ISSN : ISSN: 2576-1412
Journal of Applied Microbiology and Biochemistry
Antioxidant and Antiplasmodial Activity of Newbouldia laevis, Cnestis ferruginea and Dialium dinklagei, Three Traditional Plants used for Malaria Treatment in Côte d’Ivoire
Karim Tuo1*, Sylvain Beourou1, Kigbafori Dieudonné Silue2,3, Karamoko Ouattara4, Allico Joseph Djaman4,5, Adama Coulibaly4 and Offianan Andre Toure1
1Department of Parasitology and Mycology, Institut Pasteur of Côte d'Ivoire, 01 BP 490 Abidjan 01, Côte d'Ivoire
2Ex-vivo Chemosensitiivity Platform, Centre Suisse de Recherches Scientifiques en Côte d'Ivoire, 01 BP 1303 Abidjan 01, Côte d'Ivoire
3Laboratory of Biology and Health, Félix Houphouët-Boigny University, 01 BP V 34 Abidjan 01, Côte d'Ivoire
4Laboratory of Pharmacodynamics-Biochemistry, Félix Houphouët-Boigny University, 01 BP V 34 Abidjan 01, Côte d'Ivoire
5Department of Clinical and Basic Biochemistry, Institut Pasteur of Côte d'Ivoire, 01 BP 490 Abidjan 01, Côte d'Ivoire
- *Corresponding Author:
- Tuo Karim
Department of Parasitology and Mycology,
Institute Pasteur of Côte d’Ivoire,
Côte d'Ivoire
Tel: (00225) 0708355392;
Email: karimtuo@pasteur.ci
Received date: August 06, 2022, Manuscript No. IPJAMB-22-14293; Editor assigned date: August 08, 2022, Pre QC No. IPJAMB-22-14293 (PQ); Reviewed date: August 19, 2022, QC No. IPJAMB-22-14293; Revised date: August 29, 2022, Manuscript No. IPJAMB-22-14293 (R); Published Date: October 12, 2022, DOI: 10.36648/2576-1412.6.9.75
Citation: TUO K, Beourou S, Silué KD, Ouattara K, Djaman, et al. (2022) Antioxidant and antiplasmodial activity of Newbouldia laevis, Cnestis ferruginea and Dialium dinklagei, three traditional plants used for malaria treatment in Côte d’Ivoire. J Appl Microbiol Biochem Vol.6 No.9
Abstract
Background: Resistance of human malaria parasites to antimalarial compounds remains a crucial public health concern. In addition, the oxidative stress induced during malaria infection would seem to result from an imbalance between this decrease in antioxidant enzymes and the increased production of Reactive Oxygen Species (ROS). Treatment with antioxidants would aim to strengthen the antioxidant systems already present and protect the host. Investigating plants used in traditional medicine to treat malaria remains a credible option for new anti-malarial drug development. The study aims to identify local natural compounds with antimalarial and antioxidant activity with a strong potential for the development of new treatments against malaria.
Materials and methods: SYBR GREEN fluorescence method was used to evaluate the In vitro inhibitory activity of the extracts, chloroquine, artesunate and quinine against Plasmodium falciparum field isolates and two laboratory references strains of Plasmodium falciparum (3D7 and Dd2). The haemolytic activity of extracts showing good antiplasmodial activity was also evaluated. The IC50 and the corresponding correlation coefficients were determined graphically, using In Vitro Analysis and Reporting Tool (IVART) software of WWARN.
Results: Our findings indicate that only decoction crude extracts of Dialium dinklagei and Cnestis ferruginea and methanolic crude extract of Newbouldia laevis had a promising antiplasmodial activity. The liquid-liquid partition improved the antiplasmodial activity to 1.22 ± 0.37 μg/mL, 6.11 ± 1.3 μg/mL and 4.37 ± 0.77 μg/mL respectively for the F3 extract of Dialium dinklagei, F3 of Newbouldia laevis and F2 of Cnestis ferruginea.
The DPPH quantitative test showed good antioxidant activity of the plant extracts with dose dependent DPPH radical scavenging activity. The antioxidant test carried out on TLC plates gave yellow spots on a purple background indicating the anti-radical activity of the fractions. There was less than 1% hemolysis at the concentration of 200 μg/mL of plant extracts.
Conclusion: This work provides an opportunity to find molecules that can neutralize both the parasite and the oxidative stress induced by the disease.
Keywords
Plasmodium falciparum; Haemolytic; In vitro; Antioxidant; Antiplasmodial; Côte d’Ivoire
Introduction
Malaria is an infectious disease which is caused by the protozoan Plasmodium parasite and responsible for about 229 million clinical cases, killing 409,000 people each year over the world [1]. The extremely rapid development of resistance to antimalarial drugs such as chloroquine, mefloquine, pyrimethamine, the proguanil-atovaquone combination, and the most recent artemisinin derivatives requires the prompt identification of new compounds [2,3]. Host defense against infection is controlled by an innate and adaptive immune system. Overall, immune responses to Plasmodium infections consist of parasite destruction, but these responses are also very often exacerbated, leading to host damage [4]. During Plasmodium infections in humans as well as in experimental models (primates, mice), a strong oxidative stress is generated, due in part to the metabolism of iron-rich components [5-7]. One of the effects of malaria is a decrease in antioxidant enzymes such as catalase, Glutathione peroxidase (GSH), Superoxide Dismutase (SOD) and many others as well as an increase in the production of Reactive Oxygen Species (ROS) [8-11].
The serum concentration of malondialdehyde, one of the main markers of oxidative stress is higher in malaria subjects [12]. Thus, the oxidative stress induced during malaria infection would seem to result from an imbalance between this decrease in antioxidant enzymes and the increased production of ROS. Treatment with antioxidants would aim to strengthen the antioxidant systems already present and protect the host. Indeed, it has been suggested that the administration of Vitamin E or trolox would partially protect against cerebral malaria [12,13]. The use of traditional medicinal plants may be an interesting therapy to explore to treat malaria and fight against oxidative stress. Therefore, we are interested in Newbouldia laevis, Cnestis ferruginea and Dialium dinklagei which are plants traditionally used in Cote d'Ivoire against malaria.
Cnestis ferruginea Vahl ex DC is a perennial shrub or tree belonging to the Conaraceae family. Cnestis ferruginea has a wide distribution in West Africa, particularly in Gambia, Ghana, Guinea-Bissau, Côte d’Ivoire, Liberia, Nigeria, Sierra Leone, Benin, Niger and Gabon, especially in semi-deciduous forests [14,15]. According to Bouquet and Debray, the leaves are very active vermifuges against ascaris. These leaves are also used to treat scabies, asthenia, and would have purgative properties [16]. Several works have proved the anti-inflammatory and analgesic activities and the anti-depressive and anxiolytic activities of Cnestis ferruginea [17-19].
Dialium dinklagei belongs to the Fabaceae family. This plant is found in forest areas from Guinea to Congo. It is used in Côte d'Ivoire to treat malaria. Very little work has been done on this plant, but we noted that Bouquet and Debray have described the presence of tannins in the leaves of this plant [16]. Newbouldia laevis is native to tropical Africa and thrives on moist, well-drained soils. In Nigeria, the bark is chewed and swallowed for stomach pain, diarrhea and toothache [20]. This plant is known to be effective in the treatment of elephantiasis, dysentery, rheumatic swelling, syphilis, constipation and as a dewormer. It is also used for earaches, sore feet, chest pain, epilepsy and convulsions of children [21]. The leaf, stem and fruit are used as febrifuge and for the treatment of wounds and stomach ache [22]. The roots of Newbouldia laevis are used in Benin for the treatment of Buruli ulcer [23].
The main objective of this work is to identifiy natural compounds with antimalarial and antioxidant activity with a strong potential for the development of new treatments against malaria.
Materials and Methods
Plant material
The plant material consists of the leaves of Dialium dinklagei, Newbouldia laevis and Cnestis ferruginea. These three plants have not been investigated for their antiplasmodial activity. The leaves of Dialium dinklagei, Newbouldia laevis et Cnestis ferruginea were harvested in march 2013. Ethnobotanical data (local name, method of preparation, traditional use and combination of plants, indications, dosage, contraindications and side effects) are obtained through semi-structured interviews with traditional healers. The timing of the plant harvest was the morning at 9 AM. Samples collected were identified at Centre National de Floristique (CNF) or National Floristry Center of Felix Houphouët-Boigny University, Abidjan, (Côte d’Ivoire) by Professor Ake-Assi Laurent.
Biological material
The Biological samples are constituted of group O blood samples with a positive rhesus for an inoculum dilution with clinical and reference strains of Plasmodium falciparum.
Two ATCC reference strains were used: 3D7 chloroquino-sensitive was provided by Biochemistry and Molecular Department University of Legon, Ghana and Dd2 chloroquino-resistant provided by, MR4 ATCC®Manassas, Virginia, USA.
Methods
Extracts preparation
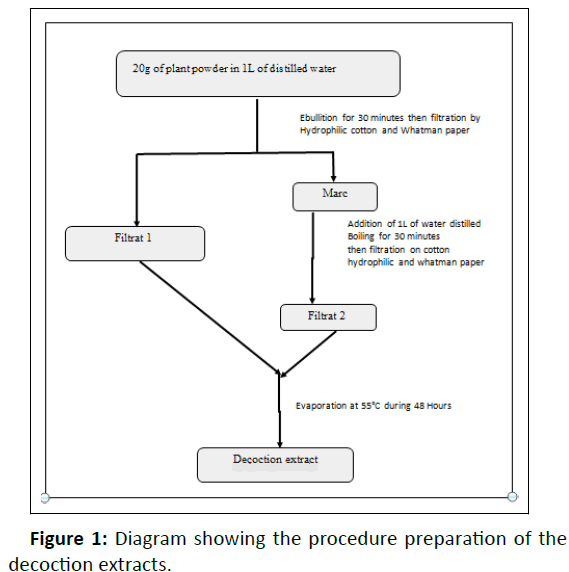
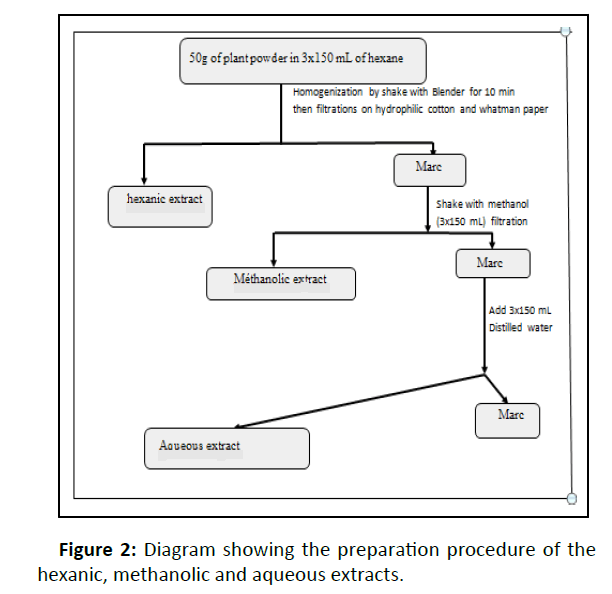
The leaves were dried out of the sun for one week at room temperature before being reduced to fine powder using a mechanical grinder (Retsch M6951). From the powder obtained, the various crude extracts were prepared. Decoction of each plant was made as close as possible to the traditional healer‘s preparation. Then, three successive extractions by solvents of increasing polarity (hexan, methanol and water), have been done according to the protocols made (Figures 1, 2) [24,25].
In order to improve antiplasmodial activity, crude extracts were separated by partition chromatography using solvents of increasing polarity (diethyl ether, butanol and ethyl acetate) (Figure 3) [26-28].
Antioxidant activity
Free radical scavenging activity-quantitative test: The evaluation of the anti-oxidant activity of the plant extracts was performed using the 2.2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging assay according to the method of Parejo et al. [29]. Hydrogen atom or electron donating abilities of the compounds were measured from the bleaching of the purple-coloured methanol solution of 2,2-Diphenyl-1-Picryl Hydrazyl (DPPH). Different concentrations of each extract were added, at an equal volume, to methanolic solution of DPPH (100 μM). After 30 min at room temperature, the absorbance was recorded at 517 nm. Test was repeated for three times. Vitamin C was used as standard control. The DPPH radical scavenging effect was calculated as inhibition of percentage (1%) using the following formula: I A blank is the absorbance of the control reaction (containing all reagents except the test compound) and a sample is the absorbance of the test compound. The values of inhibition were calculated for various concentrations of the extract. IC50 values denote the concentration of sample, which is required to scavenge 50% of DPPH free radicals.
Free radical scavenging activity-qualitative test: A Thin Layer Chromatography (TLC) plate was used to detect the antioxidant activity of extracts. This test is based on the principle of the reduction of a stable radical, 1, 1-Diphenyl-2-Picryl-Hydrazyl (DPPH) which presents a specific absorption at 517 nm which gives it a purple color. When DPPH is reduced by a radical scavenger, its color disappears to give yellow spots on a purple background [30,31]. The plates used are aluminum coated with silica (Silicagel 60 F254, Merck). On these silicagel plates, 5 μL of a 5 mg/mL concentration solution of extract was spotted. The plates were developed in vertical chambers pre-saturated for 15 min with the optimized mobile phase- butanol: acetic acid: water (60:15:25, v/v/v). The plates were dried in a hood for 30 min before derivatization. TLC plates were immersed for 5 s in freshly prepared 0.1% (w/v) methanolic DPPH solution. After removing DPPH excess, plates were kept in the dark for 30 min. Antioxidant activities of separation zones were observed almost immediately after spraying as yellow spots on a purple background.
Antiplasmodial assay
Field isolates collection: Plasmodium falciparum isolates were collected from i patients with uncomplicated malaria and sent to the laboratory. Blood samples collection were done at the health center of Wassakara by venipuncture in heparinized tubes from patients older than 18 years and infected with P. falciparum malaria after informed consent was obtained. Samples were collected then transferred at 4°C to the Ex-vivo chemosensitivity platform established in 2013 at Centre Suisse de Recherches Scientifiques en Côte d’Ivoire with the financial support of Medicines for Malaria Venture (MMV) for In vitro assays.
In vitro antiplasmodial assay for crude extracts and fractions: A SYBR Green I-based In vitro IC 50 drug sensitivity assay, described earlier, was used to test each P. falciparum field isolate and reference strain. All assays were carried out on 96-well plates filled with an infected red blood cells in the following proportions of parasitaemia<0.3% and hematocrit 5%. The In vitro P. falciparum continuous culture used for assays is derived from that developed by Trager et al. Jensen [32]. Inhibition of parasite growth was measured using the SYBR Green method [33-35]
The reading was done with the Spectra Max GEMINI XPS spectrofluorometer (Molecular Devices) at 535 nm after excitation at 485 nm. The IC50 are determined graphically, using In Vitro Analysis and Reporting Tool (IVART) software developed by WWARN [34,36]. Based on WHO guidelines and previous data [37] antiplasmodial activity was classified as follows: high (IC50<5 µg/mL), promising (5
In vitro hemolysis assay
A stock solution of samples was prepared in an appropriate solvent at concentrations of 100 μg/mL and 50 μg/mL, taking into account that the solvent volume must not be greater than 1% in the final solution.
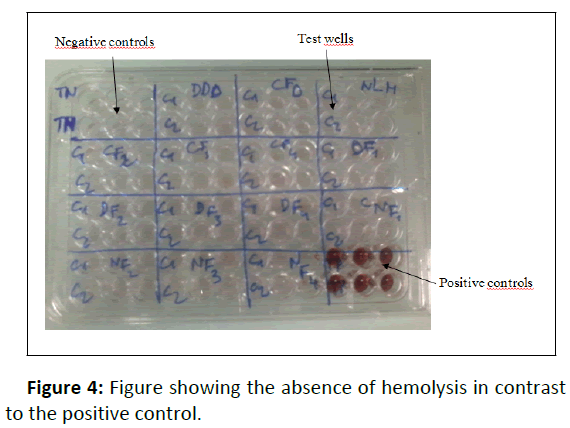
To perform hemolysis assay, 10 μL of stock solution was placed in an Eppendorff microtube and mixed with 190 μL of RBC (10%) as control. The negative control comprised 10 μL of PBS+190 μL of 10% RBC and the positive control was prepared with 10 μL of 20% Triton X-100+190 μL of 10% RBC. Tubes were centrifuged for 5 minutes at 2200 rpm and 150 μL of supernatants were placed in a 96-well plate. The absorbance was read at 550 nm with a plate reader (Multiskan FC, Thermo Scientific) (Figure 4).
The following formula was used to calculate the percentage of hemolysis:

Abs= absorbance at 550 nm
TN: Negatif controls;
TP: Positif controls;
D: Dialium dinklagei ;
NL: Newbouldia laevis ;
CF: Cnestis ferruginea
Statistical analyses
Results are expressed as mean ± SEM of three determinants. Comparisons among the groups were tested by two-way ANOVA using Graph Pad Prism, Version 5.0 (Graph Pad Software, San Diego, CA, USA). Differences with P values<0.05 were considered as statistically significant.
Ethical issues
The study was conducted in accordance with the local laws and regulations, and International Conference on Harmonization-Good Clinical Practice (ICH-GCP). The protocol was reviewed and approved by the National Ethical Committee for Research (Reference: 038/MSLS/CNER-dkn). Written informed consent was obtained from participants for blood collection and from traditional healers. In case of an illiterate participant, his/her thumb impression and signature of an independent witness were sought .
Results
Antioxidant activity
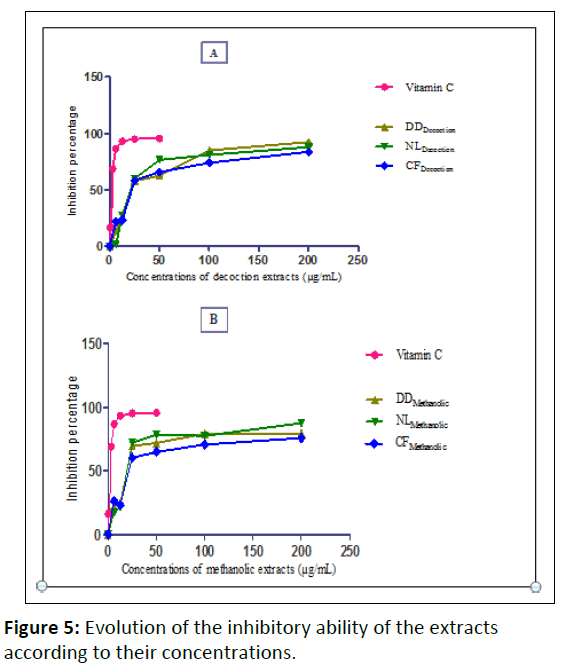
Free radical scavenging activity-quantitative test: Only decoction and methanolic extracts were tested. The antioxidant activity exerted on the DPPH free radical by the crude extracts is dose dependent in all extracts demonstrating that all three plants exhibit antioxidant activity (Figure 5).
DDDecoction: Decoction of Dialium dinklagei
NLDecoction: Decoction of Newbouldia laevis
CFDecoction: Decoction of Cnestis ferruginea
DDMethanolic: Methanolic extract of Dialium dinklagei
NLMethanolic: Methanolic extract of Newbouldia laevis
CFMethanolic: Methanolic extract of Cnestis ferruginea
A: Percentage of inhibition of decoction extracts
B: Percentage of inhibition of methanolic extracts
The results are expressed as IC50 and are shown in Table 1. The extract with the lowest IC50 value has the highest antiradical activity. According to Table 1, all extracts have antioxidant activity, which varies from one extract to another and from one plant to another (Table 1).
| Decoction | Methanolic extracts | |
|---|---|---|
| Dialium dinklagei | 21.85 ± 0.15 | 0.30 ± 0.30 |
| Newbouldia laevis | 19.50 ± 0.50 | 19.85 ± 0.15 |
| Cnestis ferruginea | 21.55 ± 0.15 | 20.65 ± 0.15 |
| Vitamine C (Standard) |
3.71 ± 0.38 | |
Table 1: Inhibitory ability (IC50) of extracts (μg/mL) of methanolic extracts and decoctions.
Differences between the extracts and standard molecule (Vitamin C) and between extracts themselves are statistically significant (P<0.05). Newbouldia laevis appears as the most active plant on the DPPH radical with an IC50 of 19 µg/mL with its methanolic and decoction extracts. As for the reference molecule (Vitamin C), it has an IC50 of 3.71 µg/mL
Free radical scavenging activity-qualitative test: Extracts migrated on a silica gel plate with butanol-acetic acid-water (60/15/25) solvent system were revealed with DPPH. Only fractions with good antiplasmodial activity were tested.
All the crude extracts and fractions from the partitions that we tested were active with DPPH giving a yellow spot on purple background (Figure 6).
DDF3: Partition F3 (acetatic) of Dialium dinklagei
NLF3: Partition F3 (acetatic) of Newbouldia laevis
CFF2: Partition F2 (butanolic) of Cnestis ferruginea
AG: Gallic acid
Antiplasmodial activity
Plasmodium falciparum isolates and reference strain 3D7 and Dd2 were tested first on the crude extracts. Table 2 shows the IC50 values of the crude extracts after data analysis. Among the 12 extracts from the 3 plant species, 6 are without antiplasmodial activity, 6 show antiplasmodial activities on the erythrocytic cycle of Plasmodium falciparum. Among these 6 extracts, 4 have promising antimalarial activity (IC50<15 μg/mL).
The four ï¬eld isolates tested were CQ sensitive. Quinine and Artesunate whowed good activity against field isolates (Table 2).
| Strains /CI50 (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical isolates | Reference strain | |||||||
| Plants | Extracts | Extraction yield (%) | W536 | W539 | W552 | ANK02 | 3D7 | Dd2 |
| Dialium dinklagei | Dec | 18.5 | 13.19 ± 3.17 | 13.74 ± 4.12 | 12.8 ± 2.4 | 12.9 ± 3.01 | 14.35 ± 0.79 | 13.82 ± 2.7 |
| Hex | 1 | >50 | >50 | >50 | >50 | >50 | >50 | |
| Met | 4.5 | 17.66 ± 2.65 | 15.76 ± 5.63 | 19.15 ± 3.27 | 15.29 ± 3.76 | 14.97 ± 2.59 | 15.11 ± 2.26 | |
| Aq | 4 | 18.34 ± 3.01 | 15.43 ± 2.24 | 17.33 ± 4.21 | 21.67 ± 3.29 | 19.54 ± 1.58 | 17.47 ± 1.26 | |
| Newbouldia laevis | Dec | 10.9 | >50 | >50 | >50 | >50 | >50 | >50 |
| Hex | 1.2 | >50 | >50 | >50 | >50 | >50 | >50 | |
| Met | 4.4 | 11.51 ± 0.43 | 11.45 ± 0.36 | 12.85 ± 0.74 | 10.21 ± 3.22 | 13.35 ± 1.46 | 10.79 ± 2.15 | |
| Aq | 5 | >50 | >50 | >50 | >50 | >50 | >50 | |
| Cnestis ferruginea | Dec | 17.95 | 12.85 ± 2.29 | 13.1 ± 3.19 | 11.96 ± 1.62 | 13.94 ± 3.23 | 11.78 ± 2.21 | 11.85 ± 1.43 |
| Hex | 1 | >50 | >50 | >50 | >50 | >50 | >50 | |
| Met | 5.4 | >50 | >50 | >50 | >50 | >50 | >50 | |
| Aq | 5.8 | 13.68 ± 2.76 | 13.74 ± 2.72 | 12. 35 ± 1.44 | 13.56 ± 3.1 | 12.15 ± 1.91 | 12.35 ± 4.21 | |
| Chloroquine (nM) | 33.01 ± 0.92 (0.01 µg/mL) |
42.71±1.32 (0.013 µg/mL) |
37.31 ± 3.24 (0.011 µg/mL) |
35.38 ± 4.92 (0.011 µg/mL) |
51.07 ± 2.23 (0.016 µg/mL) |
116.71 ± 5.11 (0.037 µg/mL) |
||
| Quinine (nM) | 5.76 ± 0.95 (0.0019 µg/mL) |
23.87 ± 1.12 (0.008 µg/mL) |
44.37 ± 2.15 (0.015 µg/mL) |
|||||
| Artesunate (nM) | 3.43 ± 0.49 (0.0013 µg/mL) |
2.22 ± 0.16 (0.0008 µg/mL) |
6.31 ± 3.01 (0.0024 µg/mL) |
|||||
Dec: Decoction; Hex: Hexanic; Met: Methanolic; Aq: Aqueous
Table 2: Antiplasmodial activity of crude extracts.
Crude extracts with promising activity (IC50<15 μg/mL) were subject to liquid-liquid partitions to improve their activity. The highest selective antiplasmodial activity was found with Newbouldia laevis acetate fraction. Methanolic and aqueous fractions also had a good activity on field isolates and Dd2 strains.
Table 3 shows the IC50 values of the extracts obtained after partition. Liquid-liquid partitioning significantly improved the antiplasmodial activity obtained with the crude extracts from around 10 µg/mL to about 1 µg/mL. Extracts F3 of Dialium dinklagei, F3 of Newbouldia laevis, and F2 of Cnestis ferruginea gave the best results 1.22 ± 0.37 µg/mL, 6.11 ± 1.3 µg/mL, and 4.37 ± 0.77 µg/mL, respectively (Table 3).
| Clinical isolates | Reference strain | ||||
|---|---|---|---|---|---|
| Plants | Extracts | Yield (%) | W639 | A149 | Dd2 |
| Dialium dinklagei | F1 Decoction | 12.66 | 6 .05 ± 0.88 | 14.63 ± 4.5 | 17.23 ± 2.3 |
| F2 Decoction | 33.2 | 12.47 ± 1.06 | 11.68 ± 2.39 | 12.61 ± 1.39 | |
| F3 Decoction | 27 | 1.47 ± 0.46 | 4.56 ± 1.97 | 1.22 ± 0.37 | |
| F4 Decoction | 24.33 | 5.71 ± 0.79 | 11.88 ± 1.06 | 10.38 ± 3.06 | |
| Newbouldia laevis | F1 Methonolic | 52 | 9.63 ± 2.21 | 9.39 ± 2.83 | 13.29 ± 2.71 |
| F2 Methonolic | 16.5 | 9.26 ± 1.74 | 22.5 ± 1 | 19.36 ± 3.05 | |
| F3 Methonolic | 5.2 | 6.11 ± 1.3 | 12.62 ± 1 | 6.32 ± 1.7 | |
| F4 Methonolic | 23.8 | 8 ± 0.9 | 7.32 ± 1.18 | 17.51 ± 2.31 | |
| Cnestis ferruginea | F1 Decoction | 2.66 | Not tested | ||
| F2 Decoction | 30 | 5.51 ± 0.94 | 7.07 ± 1.11 | 4.37 ± 0.77 | |
| F3 Decoction | 30 .33 | 5.76 ± 1.06 | 7.41 ± 1.21 | 8.35 ± 2.12 | |
| F4 Decoction | 36.9 | 5.83 ± 2.67 | 6.76 ± 1.13 | 6.62 ± 2.02 | |
| Chloroquine | 26.49 ± 2.33 nM (0.008 µg/mL) |
52.49 ± 3.82 nM (0.016 µg/mL) |
129 ± 7.32 nM (0.041 µg/mL) |
||
| Artesunate | 1.59 ± 0.46 nM (0.0006 µg/mL) |
3.16 ± 0.38 nM (0.0012 µg/mL) |
1.36 ± 0.56 nM (0.0005 µg/mL) |
||
F1: Ether fraction; F2: Butanol fraction; F3: Acetatic fraction; F4: Aqueous fraction
Table 3: Antiplasmodial activity of Dialium dinklagei, Newbouldia laevis and Cnestis ferruginea chromatography fractions.
Hemolytic activity
No fraction extract was found to exhibit significant red blood cells lysis activity with a percentage of hemolysis <1% for all tested extracts (conc=100 µg/mL and 200 µg/mL). This indicates that anti-plasmodial activity is not correlated with hemolysis of red blood cells but with a real action against the parasite (Table 4).
| Plants | Extract | Concentration (µg/mL) | Hemolytic activity (%) |
|---|---|---|---|
| Dialium dinklagei | DD Decoction | 200 | 1.36 |
| 100 | 0.90 | ||
| F1 Decoction | 200 | 1.07 | |
| 100 | 0.80 | ||
| F2 Decoction | 200 | 0.85 | |
| 100 | 0.79 | ||
| F3 Decoction | 200 | 0.96 | |
| 100 | 0.92 | ||
| F4 Decoction | 200 | 0.61 | |
| 100 | 0.57 | ||
| Newbouldia laevis | NLMethanolic | 200 | 1.04 |
| 100 | 0.97 | ||
| F1 Methanolic | 200 | 0.85 | |
| 100 | 0.70 | ||
| F2 Methanolic | 200 | 1.09 | |
| 100 | 0.77 | ||
| F3 Methanolic | 200 | 1.04 | |
| 100 | 0.95 | ||
| F4 Methanolic | 200 | 0.82 | |
| 100 | 0.63 | ||
| Cnestis ferruginea | CF Decoction | 200 | 0.97 |
| 100 | 0.68 | ||
| F2 Decoction | 200 | 1.05 | |
| 100 | 0.39 | ||
| F3 Decoction | 200 | 0.65 | |
| 100 | 0.46 | ||
| F4 Decoction | 200 | 0.79 | |
| 100 | 0.65 | ||
| Negative control (PBS) | - | - | 0% |
| Positive control (Triton X-100 20%) | - | - | 100% |
Table 4: Haemolytic activity of Dialium dinklagei, Newbouldia laevis and Cnestis ferruginea.
Discussion
The increasing prevalence of malaria exhibiting resistance of Plasmodium falciparum to standard treatments has led to the identification of new antimalarial compounds [38]. Screening of plants used in traditional medicine for malaria treatment is one way to discover promising drugs/compounds [39,40]. They are inexpensive and easily available, particularly if people grow them themselves. In vitro inhibitory activity of hexanic, methanolic and aqueous extracts of Dialium dinklagei. Newbouldia laevis and Cnestis ferruginea leaves on chloroquine sensitive and chloroquine resistant laboratory strains and field isolates P. falciparum were tested. For the purpose of this study, an IC50 value of ≤ 10g/mL was classified as promising activity, and ≤ 5g/mL was considered to be highly active. When IC50>50 μg/mL, the extract is classified as inactive and rejected [37].
Our findings indicated that only decoction crude extracts of Dialium dinklagei and Cnestis ferruginea and methanolic crude extract of Newbouldia laevis had a promising antiplasmodial activity on Dd2 and 3D7 laboratory strains. So these extracts have been fractionated.
The liquid-liquid partition improved the antiplasmodial activity to 1.22 ± 0.37 µg/mL, 6.11 ± 1.3 µg/mL and 4.37 ± 0.77 µg/mL respectively for the F3 extract of Dialium dinklagei, F3 of Newbouldia laevis and F2 of Cnestis ferruginea. The activity of the F3 extract of Dialium dinklagei is higher than that obtained with the ethanolic extract of Artemisia annua (IC50=3.9 μg/mL), the plant from which artemisinin is isolated (reference molecule used against malaria nowadays) [41-43]. This work is the first report of antiplasmodial activity of Cnestis ferruginea and Dialium dinklagei. The observed activity could be due to the richness of these plants in secondary metabolites [44,45]. Antiplasmodial activity of Newbouldia laevis was confirmed by previous studies [46-48]. The antiplasmodial activity of Newbouldia laevis leaves could be due to his chemical compounds. In a previous study, we demonstrated that the leaves of Newbouldia laevis contain plenty of saponins and alkaloids, moderate flavonoids, polyphenols, anthocyanins, tannins, and in low quantity sterols, polyterpenes, coumarins, quinones and leucoanthocyanins [44].
In this study, Chloroquine was active against field’s isolates and Dd2 strains. Values of IC50 obtained were lower compared to those found in previous studies. In Côte d’Ivoire chloroquine has been officially withdrawn from malaria treatment guidelines since 2003. It seemed that this molecule became currently active on falciparum isolates. However, this reversion of chloroquine resistance should be confirmed by future In vitro and In vivo and molecular studies. Haemolytic activity represents a useful starting point as it provides the primary information on the interaction between molecules and biological entities at cellular level. The results of haemolytic activity indicated that extracts tested have no or low hemolytic activity. This indicates that anti-plasmodial activity was not due to hemolysis of red blood cells but with a real effect of the extracts against the parasite. Therefore, we can conclude that the results obtained during the antiplasmodial activity are not influenced by this weak haemolytic action [48]. The DPPH quantitative test showed good antioxidant activity of the plant extracts probably due to abundance of total polyphenol [44,45]. These Ivorian plants could therefore be considered as sources of natural antioxidants for medicinal purposes. The extracts showed dose-dependent DPPH radical scavenging activity.
The antioxidant test carried out on TLC plates gave yellow spots on a purple background indicating the anti-radical activity of the fractions. All extracts submitted to this test reacted positively. The antioxidant activity of these extracts could be explained by the presence of polyphenolic substances: phenols, flavonoids and tannins. The antioxidant activity of extracts could be an important contribution to successful of malaria treatment. Indeed, neutrophils activated by parasites induce a destruction of endothelial cells which can be prevented by an addition of antioxidants and proteolytic enzyme inhibitors In vitro [49]. Malaria parasites would cause oxidative stress in the patients by massive lysis of red blood cells. Iyawe et al showed that the combination of chloroquine and ascorbic acid significantly reduced the oxidative stress caused by P. berghei in mice compared to chloroquine alone. Antioxidants could attenuate endothelial cell damage due to parasite-induced leukocyte activation [50]
This work provides an opportunity to find molecules that can neutralize both the parasite and the oxidative stress induced by the disease.
Conclusion
Overall, the In vitro activities of these three Ivorian plant extracts are compatible with their use as traditional remedies for malaria. This work could be a starting point for the development of traditionally improved drugs in the treatment of malaria, after deepening aspects of this study and conducting clinical trials. We are currently attempting to isolate compound with antiplasmodial activity from the most promising of these plants, prior to testing in animal models of malaria. In addition, the haemolytic activity tests of the extracts did not reveal hemolytic activity which could interfere with the antimalarial activity.
Acknowledgements
We thank the traditional practitioners who collaborated with us by sharing information on traditional plants. Following institutions is gratefully acknowledged for their assistance during the study: Institut Pasteur of Côte d’Ivoire, Centre Suisse de Recherches Scientifiques en Côte d'Ivoire (CSRS) and National Floristic Center (CNF) at University of Felix Houphouët-Boigny, Abidjan (Côte d’Ivoire), the Department of Biochemistry and Molecular at University of Legon, Ghana and MR4 ATCC®Manassas, Virginia, USA.
Funding
The funding was provided by Institut Pasteur of Côte d’Ivoire Dr. Kigbafori Dieudonné SILUE is grateful to Dr. Xavier Ding for his contribution to the Ex-vivo Chemosensitivity Platform establishment and technology transfer at Centre Suisse de Recherches Scientifiques en Côte d'Ivoire (CSRS), through the financial support provided by Medicines for Malaria Venture (MMV), Geneva, Switzerland (Project n° MMV 11/0049)
Compliance with Ethical Standards
Conflict of interest: The authors declare no conflict of interest.
References
- WHO. World malaria report (2020) Geneva, Switzerland.
- Pradines B, Dormoi J, Briolant S, Bogreau H, Rogier C (2010) La résistance aux antipaludiques. Revue Francophone des Laboratoires 2010:51–62.
- Jambou R, Legrand E, Niang M, Khim N, Lim P, et al. (2005) Resistance of Plasmodium falciparum field isolates to In vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 366:1960-1963.
[Crossref] [Google Scholar] [Indexed]
- Lamb T, Brown D, Potocnik A, Langhorne J (2006) Insights into the immunopathogenesis of malaria using mouse models. Expert Rev Mol Med 28:1–22.
[Crossref] [Google Scholar] [Indexed]
- Schwarzer E, Kühn H, Valente E, Arese P (2003) Malaria-parasitized erythrocytes and hemozoin nonenzymatically generate large amounts of hydroxy fatty acids that inhibit monocyte functions. Blood 101:722-728.
[Crossref] [Google Scholar] [Indexed]
- Ackerman HC, Beaudry SD, Fairhurst RM (2009) Antioxidant therapy: reducing malaria severity? Crit Care Med 37:758.
[Crossref] [Google Scholar] [Indexed]
- Becker K, Tilley L, Vennerstrom J, Roberts D, Rogerson S, et al. (2004) Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int J Parasitol 34:163–189.
[Crossref] [Google Scholar] [Indexed]
- Delmas-Beauvieux M, Peuchant E, Dumon M, Receveur M, Le Bras M, et al. (1995) Relationship between red blood cell antioxidant enzymatic system status and lipoperoxidation during the acute phase of malaria. Clin Biochem 28:163–169.
[Crossref] [Google Scholar] [Indexed]
- Luersen K, Walter RD, Muller S (2000) Plasmodium falciparum-infected red blood cells depend on a functional glutathione de novo synthesis attributable to an enhanced loss of glutathione. Biochem J 346:545–552.
- Müller S (2015) Role and regulation of glutathione metabolism in Plasmodium falciparum. Molecules 20:10511–34.
[Crossref] [Google Scholar] [Indexed]
- Selvam R, Mathews ST (1992) Biochemical alterations in Plasmodium vivax-infected malarial patients before and after radical treatment. Indian J Malariol 29:103–111.
- Raza A, Kumar VS, Shahid M, Khan HM, Ashraf Malik M, et al. (2013) Lipid peroxidation in cerebral malaria and role of antioxidants. IOSR J Pharm 3:15–18.
- Postma NS, Mommers EC, Eling WMC, Zuidema J (1982) Oxidative stress in malaria; implications for prevention and therapy. Pharm World Sci 18:121–129.
- Olugbade TA, Oluwadiya JO, Yisak WA (1982) Chemical constituents of cnestis ferruginea DC. I. petroleum ether fraction. J Ethnopharmacol 6:365–370.
[Crossref] [Google Scholar] [Indexed]
- Aschfalk A, Steingass H, Müller W, Drochner W (2000) Acceptance and digestibility of some selected browse feeds with varying tannin content as supplements in sheep nutrition in West Africa. J Vet Med Series A 47:513–524.
[Crossref] [Google Scholar] [Indexed]
- Bouquet A, Debray M (1974) Plantes médicinales de la Côte d’Ivoire. O.R.S.T.O.M., Office de la recherche scientifique et technique outre-mer. Paris. [Crossref]
- Ishola IO, Akindele AJ, Adeyemi OO (2011) Analgesic and anti-inflammatory activities of Cnestis ferruginea Vahl ex DC (Connaraceae) methanolic root extract. J Ethnopharmacol 135:55–62.
[Crossref] [Google Scholar] [Indexed]
- Ishola IO, Chaturvedi JP, Rai S, Rajasekar N, Adeyemi OO, et al. (2013) Evaluation of amentoflavone isolated from Cnestis ferruginea Vahl ex DC (Connaraceae) on production of inflammatory mediators in LPS stimulated rat astrocytoma cell line (C6) and THP-1 cells. J Ethnopharmacol 146:440–448.
[Crossref] [Google Scholar] [Indexed]
- Ishola IO, Chatterjee M, Tota S, Tadigopulla N, Adeyemi OO, et al. (2012) Antidepressant and anxiolytic effects of amentoflavone isolated from Cnestis ferruginea in mice. Pharmacol Biochem Behav 103:322–331.
[Crossref] [Google Scholar] [Indexed]
- Lewis WH, Elvin-Lewis MPF (2003) Medical botanyâ?¯: plants affecting human health. J Wiley
- Akunyili DN, Akunyili DN (2000) Anticonvulsant activity of the ethanol extract of Newbouldia laevis proceedings of the 2nd NAAP Scientific Conference, Zaria, 155-158. Scientific Research Publishing. [Crossref]
- Iwu MM (2014) Handbook of African Medicinal Plants. Handbook of African Medicinal Plants, Second Edition 1–431.
- Yemoa AL, Gbenou JD, Johnson RC, Djego JG, Zinsou C, et al. (2008) Identification et étude phytochimique de plantes utilisées dans le traitement traditionnel de l’ulcère de Buruli au Bénin. Ethnopharmacologia 48–55.
- Zirihi GN, Kra AKM, Guede-Guina F (2003) Évaluation de l’activité antifongique Microglossa Pirifolia (Larmarck) O. Kuntze (Asteraceae) «â?¯PYMIâ?¯» sur la croissance In vitro de Candida albicans. Revue de Medicine et Pharmacie Africaine 17:11–8.
- Bekro YA, Mamyrbekova J, Boua B, Tra Bi F, Ehile E (2007) Étude ethnobotanique et screening phytochimique de Caesalpinia benthamiana (Baill.) Herend. et Zarucchi (Caesalpiniaceae) Sciences and Nature 4:217–225.
- Bolou G, Attioua B, N’guessan Ac, Coulibaly A, N’guessan JD, et al. (2011) Glaucescens planch sur Salmonella typhi et Salmonella typhimurium. Bull Soc R Sci Liège 80:772–790.
- N’Guessan JD, Bidié AP, Lenta BN, Weniger B, André P, et al. (2007) In vitro assays for bioactivity-guided isolation of antisalmonella and antioxidant compounds in Thonningia sanguinea flowers. Afr J of Biotechnol 6:1685–1690.
- Sanogo R, Diallo D, Diarra S, Ekoumou C, Bougoudogo F, et al. (2006) Activite antibacterienne et antalgique de deux recettes traditionnelles utilisees dans dans le traitement des infections urinaires et la cystite au Mali. Mali Médical 21:18–24.
- Parejo I, Codina C, Petrakis C, Kefalas P (2000) Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH* (2,2-diphenyl-1-picrylhydrazyl) free radical assay. J Pharmacol Toxicol Methods 44:507–512.
[Crossref] [Google Scholar] [Indexed]
- Kowalska I, CieÅ?la Å, Oniszczuk T, Waksmundzka-Hajnos M, Oleszek W, et al. (2013) Comparison of two TLC-DPPH•-image processing procedures for studying free radical scavenging activity of compounds from selected varieties of Medicago sativa. J Liq Chromatogr Relat Technol 36:2387–2394.
- CieÅ?la Å, KryszeÅ? J, Stochmal A, Oleszek W, Waksmundzka-Hajnos M (2012) Approach to develop a standardized TLC-DPPH test for assessing free radical scavenging properties of selected phenolic compounds. J Pharm Biomed Anal 70:126–135.
[Crossref] [Google Scholar] [Indexed]
- Trager W, Jensen J (1976) Human malaria parasites in continuous culture. Science 193:673–675.
[Crossref] [Google Scholar] [Indexed]
- Smilkstein M, Sriwilaijaroen N, Kelly J, Wilairat P, Riscoe M (2004) Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806.
[Crossref] [Google Scholar] [Indexed]
- Basco LK (2007) Field application of In vitro assays for the sensitivity of human malaria parasites to antimalarial drugs. Geneva, Switzerland: World Health Organization.
- Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, et al. (2007) Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother 51:1926.
[Crossref] [Google Scholar] [Indexed]
- Akala H, Eyase F, Cheruiyot A, Omondi A, Ogutu B, et al. (2011) Antimalarial drug sensitivity profile of western Kenya Plasmodium falciparum field isolates determined by a SYBR Green I In vitro assay and molecular analysis. American J Trop Med 85:34–41.
[Crossref] [Google Scholar] [Indexed]
- Jonville M, Kodja H, Humeau L, Fournel J, De Mol P, et al. (2008) Screening of medicinal plants from Reunion Island for antimalarial and cytotoxic activity. J Ethnopharmacol 120:382–386.
[Crossref] [Google Scholar] [Indexed]
- Soh PN, Benoit-Vical F (2007) Are West African plants a source of future antimalarial drugs? J Ethnopharmacol 114:130–140.
- Eze UA, Bello S, Etuk E, Ameh GI, Ugwah OM, et al. (2013) Phytochemical and preliminary toxicological studies of the aqueous leave extract of Leucas martinicensis in wistar rats. Int J Med Plants Res 2:166-169. [Crossref]
- Rodrigues E (2007) Plants of restricted use indicated by three cultures in Brazil (Caboclo-river dweller, Indian and Quilombola). J Ethnopharmacol 111:295–302.
[Crossref] [Google Scholar] [Indexed]
- Ferreira JFS, Luthria DL, Sasaki T, Heyerick A (2010) Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 15:3135.
[Crossref] [Google Scholar] [Indexed]
- Phillipson DJ, Wright CW (1991) Can ethnopharmacology contribute to the development of antimalarial agents? J Ethnopharmacol 32:155–65.
[Crossref] [Google Scholar] [Indexed]
- O’Neill MJ, Bray DH, Boardman P, Phillipson JD, Warhurst DC, et al. (1986) Plants as sources of antimalarial drugs: In vitro antimalarial activities of some quassinoids. Antimicrob Agents Chemother 30:101.
[Crossref] [Google Scholar] [Indexed]
- Béourou S, Tuo K, Ouattara K, André TO, Meité S, et al. (2014) International Journal of Chemistry and Phytochemical screening of some medicinal plants used to treat malaria in Côte d ’ Ivoire (West Africa) 2:919–925.
- Tuo K, Béourou S, Toure OA, Ouattara K, Silué Kigbafori D, et al. (2015) Phytochemical Screening and Polyphenolic Contents of Dialium dinklagei and Diospyros monbuttensis, two ivorian medicinal plants used to treat malaria. J Adv Med Pharm Sci 2:144-153.
- Bero J, Quetin-Leclercq J (2011) Natural products published in 2009 from plants traditionally used to treat malaria. Planta Med 77:631–640.
[Crossref] [Google Scholar] [Indexed]
- Bero J, Frédérich M, Quetin-Leclercq J (2009) Antimalarial compounds isolated from plants used in traditional medicine. J Pharm Pharmacol 61:1401–1433.
- Jansen O, Tits M, Angenot L, Nicolas JP, De Mol P, et al (2012) Anti-plasmodial activity of Dicoma tomentosa (Asteraceae) and identification of urospermal A-15-O-acetate as the main active compound. Malaria J :11.
- Hemmer C, Lehr H, Westphal K, Unverricht M, Kratzius M, et al. (2005) Plasmodium falciparum Malaria: reduction of endothelial cell apoptosis In vitro. Infect Immun 73:1764–1770.
[Crossref] [Google Scholar] [Indexed]
- Iyawe HOT, Onigbinde AO, Aina OO (2006) Effect of chloroquine and ascorbic acid interaction on the oxidative stress status of Plasmodium berghei infested mice. Int J Pharmacol 2:1–4.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences