Assessing Immuno-Expression of p53 Protein and TP53 Gene Amplification in Histologically Negative Surgical Margins of Oral Squamous Cell Carcinoma Patients and Normal Oral Mucosa
Shankargouda Patil1*, Mamata S Kamat2, Rudrayya S Puranik3, Bhagavan Das Rai A4 and BR Patil5
1Department of Oral Pathology and Microbiology, Bharati Vidyapeeth (Deemed to be University) Dental College and Hospital, Sangli, India
2Department of Oral Pathology and Microbiology, PMNM Dental College & Hospital, Bagalkot, India
3Department of Oral and Maxillofacial Surgery, Pacific Dental College and Hospital, Udaipur, India
4Department of Surgical Oncologist, Karnataka Cancer Therapy and Research Institute, Navanagar, Hubli, India
5Department of Maxillofacial Surgery and Diagnostic Sciences, Division of Oral Pathology, College of Dentistry, Jazan University, Jazan, Saudi Arabia
- *Corresponding Author:
- Shankargouda Patil
Department of Oral Pathology and Microbiology, Bharati Vidyapeeth (Deemed to be University) Dental College and Hospital, Sangli, India
Tel: 0913999128
E-mail: dr.ravipatil@gmail.com
Received Date:January 25, 2022, Manuscript No. IPMO-22-12388; Editor assigned date: January 28, 2022, Pre QC No. IPMO-22-12388 (PQ); Reviewed date: February 14, 2022, QC No. IPMO-22-12388; Revised date: March 27, 2022, Manuscript No. IPMO-22-12388 (R); Published date: April 05, 2022, DOI: 10.36648/ipmo/22.4.001
Citation: Patil S, Puranik RS, Das Rai B, Patil BR Kamat M (2022) Assessing Immuno-Expression of p5 3 protein and TP53 Gene Amplification in Histologically Negative Surgical Margins of Oral Squamous Cell Carcinoma Patients and Normal Oral Mucosa. J Med Oncol Vol:5 No:4
Abstract
Background
About 30% of patients with Oral Squamous Cell Carcinoma (OSCC) develop a Local Recurrence (LR) even with histologically tumor-free surgical margins (HNMs). Hence, molecular analysis of the HNMs before the occurrence of any morphological changes is of paramount importance. The present study aimed to assess and compare the immunoexpression of p53 and TP53 gene amplification in normal oral mucosa and histologically negative surgical margins of Oral Squamous Cell Carcinoma (OSCC) along with the correlation with LR and survival.
Methods
Formalin-fixed paraffin-embedded tissue blocks of 40 HNMs of OSCC and 40 normal oral mucosa samples were analyzed for p53 immunostaining and TP53 gene amplification by PCR.
Results
Significantly higher positivity was noted with p53 immuno-expression, TP53 gene amplification, and combined p53 and TP53 expression in the study group than the control group (p<0.05). Although, statistically insignificant, most of the cases that showed LR and succumbed to mortality; were positive for p53 immuno-expression, TP53 gene amplification, and combined p53 and TP53 expression. Kaplan-Meier survival analysis showed that subjects with TP53 and combined p53 and TP53 positivity had decreased survival rate than their negative counterparts.
Conclusion
Detection of p53 in HNMs of OSCC can be used as a biomarker to identify those patients who are at high risk to develop LR and to predict survival. Combined p53 and TP53 assessment to be more reliable for predicting LR to help clinicians/surgeons for treatment planning.
Keywords
Oral squamous cell carcinoma; p53; Recurrence; Survival
Introduction
Globally, OSCC ranks sixth to eighth most frequent cancer and the incidence varies according to the geographic locations; with developing countries nearly contributing for 75% of the cases [1,2]. Literature has revealed that approximately 30% of all new cases are reported annually in India.
In India, OSCC commonly occurs in the gingival-buccal complex (alveolar ridge, gingival-buccal sulcus, and buccal mucosa), whereas in the Western world, the tongue is frequently affected. This has been attributed to the rampant use of chewable tobacco products which expose the buccal mucosa to high doses of carcinogens, especially in the younger generation [2].
Traditionally, the therapeutic planning and prognostic evaluation of OSCC is highly based on TNM staging; and surgery and radiotherapy remain the elective therapy for oral cancer. However, even with advanced diagnostic and therapeutic procedures, the 5-year survival remains less than 50% [3]. Hence, complete removal of the primary tumor along with free margins is a crucial prognostic and predictive step for successful treatment outcomes for OSCC [4,5]. Apart from the stage, the surgical margin status can have a significant impact on post-surgical treatments like chemotherapy and radiotherapy [6-8].
Moreover, “Minimal Residual Cancer (MRC) cells” or “fields of genetically altered cells” may not be recognized with conventional histopathology even after the complete excision of the tumor as indicated by microscopic assessment of surgical margin. Approximately about 30% of patients with OSCC develop recurrence even with tumor-free surgical margins [9,10].
The sampling of resected surgical margins for analysis (i.e. defect-driven sampling or specimen-driven sampling) and the methods employed to assess these margins have a great impact on the treatment outcome. To address this challenging issue, both intra and postoperative techniques like the use of intraoperative frozen section analysis, optical imaging and molecular assessment of margins, etc. have come into practice [11].
For precise identification of tumor cells at the surgical margins postoperatively, molecular margin analysis has shown promising results [11]. Studies have shown that accumulated genealogical alterations in the histologically normal epithelium of the resected surgical margins are involved in local re-appearance [12]. Hence, evaluating the resected surgical margins for their molecular nature before the occurrence of any clinically evident changes is of paramount importance. Consequently, designing a reliable path to ascertain the existence of malignantly transformed cells in Histologically Negative Surgical Margins (HNMs) will be possible [10].
P53, well known as “the g protein is a product of the TP 53 gene and their accumulation in OSCC is most frequently seen in >50% of OSCC [13]. Similarly, recent studies have focussed on investigating p53 at the cellular level and TP53 at the molecular level in HNMs and found an association with local recurrence [14].
Considering the above-mentioned facts, the present research was undertaken to evaluate the immuno-expression of p53 protein and TP 53 gene amplification in HNMs of OSCC and to correlate the expression with local recurrence and survival status.
Materials and Methods
Patients and tissue samples
After obtaining institutional ethical clearance, records from the histopathology Department, Karnataka Cancer Therapy and Research Institute (KCTRI), Navanagar, Hubli were searched for OSCC cases affecting lower gingivo-buccal sulcus or buccal mucosa that underwent resection of the primary OSCC. The present study included two groups; Group I (Control group): 40, age and gender-matched normal oral mucosal tissue samples obtained during routine dental procedures, and Group II (Study group): 40 Histologically Negative Surgical Margins (HNMs) of primary OSCC patients.
The criteria for inclusion of cases were as follows; i) OSCC cases affecting the lower gingivo-buccal sulcus or buccal mucosa; ii) OSCC cases classified as T1/T2/T3, N0/N1/N2, M0, with adequate clinical, histological, and a follow-up data and; iii) distance between tumor and HNM should be equal to or more than 5 mm as assessed by conventional histological examination were included in the study. Margins with the presence of a potentially malignant lesion, such as Epithelial Dysplasia (ED), subjects who were given preoperative chemotherapy or radiotherapy, Microscopic subtypes, and recurrent OSCCs were excluded. The Haematoxylin and Eosin-stained sections of the selected cases were reviewed to confirm tumor-free surgical margins.
Local recurrence was considered when the tumor regrowth of <2 cm away from the primary site seen within 3 years. Survival status was assessed based on the interval between the time of treatment and mortality.
IHC staining
The formalin-fixed paraffin-embedded tissue blocks from both groups were sectioned using a soft tissue microtome and were mounted on slides coated with 3-Aminopropyl Triethoxy Silane (APES). One section was stained with Harris hematoxylin and eosin. The other section was stained with p53 (ready to use, Monoclonal Mouse Anti-p53, Clone DO-7; Dako) antibody.
Evaluation of IHC stained slides
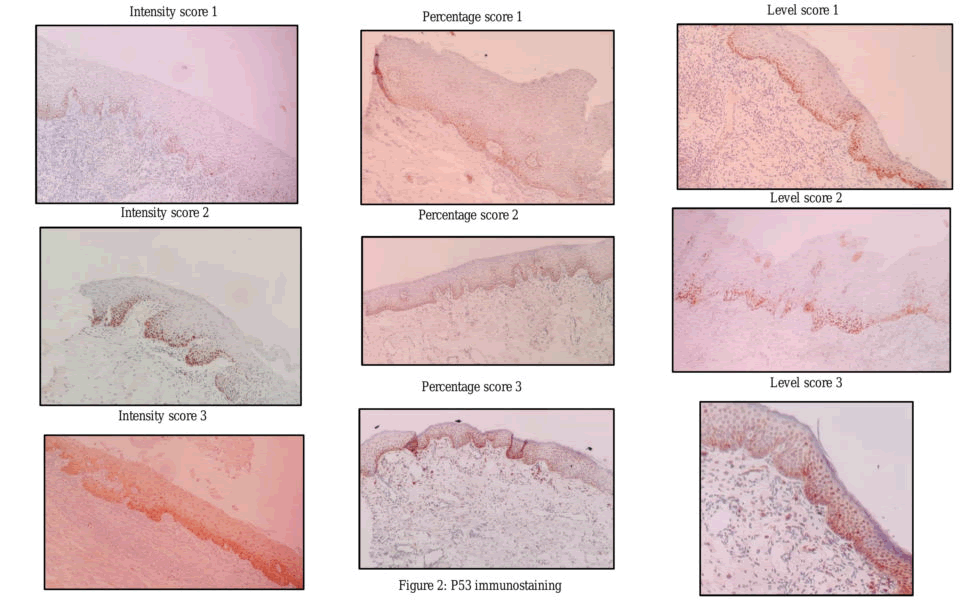
p53 stained sections were evaluated for intensity, percentage of nuclear staining as well as the level of expression in the epithelium. The nuclear staining of tumor cells of known p53 positive OSCC tissue represented internal positive control.
Scores for Intensity of p53 nuclear staining included 0: No nuclear staining,
1: Focal nuclear staining not easily visible in low power,
2: Dark and focal nuclear staining easily visible under low power, and
3: Dark and diffuse nuclear staining easily seen in low power.
Percentage of p53 nuclear staining was scored as; 0: nuclear staining absent,
- 1: <25% of cells showing nuclear staining,
- 2: 25–50% of cells showing nuclear staining, and
- 3: >50% of cells showing nuclear staining [16].
Additionally, we also scored the level of p53 nuclear staining within the epithelium; Score 0: no staining, score 1: p53 nuclear staining limited to the basal layer of epithelium, score 2: p53 nuclear staining confined to basal and supra-basal layers of epithelium and Score 3: p53 nuclear staining seen in the entire thickness of the epithelium [15].
Real-time polymerase chain reaction (RT-PCR)
Samples from both groups were further subjected for p53 specific RT-PCR (real-time Polymerase Chain Reaction). DNA extraction was done from the selected samples of both groups using the modified Proteinase-K method [17]. PCR primers specific to TP53 included the following (Bioserve India Pvt Ltd, Hyderabad, INDIA);
TP53-F: 5’-TGGATCCTCTTGCAGCAGCC-3’
TP53-R: 5’-AACCCTTGTCCTTACCAGAA-3’
In the preparation of the PCR reaction mixture, a Universal SYBR green master mix (Roche, Basel, Switzerland) was used (Roche FastStart Universal SYBR green PCR master mix containing 2.5 mM MgCl2 was used). Additionally, the master mix is also comprised of dNTP Mix, Taq polymerase enzyme, and Syber green dye.
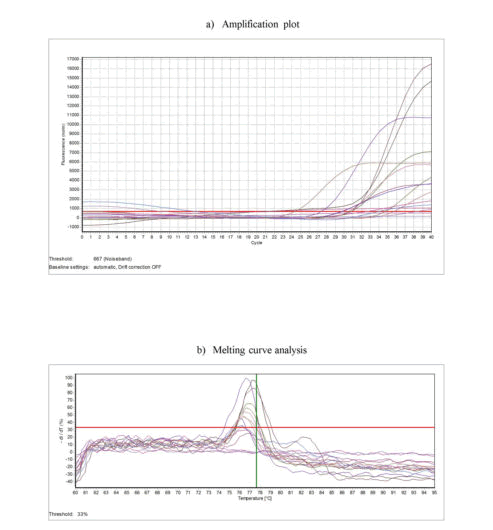
Initial denaturation was done at 95°C for 5 minutes and this was followed by 40 cycles of denaturation at 95°C, annealing at 54°C, and extension at 72°C for 20 seconds each. Melting curve analysis was carried from 60°C to 95°C in 20 minutes duration. Samples of OSCC with confirmed p53 gene as positive control and sterile molecular grade water as negative control were included in each PCR run. Melting curve analysis was done to confirm the specific amplification of target DNA.
Statistical analysis
All the statistical analysis was performed using SPSS version 16 software. A Chi-square test or unpaired t-test was employed to compare the immuno-expression of p53 and TP53 gene amplification depending on the type of data. Bivariate and multivariate logistic regression analysis was employed to predict the local recurrence based on the immuno-expression of p53 and TP53 amplification and also to adjust known confounders. Kaplan-Meier analysis was used for survival analysis.
Results
The mean ages in study and control groups were 49.83 years (SD ± 10.43) and 37.28 years (SD ± 8.61) respectively. The maximum number of subjects in both groups was males. Among the study group, tobacco with areca nut quid chewing habit was the most frequently practiced habit.
p53 and TP 53 expression in HNMs: p53 protein expression by IHC was seen in Thirty-four (85%) HNMs of the study group and 16 (40%) samples of the control group and the difference was statistically significant (p=0.0001).
Further, TP 53 gene analysis by PCR (Figure 1), showed positivity in 27(67.5%) of HNMs and 14 (35%) of controls with statistically significant difference (p=0.0040).
Overall, 38(95%) of HNMs and 18 (45%) of controls showed either p53 protein-expression (IHC) or TP 53 gene amplification (PCR) or both, with statistically significant difference (p=0.0001). The types of tobacco chewing habit in the study group did not show a significant association with p53 immunoreaction.
In the present retrospective study, the follow-up data were retrieved through patient data sheets and contacting the patients or their relatives.
Of the 40 patients, local recurrence was seen in 21 patients. Although statistically insignificant, higher rate of local recurrence was noted in p53 (n=19), TP53 (n=16) and p53+TP53 (n=21) positive cases than p53 (n=2), TP53 (n=5) and p53+TP53 (n=0) negative cases respectively as assessed by logistic regression.
During the follow-up period (9-53 months), a total of 15 out of 40 patients succumbed to mortality. Among these 15 patients, p53 positivity was observed in 13 cases, TP53 positivity in 11 cases, and p53+TP53 positivity in all 15 cases.
The intensity, percentage, and level of p53 immuno-expression in HNMs (Figure 2) did not show any association with local recurrence and survival.
According to TNM staging, 33 cases showed lymph node involvement. All 33 cases showed positivity for both p53 and TP53 expression (n=33) and the association was statistically significant (p<0.05).
Further, the logistic regression analysis showed that the likelihood of LR varied among the various clinical characteristics; age (<50/>50), gender (M/F), smoking (Yes/No), alcohol (Yes/No), and the following groups; p53 Vs. no p53 in HNMs, the intensity of p53 staining i.e. score0-1 Vs. score 2-3, percentage of p53 staining i.e. score0-1 Vs. score 2-3, level of p53 staining i.e. score0-1 Vs. score 2-3, TP53 Vs. no TP53 in HNMs and p53+TP53 Vs. no p53+TP53 in HNMs.
Cox regression analysis for prediction of the status of survival was employed. Although statistically not significant, the habit of smoking (1.06 OR, p=0.8900), alcohol consumption (1.85 OR, p=0.1450), TP 53 positivity (1.66 OR, p=0.2460), and p53+TP 53 positivity (1.40 OR, p=0.6530) were good predictors of the status of survival in OSCC with HNMs.
Kaplan-Meier curves of disease-free survival rates among OSCC patients with HNMs showed the difference in the status of survival between positive and negative groups for p53 (Figure 3A, p=0.0030) TP 53, (Figure 3B, p=0.4230), and any positivity of p53 or TP53 (Figure 3C, p=0.0120). However, the difference was statistically insignificant.
Figure 3: Kaplan-Meier survival analysis.
The combined p53 and TP53 assessment showed the highest sensitivity with good accuracy figures. The Positive Predictive Value (PPV) was almost similar for all three parameters (p53, TP53, and p53+TP53).
Discussion
Mutation of p53 tumor suppressor gene and protein is known to occur in early cancerous development and is observed in more than 50% of OSCC cases [18-20]. Also, its overexpression has been shown to predict prognosis [21]. Were the pioneers to evaluate p53 gene alteration in surgical margins of HNSCC and found an association with recurrence and survival? Recently, it has been shown that assessment of p53 overexpression by IHC (that gives an insight into histology as well as the distribution of the marker within the tissue) along with TP53 gene analysis of HNMs has a promising role to identify recurrence and predict survival [22]. Hence we aimed to evaluate HNMs for expression p53 oncoprotein and TP53 gene amplification by PCR and compared with controls.
In the present study, the maximum number of OSCC patients was above 40 years [6,8,22]. Studies also found similar findings. Literature reports that OSCC commonly occurs in higher age group mostly above 40 years and India, the peak incidence is between 5th to 6th decades of life as cited in the IARC database [23-25]. In the present study, the majority of OSCC cases were noted in males (85%) than females (15%) Similar to studies, cancer is frequently seen in men than women. Heavy indulgence of tobacco and other risk habits by men has been suggested as a possible reason for this gender difference in OSCC [23,24].
In our study, tobacco and areca nut quid habit (82.5%) was most frequently practiced among the OSCC patients than tobacco or areca nut alone. The rampant usage of tobacco in smokeless form; pan containing betel nut along with lime and tobacco leaf is a very commonly practiced method of tobacco consumption in India [25]. The quid is usually kept for a prolonged time in the gingivo-buccal vestibule thus accountable for the high prevalence of gingival-buccal cancer in India.
In the present study, p53 immunostaining showed a statistically significant difference among the cases and controls; 85% of cases and 40% of controls showed positive immunostaining. It is suggested that the wild-type p53 protein is difficult to detect in normal tissue due to its extremely short-lived half-life of 6 to 20 minutes. But, due to mutation/defect in the degradation pathway, the protein may persist in tissues for a longer period, leading to expression in the altered tissue [26]. A similar mechanism may occur in the HNMs generating a more stabilized mutant p53 protein subsequently leading to its overexpression. Previous studies have shown p53 immunostaining ranging from 22% to 96%, in HNMs of OSCC [6,8,18,22]. This variation in p53 expression among the studies may be due to discrepancy in methods employed, criteria of case selection, anatomical sites included. Moreover, lack of antibody detection may be due to;
- Biallelic deletion of TP53 gene
- Mutant or wild-type p53 existing in low quantity
- N-terminal portion showing truncated p53 protein or a nonsense mutation
However, considering the drawback of IHC that include; impairment of antigenic alterations, variation in tissue handling methods, we employed analysis of TP53 gene amplification by PCR to detect the gene alterations [27].
TP53 gene amplification by PCR showed positivity in 67.5% and 35% in study and control groups respectively. Found positivity in 23.9%, 52%, and 66% of HNMs as detected by PCR respectively. By FISH assay for analysis of TP53 gene aberrations, found positivity in 65% of cases. Overall, TP53 gene amplification was noted in quite a sufficient number of HNMs, thus indicating its significant application in HNMs of OSCC [4,18,21,22].
On the whole, 38 cases were either positive for p53 protein or TP53 gene, or both. Among these 38 cases, 23 HNMs showed positivity for both p53 immuno-expression and TP53 gene amplification. Including the present study, only have studied both p53 and TP53 analysis in HNMs [18,22].
Eleven cases showed positivity only for IHC immuno-expression without gene amplification. This indicates the involvement of various additional biologic events like active TP53 gene of wild-type etc. causing genomic instability or genetic stability in such cases may be maintained by wild-type TP 53 gene overexpression. Four cases showed only TP 53 gene amplification (PCR) without expression for p53 immunoprotein. The possible reason suggested is the occurrence of mutated TP53 in a gene locus that results in a lack of p53 protein accumulation [18].
The Lymph node involvement showed a statistically significant association with p53 and TP53 positivity (p<0.05). However, studies by Singh et al and Wang et al. did not such association. This could be due to disparity in the study population, anatomical location of the lesion, type of habit practiced, etc. Moreover, as this study focused primarily on the predictive role of p53 protein status in HNMs with LR and survival, we did not attempt to obtain the histologic status of lymph nodes.
Out of 40 cases, LR was observed in 21 patients. By Logistic regression analysis, although statistically insignificant, patients of >50 years of age and smokers had a higher risk of local recurrence than patients of <50 years and non-smokers respectively. However, did not find any relation between age and smoking. It is a well-known fact that factors like progressive aging and smoking deteriorate the effectiveness of limbs of an immune response, thus paving way for decrease host response resulting in increased LR. Moreover, smoking is a major etiological factor for OSCC. However, gender and alcohol consumption did not show any relation with local recurrence in the present study. This is following study by [22].
Although statistically insignificant, a higher rate of LR was noted in p53 (90.4%), TP53 (76.19%) positive HNMs than negative cases. Results with significance were observed who have employed only p53 immuno-expression found that 42.8% of cases with LR had p53 positive HNMs [8,22].
In HNMs, p53 immunostaining was found to be distributed in basal, supra-basal, para-basal as well as in the entire thickness of the epithelium, whereas in controls it was seen restricted to basal layer only. However, we did not find any significant association between the level of p53 expression and LR in the present study. The study by, found that lesions that expressed p53 beyond basal cell layers developed LR more frequently [28,29].
When logistic regression analysis was employed, it was found that the probability of LR increased among p53, TP53, and p53+TP53 positive HNMs compared to negative HNMs (Odds ratio; 1.27, 1.46, and 1.24 respectively).
Interestingly, we found that all of the cases with LR were either positive for p53 or TP53 or both, indicating that combined p53+TP53 assessment to be more reliable for predicting LR. The combined p53 and TP53 assessment showed the best sensitivity (100%) and NPV (100%) with good accuracy figures. The positive predictive value (PPV) was almost similar for all the three parameters (p53, TP53, and p53 with TP53, i.e. 56%, 59%, and 55% respectively). These findings are similar to the results of a study hence, it is advisable to initially subject the HNMs to p53 by IHC analysis followed by TP 53 gene amplification to assist the surgeons to precisely recognize high-risk patients for LR.
However, even though p53 positivity was seen in a relatively greater number of cases, only a few developed LR. This is in agreement with the results of Singh et al 8 and This could be due to lack of adequate follow-up period and post-surgical radiotherapy and chemotherapy, which may kill residual cancer cells. Role of a variety of factors other than p53 that allow LR should be taken into consideration in this context. Moreover, it is a well-known fact that factors like depth of invasion; local site (i.e. tongue being more likely to exhibit LR than other sites) has a significant role in regulating LR [13].
Concerning the status of survival, cox regression analysis showed that, although statistically not significant, habit of smoking (1.06 OR, p=0.8900), alcohol consumption (1.85 OR, p=0.1450), TP 53 positivity (1.66 OR, p=0.2460) and p53+TP 53 positivity (1.40 OR, p=0.6530) were good predictors of the status of survival in OSCC with HNMs. Similar results were noted, except that they found no association with smoking or alcohol consumption.
Although statistically insignificant, Kaplan-Meier curves of disease-free survival rates among OSCC patients with HNMs showed the difference between positive and negative groups for p53, TP53, and p53+TP53. Although our study results showed that analysis of p53 has a good predictive value for survival rate analysis, a study with a larger sample size and increasing the number of examined margins is essential to reach a consensus.
In near future, a prospective study with diverse patient populations and larger cohorts along with a longer follow-up period will be necessary to unmask the influence of p53 and TP53 on recurrence and overall survival. Owing to the asymmetrical spread of field cancerization, the number of examined margins could be increased which may increase the likelihood of detection of genetic changes.
In the present scenario, the employment of selected and reliable studied markers consisting of a band of biomarkers is needed to establish “Molecular Grading” of surgical margins of OSCC as an adjunct to histopathological grading. Understanding the concept of “Molecular Surgical Margin (MSM)” thus can aid to set up a standardized criterion to define the resected SMs.
Author contribution
Mamata S. Kamat, Rudrayya S. Puranik: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing-original draft; Writing-review and editing. Bhagavan Das Rai A: Data curation; Formal analysis; Investigation; Methodology; Writing review and editing. B.R. Patil: Investigation; Methodology; Supervision; Writing-review and editing. Shankargouda Patil: Formal analysis; Supervision; Project Administration; Writing-original draft; Writing-review and editing.
References
- Angadi PV, Patil PV, Hallikeri K, Mallapur MD, Hallikerimath S, et al. (2015) Tumor budding is an independent prognostic factor for prediction of lymph node metastasis in oral squamous cell carcinoma. Int J Surg Pathol 23:102–110
- More Y, D’Cruz AK (2013) Oral cancer: review of current management strategies. Natl Med J India 26:152–158
- Oliveira LR, Ribeiro-Silva A, Costa JPO, Simões AL, Matteo MAS Di, et al. (2008) Prognostic factors and survival analysis in a sample of oral squamous cell carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:685–695
- Jelovac DB, Tepavcevic Z, Nikolic N, Ilic B, Eljabo N, et al. (2016) The amplification of c-erb-B2 in cancer-free surgical margins is a predictor of poor outcome in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 45:700–705
- van Houten VMM, Leemans CR, Kummer JA, Dijkstra J, Kuik DJ, et al. (2004) Molecular Diagnosis of Surgical Margins and Local Recurrence in Head and Neck Cancer Patients. Clin Cancer Res 10:3614–3620
- Mognetti B, Trione E, Corvetti G, Pomatto E, Di Carlo F, et al. (2005) ?Np63a as early indicator of malignancy in surgical margins of an oral squamous cell carcinoma. Oral Oncol Extra 41:129–131
- Bilde A, von Buchwald C, Dabelsteen E, Therkildsen MH, Dabelsteen S, et al. (2009) Molecular markers in the surgical margin of oral carcinomas. J Oral Pathol Med 38:72–78
- SINGH J, JAYARAJ R, BAXI S, MILEVA M, SKINNER J, et al. (2016) Immunohistochemical expression levels of p53 and eIF4E markers in histologically negative surgical margins, and their association with the clinical outcome of patients with head and neck squamous cell carcinoma. Mol Clin Oncol 4:166–172
- Yang X-H, Ding L, Fu Y, Chen S, Zhang L, et al. (2019) P53-positive expression in dysplastic surgical margins is a predictor of tumor recurrence in patients with early oral squamous cell carcinoma. Cancer Manag Res 11:1465–1472
- De Carvalho AC, Kowalski LP, Campos AHJFM, Soares FA, Carvalho AL, et al. (2012) Clinical significance of molecular alterations in histologically negative surgical margins of head and neck cancer patients. Oral Oncol 48:240–248
- Li MM, Puram SV, Silverman DA, Old MO, Rocco JW, et al. (2019) Margin Analysis in Head and Neck Cancer: State of the Art and Future Directions. Ann Surg Oncol 26:4070–4080
- Shah AK (2018) Postoperative pathologic assessment of surgical margins in oral cancer: A contemporary review. J Oral Maxillofac Pathol 22:78–85
- Payne K (2017) Factors Influencing the Status of the Surgical Margin in the Resection of Oral Squamous Cell Carcinoma. Biomed J Sci Tech Res 1:1835–1838
- Clark DJ, Mao L (2017) Understanding the Surgical Margin: A Molecular Assessment. Oral Maxillofac Surg Clin North Am 29:245–258
- Adams EJ, Green JA, Clark AH, Youngson JH (1999) Comparison of different scoring systems for immunohistochemical staining. J Clin Pathol 52:75–77
- Stoicanescu D, Andreescu N, Belengeanu A, Meszaros N, Cornianu M, et al. (2013) Assessment of p53 and HER-2/neu genes status and protein products in oral squamous cell carcinomas. Rom J Morphol Embryo 54:1107–1113
- Elizabeth van Pelt-Verkuil, Alex van Belkum, John P Hays (2008) Principles and Technical Aspects of PCR Amplifi cation. Springer, Netherlands.
[Crossref]
- Heah KG, Hassan MIA, Huat SC (2011) p53 Expression as a marker of microinvasion in oral squamous cell carcinoma. Asian Pac J Cancer Prev 12:1017–1022
- Gasco M, Crook T (2003) The p53 network in head and neck cancer. Oral Oncol 39:222–231
- Nylander K, Dabelsteen E, Hall PA (2000) The p53 molecule and its prognostic role in squamous cell carcinomas of the head and neck. J Oral Pathol Med 29:413–425
- Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, et al. (1995) Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med 332:429–435
- Wang X, Chen S, Chen X, Zhang C, Liang X, et al. (2016) Tumor-related markers in histologically normal margins correlate with locally recurrent oral squamous cell carcinoma: a retrospective study. J Oral Pathol Med 45:83–88
- Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45:309–316
- Slootweg PJE (2005) Tumors of oral cavity and oropharynx: Introduction. In: Leon Barnes John W. Eveson Peter Reichart David Sidransky, editor Pathol Genet Head Neck Tumours. (2005th ed) IARCPress International Agency for Research on Cancer (IARC) 69008 Lyon, France.
- Prabhu SR (1992) Oral diseases in the tropics. Oxford; New York: Oxford University Press. Oxford University Press, New York.
- Chandra A, Singh A, Sebastian B, Bali R, Verma P, et al. (2013) Oral squamous cell carcinomas in age distinct population: A comparison of p53 immunoexpression. J Cancer Res Ther 9:587-591
- Sá CT, Fonseca LMS, Cardoso SV, Aguiar MCF, Carmo MAV do, et al. (2006) p53 Immunoexpression in Oral Squamous Cell Carcinomas from Different Anatomical Sites: A Comparative Study. Int J Morphol 24:231-238
- Cruz IB, Meijer CJ, Snijders PJ, Snow GB, Walboomers JM, et al. (2000) p53 immunoexpression in non-malignant oral mucosa adjacent to oral squamous cell carcinoma: potential consequences for clinical management. J Pathol 191:132–137
- Cruz I, Napier SS, van der Waal I, Snijders PJF, Walboomers JMM, et al. (2002) Suprabasal p53 immunoexpression is strongly associated with high grade dysplasia and risk for malignant transformation in potentially malignant oral lesions from Northern Ireland. J Clin Pathol 55:98–104
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences