacy of Bacillus subtilis (Bbv57) strain on Post Flowering Stalk Rot of Maize caused by Fusarium verticillioides in maize were conducted under artificial and natural epiphytotic conditions at Maize Research Station, Vagarai and in two hot spot locations, where the disease incidence was 3-10%. The experimental results revealed the reduction of 2.9% with increased yield of 6.5 tonnes/ha in Bacillus subtilis (Bbv57) treated plots compared to untreated control, which showed 5.1% disease incidence, with low yield of 5.6 tonnes/ha (Figure 1).
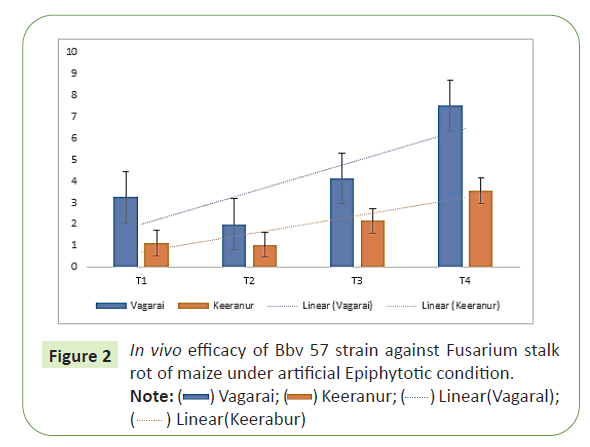
T1: Seed Treatment (4 g/kg) + Soil Application (2.5 kg/ha) with Trichoderma asperellam (Tv1), T2: Seed Treatment (10 g/kg) + Soil Application (2.5 kg/ha) with Bacillus subtilis (Bbv57), T3: Seed Treatment (2 g/kg) + Soil Application with Carbendazim (0.2%), T4: Untreated control. Among the fields of Vagarai and Keeranur, Seed treatment (10 g/kg) with Bacillus subtilis (Bbv57) and Soil Application (2.5 kg/ha) recorded lower Fusarium PFSR incidence (2.0% and 1.04%) compared with Untreated control (7.5% and 3.56%) in both hot spot areas. Bacillus subtilis one of the bio control agent found to colonize the roots of Maize and to control Fusarium at early stages of crop growth with high competitive ability in terms of colonizing the roots @ 1.4 x 107 cfu/g/root (Figure 2).
T1: Seed Treatment (4 g/kg) +Soil Application (2.5 kg/ha) with Trichoderma asperellam (Tv1), T2: Seed Treatment (10 g/kg) +Soil Application (2.5 kg/ha) with Bacillus subtilis (Bbv57), T3: Seed Treatment (2 g/kg) +Soil Application with Carbendazim (0.2%), T4: Untreated control. Bacillus subtilis one of the biocontrol agents found to colonize the roots of Maize and to control Fusarium at early stages of crop growth with highly competitive ability in terms of colonizing the roots 107 cfu/g/root. The results were in line with the findings of Bacillus subtilis CE1 at 108 and 107 CFUml-1 inocula was able to reduce rhizoplane and endorhizosphere colonization of F.verticillioides in greenhouse trials [8].
Figueroa revealed that in plantae assays with three Bacillus isolates: B. megaterium (B5), B. cereus sensu lato (B25) and Bacillus sp. (B35) displayed the highest antagonistic activity against Fv in terms of disease reduction on maize plants [9]. Antagonistic activity analysis revealed that these strains produce glucanases, proteases or chitinases, as well as siderophores and auxins and suggests these as possible control mechanisms against Fusarium verticilliodes.
Bacillus species use diverse mechanisms that may inhibit this fungal pathogen including nutrient competition, production of antifungal lipopeptides, or production of lytic enzymes such as chitinases that can degrade the fungal cell wall as a means to avoid fungal hyphal extension [10-13]. B. subtilis in maize causes a reduction in mycotoxin production and a decrease in Fv colonization. Bacillus cereus increased grain yield by 43.8 % in maize [14]. It has been reported that Bacillus velezensis BM21, a potential and efficient bio control agent in control of corn stalk rot caused by Fusarium graminearum [15].
Similar results were found in Bbv57 strain effective in controlling Fv possibly due to the several PGPR traits that the bacterium possesses. Bbv57 is currently being studied to develop a novel biological product based on spore production to reduce Fusarium stalk, ear and root rots in maize fields [16].
Our findings with Fv in vivo have been recently confirmed in field trials and the efficacy of Bacillus subtilis (Bbv57) have been proved in order to exploit as potential bio control agents suitable for widespread use in the large extensions of maize sown in dry land ecosystems in Tamil Nadu, India.
Statistical analysis
Statistical analyses were conducted using the IRRISTAT version 92-1 programmed developed by biometrics unit at International Rice Research Institute, The Philippines. Differences between treatment mean values were determined following LSD test at 0.05 probability level.
Conclusion
The present investigation highlighted the potentialities of talc- based formulations of Bacillus subtilis obtained from Department of Plant Pathology, Tamil Nadu Agricultural University, Coimbatore-641003, Tamil Nadu, and India in managing disease of maize. Seed treatment and soil application of Bacillus subtilis reduced disease incidence under in vivo conditions with high yield and the same isolate may be recommended for ecofriendly management of maize Post Flowering Stalk Rot in dry land ecosystems.
Acknowledgements
The author is thankful to Tamil Nadu Agricultural University for funding through Non-Plan Scheme and providing field to carry out experimental trials at Maize Research Station, Vagarai.
References
- Figueroa M, Kosack KEH, Solomon PS (2017) Review of wheat diseases a field perspective. Mol Plant Pathol.19(6):1523-1536.
- Rautela A, Dwivedi M (2018) Wheat Stem Rust Race Ug99: A Shifting Enemy. Int J Curr Microbial App Sci.7(1):1262-1266.
- Negassa A, Shiferaw B, Koo J, Sonder K, Smale M, et al. (2013) The Potential for Wheat Production in Africa: Analysis of Biophysical Suitability and Economic Profitability. 1(1):1-76.
- White JW, Tanner DG, Corbett JD (2001) An agro-climatological characterization of bread wheat production areas in Ethiopia.
- Cochrane L, Bekele YW. (2018) Average crop yield (2001-2017) in Ethiopia: Trends at national, regional and zonal levels. Data Brief. 16(1): 1025-1033.
- Shiferaw B, Smale M, Braun J, Duveiller E, Reynolds M, et al. (2013) Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Security. 5(3): 291-317.
- Hei N, Shimelis HA, Laing M (2017) Appraisal of farmer’s wheat production constraints and breeding priorities in rust prone agro-ecologies of Ethiopia. Afr J Agric Res. 12(12): 944-952.
- Prescott JM, Geleta AB, Bowman J, Burnett PA, De Milliano W, et al (2002) Wheat diseases and pests: a guide for field identification. CIMMYT. (2nd Eds):1-148.
- Admassu B, Lind V, Friedt W, Ordon F (2008) Virulence analysis of Puccinia graminis f. sp. tritici populations in Ethiopia with special consideration of Ug99. Plant Pathol. 58(2):362-369.
- Denbel W, Badebo A, Alemu T (2013) Evaluation of Ethiopian commercial wheat cultivars for resistance to stem rust of wheat race ‘UG99’. Intl J Agron Plant Prod. 4(1):15-24.
- Peterson RF, Campbell A, Hannah A (2011) A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can J Res. 26(1):496-500.
- Roelfs AP, Singh RP, Saari EE (1992) Rust diseases of wheat: concepts and methods of disease management. CIMMYTY. 1(1): 1-81.
- Zadoks JC (1981) Cereal rusts, dogs and stars in antiquity. Cereal Rusts Bulletin. 13(1);1-10.
- Schielzeth H, Nakagawa S (2013) Nested by design: model fitting and interpretation in a mixed model era. Methods Ecol. Evol. 4(1):14-24.
- Stokes ME, Davis CS, Koch GG (2012) Categorical data analysis using SAS, SAS institute.(3rd Eds):1-13.
- Green S, Salkind N (2017) Using SPSS for Windows and Macintosh, books a la carte. Pearson. 2, 3.
- Prank M, Kenaley SC, Bergstrom GC, Acevedo M, Mahowald NM (2019) Climate change impacts the spread potential of wheat stem rust, a significant crop disease. Environ. Res. Lett. 14(12): 1-10.
- Ferede T, Ayenew AB, Hanjra MA, Hanjra M (2013) Agroecology matters: Impacts of climate change on agriculture and its implications for food security in Ethiopia. Global food security: Emerging issues and economic implications (Eds.). 71-111.
- Mideksa T, Fininsa C, Hundie B (2018) Analysis of Climate Variability Effects on Wheat Stem Rust (Puccinia graminis f. sp tritici) Epidemics in Bale and Arsi Zones of Oromia Regional State, Ethiopia. American J Bio Environ Stat. 4(2):49-65.
- Hailu A, Woldeab G, Dawit W, Hailu E (2015) Distribution of Wheat Stem Rust (Puccinia graminis f. Sp. tritici) in West and Southwest Shewa Zones and Identification of its Phsiological Races. Adv Crop Sci Tech 3:189.
- Abebe T, Woldeab G, Dawit W (2012) Distribution and physiologic races of wheat stem rust in Tigray, Ethiopia. J Plant Pathol Microbiol. 3(6): 1-5.
- Regasa GH, Senbeta GA, Hei NB (2019) Evaluation of Ethiopian bread wheat varieties to dominant stem rust races (Puccinia graminis f. sp. tritici) at seedling stage under greenhouse condition. Int. J. Agric. Biosci. 8(4):210-216.
- Singh RP, Hodson DP, Espino JH, Jin Y, Njau P, et al. (2008) Will stem rust destroy the world's wheat crop? Adv. Agron. 98(1): 271-309.
- Fetch T, Mccallum B, Menzies J, Rashid K, Tenuta A (2011) Rust diseases in Canada. Prairie Soils and Crops.4: 87-96.
- Bhavani S, Singh R, Argillier O, Espino JH, Singh S, et al. (2011) Mapping of durable adult plant stem rust resistance in six CIMMYT wheats to Ug99 group of races. 2011 BGRI technical workshop, St Paul, Minnisota, USA, 2011. 43-53.
- Hailu E, Rani S, Deyou M (2015) Effects of land versus water based fitness program in improving aerobic fitness, muscular strength and speed among young male beginner soccer players. Turk J Kin. 1(1):15-19.
- Krupinsky JM, Bailey KL, Mcmullen MP, Gossen BD, Turkington TK (2002) Managing plant disease risk in diversified cropping systems. J Agron. 94(2):198-209.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences