Astroglial Transcriptome Dysregulation at the Core of Amyotrophic Lateral Sclerosis
Amin M1, Nisar A1 and Choudry UK2*
1Jinnah medical and dental college, Karachi, Pakistan
2Department of Post Graduate Education, Aga Khan University Hospital, Karachi, Pakistan
- *Corresponding Author:
- Choudry UK
Department of Post Graduate Education, Aga Khan University Hospital, Karachi, Pakistan
Tel: +923456165524
E-mail: uk_choudry@hotmail.com
Received date: February 20, 2017; Accepted date: February 21, 2017; Published date: February 28, 2017
Citation: Amin M, Nisar A, Choudry UK (2017) Astroglial Transcriptome Dysregulation at the Core of Amyotrophic Lateral Sclerosis. J Surgery Emerg Med 1:1.
Copyright: © 2017 Amin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Editorial
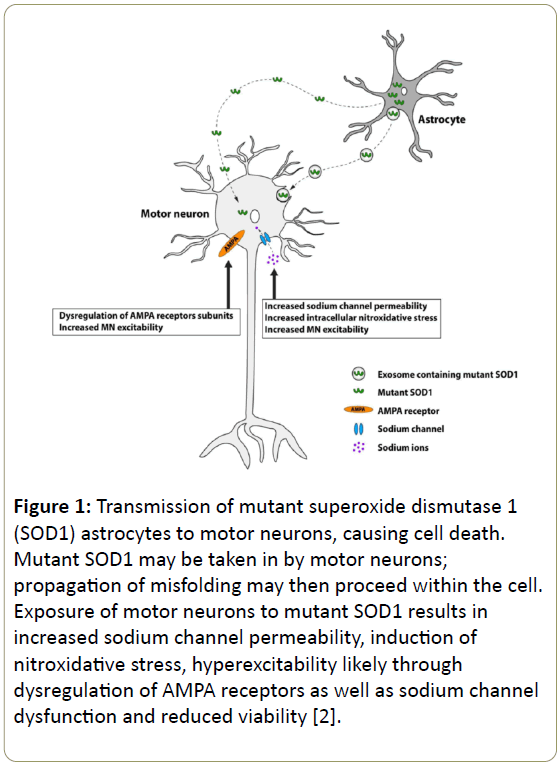
Amyotrophic lateral scl erosis (ALS) which is also known as motor neuron disease, is a neurodegenerative disorder in which upper and lower motor neuron death occurs leading to progressive neuromuscular failure, resulting in death within 3-5 years of symptom onset [1]. Despite substantial endeavors the cause of ALS remains elusive. ALS is caused by a dominant mutation in an antioxidant enzyme, superoxide dismutase 1 (SOD1) which normally protects the cell from toxic reactive oxygen species. In a recent study, it has been proposed that exposure of motor neurons to the mutant SOD 1 results in increased sodium channel permeability, induction of nitroxidative stress, hyperexcitability and motor neuron excitability through dysregulation of AMPA receptors. Dysregulation of the receptors leads to sodium channel dysfunction and astroglia toxicity to motor neurons [2]. The mechanisms behind the dysregulation of neuronal signaling remain ambiguous. Current researches suggest alternations in function of motor neurons to be associated with astrocyte mediated increase neuronal excitability and failing to regulate glutamate levels.
Astroglia derived from SOD1 ALS mouse model show significant decrease in glutamatergic response, as compared to wild type astrocytes that exhibit a vigorous response when activated with lipopolysaccarides, G5 or treated with ceftriaxone [3]. A study documented astroglial potassium channel KCNK1 down regulation and an increase in sodium cotransporter Slc41a1, prior to symptomatic phase in G93A SOD1 mice of ALS mouse model. In mid symptomatic stage, there is strongest decrease of calcium binding protein, Sparc11, and a remarkable increase in lysosomal protein, Ctss. These intriguing results are suggestive of transcriptional level dysregulation in astroglia in ALS [4]. Mutant superoxide dismutase 1 (SOD1) is secreted in the exosomes and transmitted from astrocytes to motor neurons, causing neuronal cell death (Figure 1) [2].
Figure 1: Transmission of mutant superoxide dismutase 1 (SOD1) astrocytes to motor neurons, causing cell death. Mutant SOD1 may be taken in by motor neurons; propagation of misfolding may then proceed within the cell. Exposure of motor neurons to mutant SOD1 results in increased sodium channel permeability, induction of nitroxidative stress, hyperexcitability likely through dysregulation of AMPA receptors as well as sodium channel dysfunction and reduced viability [2].
In a study on microRNAs (miRNAs), involved in post translational gene regulation, established that miRNAs expression of astrocytes as compared to miRNAs secreted via astroglial exosomes are vastly different and only selective miRNAs are recruited to exosomes. There is also evidence of central nervous system consequences in disturbance of expression of miRNAs abounding in astroglial exosomes [5]. Dysregulation of gene expression is recognized as one of the pathophysiological components triggering neurodegeneration in the disease. Alteration in properties of astrocytes leads to propagation of motoneuron injury in SOD1 related ALS. Previous reports have also demonstrated that selective silencing of mutant SOD1 expression in astrocytes has significantly slowed disease progression [6]. Data from recent studies indicates that manipulation of energy supply to motoneuron may help to reduce disease progression. Results proposed that lactate supplement can completely reverse the observed toxicity particularly as metabolic dysregulation occurs to be the cause of progression of the disease. Dietary interventions are being considered as a potential therapeutic approach to ameliorate the motoneuron energy deficit in the disease [4]. We further anticipate that emerging methodologies will enable to invesstigate on the gene expression dysregulation and will dissect the molecular events that occurs in actively translating mRNA (i.e. translatome). These hypothesis offer new avenues for development of new therapeutic strategies targeting astrocytic dysregulation in ALS.
References
- Thomas M, Alegre-Abarrategui J, Wade-Martins R (2013) RNA dysfunction and aggrephagy at the centre of an amyotrophic lateral sclerosis/frontotemporal dementia disease continuum. Brain.
- Bunton-Stasyshyn RK, Saccon RA, Fratta P, Fisher EM (2015) SOD1 function and its implications for amyotrophic lateral sclerosis pathology new and renascent themes. The Neuroscientist 21: 519-529.
- Benkler C, Benâ€ÂÂZur T, Barhum Y, Offen D (2013) Altered astrocytic response to activation in SOD1G93A mice and its implications on amyotrophic lateral sclerosis pathogenesis. Glia. 61: 312-326.
- Miller SJ, Zhang PW, Glatzer J, Rothstein JD (2016) Astroglial transcriptome dysregulation in early disease of an ALS mutant SOD1 mouse model. Journal of neurogenetics, pp: 1-12.
- JoviÄÂÂić A, Gitler AD (2017) Distinct repertoires of microRNAs present in mouse astrocytes compared to astrocyte-secreted exosomes. PloS one 12: e0171418.
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, et al. (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nature neuroscience 11: 251-253.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences